Introduction
Radiation therapy has remained the mainstay of
treatment for glioblastoma, but these tumors are often associated
with radioresistance (1). Heavy
ions represent the best tool for various tumors, especially for
radioresistant tumors mediated by hypoxia, due to several
advantages over conventional radiotherapy (gamma and X-rays) such
as an inverted depth-dose distribution, a higher relative
biological effectiveness, a reduction in oxygen enhancement ratio,
less variation in cell cycle related sensitivity and cells having
decreased ability to repair (2).
Cell apoptosis is a key mechanism through which
ionizing radiation kills tumor cells via two principal signaling
pathways, the extrinsic death pathway involving the ligation of
death receptors and the intrinsic death pathway initiated at the
mitochondrion (3). Among them,
intrinsic apoptosis seems to play a key part in the regulation of
susceptibility of tumor cells to radiation (4). Caspases, a family of cysteine
proteases, have been recognized as important mediators of apoptosis
in the intrinsic apoptotic cascade. There is accumulating evidence
indicating that heavy ion radiation can effectively trigger
apoptosis as described in previous findings (5,6) which
can be through activation of caspase-3 and caspase-9 (7); however, so far little information is
available on the caspase-independent form of apoptosis.
Apoptosis-inducing factor (AIF), a
mitochondrion-localized flavoprotein initially discovered as a
caspase-independent death effector, is released in response to
death stimuli, and subsequently results in chromatin condensation
and large-scale fragmentation of DNA (8–10).
There is definite evidence demonstrating that AIF has been shown to
associate well with induction of cell death after
ischemia/reperfusion (11),
hypoglycemia (12), brain trauma
(13) and neurodegenerative
diseases (14).
Poly(ADP-ribose) polymerase 1 (PARP-1), the major
isoform of the poly(ADP-ribose) polymerase family, is a
constitutive nuclear and mitochondrial protein with well-recognized
roles in base excision repair and DNA strand break repair (15). Over activation of PARP-1 is required
for the translocation of AIF from the mitochondria to nucleus as an
important activator of caspase-independent cell death (16–18).
PARP-1 activity was reported to affect the radiation sensitivity of
tumor cells by manipulation of the DNA repair processes (19), cell cycle (20) and autophagy (21) following exposure to ionizing
radiation.
Among all high-LET heavy ion radiations, CIB is
becoming popular and excellent in treatment of malignant tumors. In
the current study we present preliminary data which could help to
clarify the role of caspase-independent manner in CIB-induced
glioma cell death by means of caspase inhibitor Z-VAD-FMK.
Furthermore, PARP-1/AIF pathway might contribute to the development
of more effective cancer radiotherapy. This study provides the
supplement to the apoptosis mechanisms caused by the heavy ion
radiation and demonstrates a potential benefit of therapeutic
strategies for high-LET particle therapy.
Materials and methods
Cell culture and irradiation
Human glioblastoma multiform (GBM) U251 cell line
was obtained from China Center for Type Culture Collection (CCTCC),
Wuhan, China. Cells were maintained in DMEM medium supplemented
with 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin in a
humid atmosphere of 5% CO2 and 95% air at 37°C. U251
cells were irradiated with 2 Gy 12C6+ ions at
room temperature. A carbon ion beam of 350 MeV/u was supplied by
the Heavy Ion Research Facility in Lanzhou (HIRFL) at the Institute
of Modern Physics, Chinese Academy of Sciences (Lanzhou, China).
The dose rate was adjusted to be approximately 0.2 Gy/min.
Apoptosis assay
Hoechst 33258 staining was performed with a few
modifications. Briefly, after exposure to radiation, confluent
cells were incubated for 24 h at 37°C in 6-well plates. Then these
cells were fixed with 4% paraformaldehyde for 30 min at RT and
washed once with PBS. Fixed cells were stained with Hoechst 33258
of 50 ng/ml and incubated for 30 min at RT and washed with PBS.
Apoptotic cells were identified by condensation and fragmentation
of nuclei examined by fluorescence microscopy.
Quantification of apoptotic cells was obtained using
the Annexin V-FITC detection kit (Invitrogen, Eugen, Oregon, USA)
according to the manufacturer's protocol. Following controls were
used to set up compensation and quadrants: unstained cells and
cells stained with FITC-Annexin V or with PI alone. The
apoptotic/necrotic cell population was analyzed with a FACS Calibur
flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Flowsight data acquisition and
analysis
Acquisition speed was set up to low speed and the
highest resolution, an automated condition provided in Flowsight
(Amnis/Merck Millipore, Darmstadt, Germany). Approximately
1000–5000 cells were acquired. Channel 5 was used to acquire DRAQ5
and channel 2 was used to detect Alexa Fluor 488. Data were
analyzed in IDEAS software after compensation of single color
control samples using a compensation matrix. The frequency of AIF
translocation to the nuclei was analyzed using the nuclear
translocation application wizard in IDEAS software.
Gene expression analysis
Total RNA was extracted from glioma cells at 24 h
after CIB irradiation using the TRIzol Reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. The cDNA was synthesized from 1 mg total RNA using RT
reagent kit with gDNA Eraser (Takara, Tokyo, Japan) following the
manufacturer's protocols. Real-time polymerase chain reaction
(quantitative PCR) was carried out the SYBR Premix EX Taq II kit
(Takara, Dalian, China) on an FTC-3000+ instrument (Funglyn Biotech
Inc., Toronto, ON, Canada). Gene expression was detected using qPCR
primers for PARP-1 and β-actin which was used to normalize the mRNA
and cDNA quantity and quality. Sequences of the primers are as
follows: PARP-1 sense, 5′-TAGGCATGATTGACCGCTGG-3′, and antisense,
5′-ACCATGCCATCAGCTACTCG-3′; β-actin sense,
5′-TGAGCGCAAGTACTCTGTGTGGAT-3′, and antisense,
5′-TAGAAGCATTTGCGGTGCACGATG-3′. The PCR program was denatured at
95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for
30 sec. The fold changes of gene expression of the treatment groups
were calculated by the 2−∆∆Ct method.
Immunofluorescence
GBM U251 cells were fixed in 4% paraformaldehyde for
10 min and permeabilized with 0.2% Triton X-100 for 10 min. Cells
were blocked for 1 h with TBST containing 0.1% BSA followed by a 1
h incubation with rabbit polyclonal or mouse polyclonal antibody,
PARP-1 (1:400, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
and AIF (1:400, CapitalBio, Beijing, China). The primary antibody
was detected with Alexa Fluor-555 donkey anti-rabbi secondary
antibody (1:1000, Invitrogen, Carlsbad, CA, USA) or Alexa Fluor-488
goat anti-mouse (1:1000, Invitrogen). The cells were counterstained
with DAPI (Vector Laboratories, Burlingame, CA, USA). Confocal
images were acquired using a Zeiss LSM-700 confocal microscope.
Fluorescence intensity was quantificationally analyzed using ZEN
2010 software (Carl Zeiss).
Statistical analysis
Statistical analysis for each of the studied
parameters was performed on the means of the data obtained from at
least 3 independent experiments. Data are presented as means ± SD.
Student's t-test program in Microsoft Excel was used to detect
statistical significance. A P-value <0.05 was selected as a
criterion for a statistically significant difference.
Results
Cell apoptosis
Apoptotic cells were determined by Hoechst 33258 and
Annexin V/PI staining 24 h after CIB irradiation. As illustrated in
Fig. 1, it shows radiation-induced
augmentation in the proportion of apoptotic cells which displayed
the typical morphological features of cell death such as cell
shrinkage, nuclear condensation and formation of pyknotic bodies of
condensed chromatin. Z-VAD-FMK (50 µM), pan inhibitor of caspases,
blocked caspase-mediated mitochondrial membrane depolarization.
Interestingly, no distinct difference was detected in the cellular
apoptosis of cells pretreated with Z-VAD-FMK followed by CIB
irradiation (14.52%) and cells treated with CIB alone (16.16%),
indicating that CIB can trigger apoptosis in U251 cells via an
alternative caspase-independent pathway.
AIF translocation
AIF, an important caspase-independent death
effector, translocates from mitochondria to the cytosol as well as
the nucleus when apoptosis is induced. To explore the detailed
mechanism of cell death program elicited by CIB, AIF translocation
was investigated by Imaging Flow Cytometry, which is closely linked
to the caspase-independent apoptosis. Green color denotes AIF
protein and red color denotes nucleus. The merged color regions
with high density represent the AIF translocation into nucleus.
Herein, CIB radiation promoted the AIF nuclear translocation in
glioma cells as observed in Fig.
2A. Amnis data exhibit that the nuclear translocation frequency
of AIF protein was increased 3.96- or 4.81-flod in the CIB alone
group or in the combination with Z-VAD-FMK group compared with the
control group, respectively. Furthermore, incubation with or
without Z-VAD-FMK had a significant difference on the AIF nuclear
translocation (P<0.05, Fig. 2B).
These data imply that the inhibition activation of caspase may lead
to AIF translocation with compensatory enlargement.
PARP-1 mRNA expression
To ascertain the cause of the AIF translocation in
the irradiated glioma cells, we determined the PARP-1 status which
is the decisive molecule responsible for the translocation of AIF
from the mitochondria to nucleus. As given in Fig. 3, the data from qRT-PCR exhibited
that PARP-1 expression level was significantly elevated by
irradiation with CIB compared with the control (P<0.001) at 24 h
post-irradiation. Remarkably, CIB-induced PARP-1 activation is
higher 1.46-fold in the presence of Z-VAD-FMK than in the absence
of Z-VAD-FMK, suggesting that caspase inhibition could improve the
PARP-1 activation. In addition, a positive correlation between AIF
translocation with PARP-1 mRNA level was found (y=19.18x-5.826,
R2=0.951) in Z-VAD-FMK-intervened glioma cells.
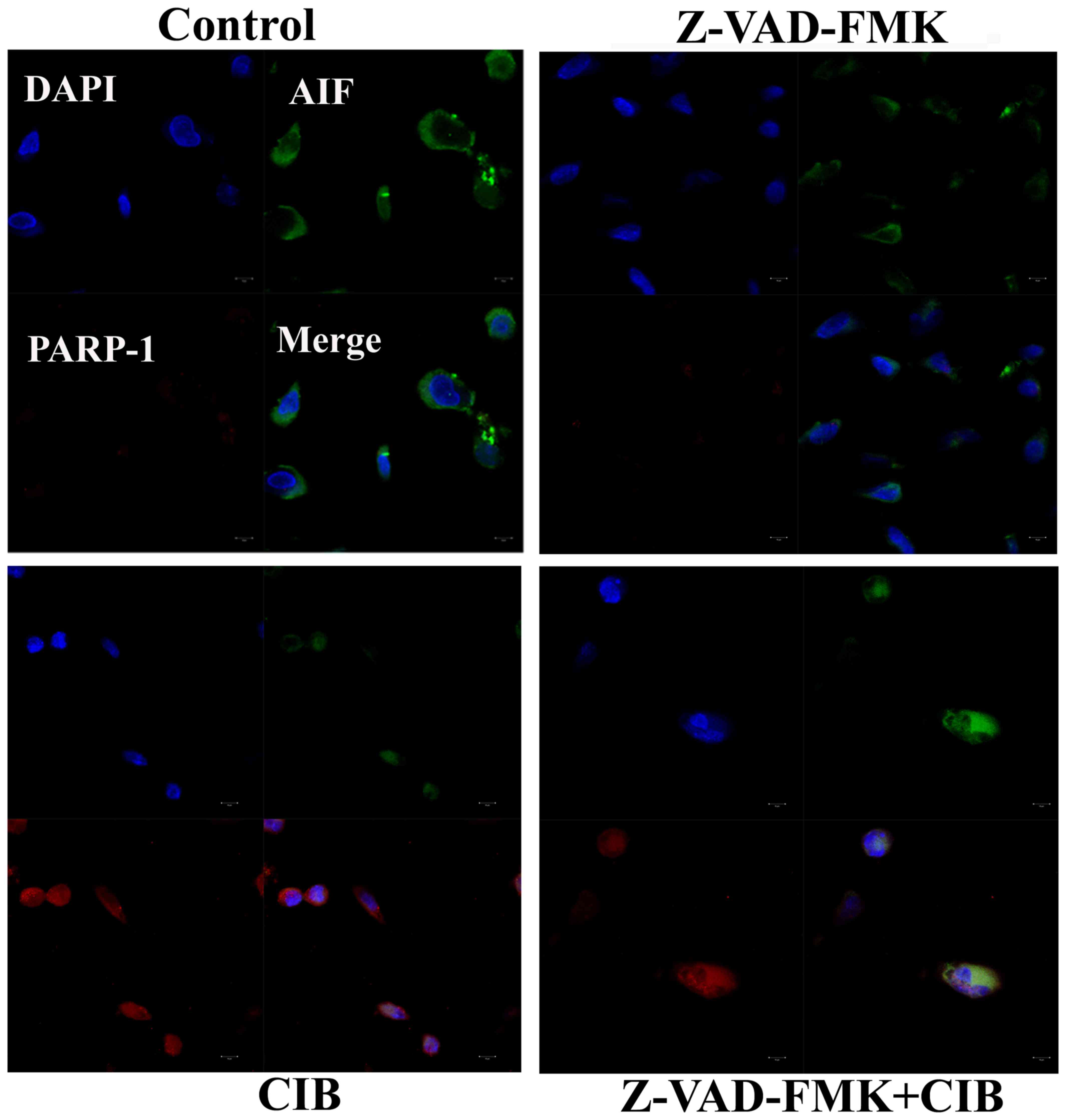
Interactions of PARP-1 with AIF
To further characterize the role of PARP-1 activity
in AIF translocation of glioma cells following CIB treatment, we
confirmed the interactions of AIF and PARP-1 by immunofluorescence
analysis. As shown in the Fig. 4,
it is evident that the colocalization of PARP-1 and AIF in the
nucleus was distinctly potentiated in the cells irradiated with CIB
as well as in those supplemented with Z-VAD-FMK prior to CIB
irradiation. In addition, immunofluorescence result showed that CIB
markedly increased the PARP-1activation, which was possibly in
response to the accumulated DNA damage.
Oxidative DNA damage
The percentage of U251 cells possessing oxidative
damage to their DNA was assessed using 8-OHdG as the marker. As
seen in Fig. 5, CIB radiation
enhanced the oxidation of DNA compared to the untreated control.
The signal intensity of 8-OHdG-positive cells prominently increased
24 h after irradiation (P<0.01), with most immunoreactivity in
the perinuclear region of the cytoplasm (Fig. 5A). The 8-OHdG content was higher in
the mitochondrial than the nuclear fraction. Moreover, the
supplement of Z-VAD-FMK had no significant effect on glioma cells
in terms of the percentage 8-OHdG.
Discussion
Currently, a few studies indicate that inhibition of
caspase did not affect apoptosis in malignant glioma cells and
various cell types (22,23). To evaluate whether high-LET CIB
irradiation-induced apoptosis is caspase-dependent or not, we
immediately applied glioma cells with 50 µM of Z-VAD-FMK, a
pan-caspase inhibitor before irradiation with 2 Gy in the present
study. The fraction of apoptosis measured at 24 h following CIB
irradiation showed that pre-administration with Z-VAD-FMK did not
significantly reduce, in particular, the percentage of Annexin V
staining cells (early apoptotic indicator, right lower quadrant of
Fig. 1B), suggesting that
caspase-independent cell death at an early stage could be triggered
effectively by high-LET CIB. Similarly, the data from Ghorai et
al also found the existence of caspase-independent signaling
pathway of apoptosis in cervical cancer HeLa cells caused by CIB
irradiation (7).
For further insight into the caspase-independent
apoptotic signal evoked by high-LET CIB, we consider that AIF as an
important factor involved in the regulation of the glioma cell
death. There is strong evidence that AIF translocation occurred in
response to neuronal stimuli, including hypoxia, cerebral ischemia
and traumatic brain injury (13,24).
Our results demonstrate that CIB prominently promoted the
mitochondrial release and translocation of AIF to the nucleus
visualized using a FlowSight, suggesting that CIB could activate
AIF-associated caspase-independent apoptosis, and contribute to the
execution of cell death. In particular, the nuclear translocation
of AIF was facilitated when caspase activation was suppressed. It
is likely that the early activation of caspase caused the
inactivation of the process responsible for AIF nuclear
translocation (25). Commonly,
activation of PARP-1, calpain, cytochrome c, Bax has been reported
to affect the mitochondrio-nuclear translocation of AIF (11,26).
However, the molecular mechanism of mitochondrial
AIF release to the nucleus induced by high-LET CIB remains obscure.
PARP-1 activation is proposed to require translocation of AIF from
the mitochondria to the nucleus and that AIF is necessary for
PARP-1-dependent cell death (parthanatos) (18,27).
In the present study, the levels of PARP-1 mRNA and protein were
significantly upregulated by CIB irradiation as seen in the
Figs. 3 and 4. Furthermore, an obvious difference of
PARP-1 activity raised by CIB irradiation was observed in glioma
cells cultured in the presence and absence of Z-VAD-FMK
(P<0.01). Prabhakaran et al also reported that inhibition
of caspase cascade with Z-VAD-FMK led to a marked increase of
PARP-1 in cyanide-treated cortical cells (28). Moreover, the administration of
Z-VAD-FMK enhanced 11′-deoxyverticillin A-elicited PAR formation
(29) which played a pivotal role
in PARP-1 induced cell death (30).
In addition, our data showed a positive correlation between PARP-1
activity and AIF translocation frequency, suggesting that PARP-1
may be strongly activated in glioma cells where it caused the
CIB-induced AIF translocation into nucleus, which is consistent
with the HeLa cells after exposure with CIB (24). As shown in Fig. 4, nuclear colocalization of AIF and
PARP-1 positive signals provided further evidence that PARP-1
initiated AIF-mediated glioma cell death with apoptotic features.
Some literature reported that oxidative damage to DNA specifically
can trigger caspase-independent apoptosis (31). Our data clearly showed that CIB
elevated the amount of 8-OHdG and pre-administration with Z-VAD-FMK
did not alter radiation-induced oxidative stress to DNA (Fig. 5), and consequently PARP-1 may be
activated to involve in base excision repair process that repairs
8-OHdG lesions (32).
Taken together, our results strongly suggested that
AIF translocation from the mitochondria to the nucleus governed by
the activated PARP-1 could be particularly effective in glioma
cells irradiated with high-LET CIB when the caspase protease
cascade is inhibited. These findings demonstrated that at 24 h
after treatment with 2 Gy CIB, caspase-independent glioma cell
death provide an outstanding contribution in cell death via
PARP-1/AIF pathway, which may be involved in DNA oxidative stress
(Fig. 6).
Acknowledgements
This work was supported by grants from the Key
Program of National Natural Science Foundation of China (U1432248),
Ministry of Science and Technology National Key R&D Project
(2016YFC0904602) and National Natural Science Foundation of China
(nos. 1120521, 11305224 and 11575262).
Glossary
Abbreviations
Abbreviations:
|
AIF
|
apoptosis inducing factor
|
|
PARP-1
|
poly(ADP-ribose) polymerase-1
|
|
CIB
|
carbon ion beam
|
|
LET
|
linear energy transfer
|
|
DAPI
|
4′, 6-diamidino-2-phenylindole
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
References
|
1
|
Kao GD, Jiang Z, Fernandes AM, Gupta AK
and Maity A: Inhibition of phosphatidylinositol-3-OH kinase/Akt
signaling impairs DNA repair in glioblastoma cells following
ionizing radiation. J Biol Chem. 282:21206–21212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H, Li S, Wang XH, Li Q, Wei SH, Gao
LY, Zhao WP, Hu ZG, Mao RS, Xu HS, et al: Results of carbon ion
radiotherapy for skin carcinomas in 45 patients. Br J Dermatol.
166:1100–1106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jänicke RU, Engels IH, Dunkern T, Kaina B,
Schulze-Osthoff K and Porter AG: Ionizing radiation but not
anticancer drugs causes cell cycle arrest and failure to activate
the mitochondrial death pathway in MCF-7 breast carcinoma cells.
Oncogene. 20:5043–5053. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park MT, Kim MJ, Kang YH, Choi SY, Lee JH,
Choi JA, Kang CM, Cho CK, Kang S, Bae S, et al: Phytosphingosine in
combination with ionizing radiation enhances apoptotic cell death
in radiation-resistant cancer cells through ROS-dependent and
-independent AIF release. Blood. 105:1724–1733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di CX, Yang LN, Zhang H, An LZ, Zhang X,
Ma XF, Sun C, Wang XH, Yang R, Wu ZH, et al: Effects of carbon-ion
beam or X-ray irradiation on anti-apoptosis ∆Np73 expression in
HeLa cells. Gene. 515:208–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu B, Zhang H, Zhou G, Xie Y, Hao J, Zhou
Q, Duan X and Qiu R: Enhanced cell death by AdCMV-p53 after
irradiation of HeLa cells with 12C6+ ions.
Eur J Obstet Gynecol Reprod Biol. 138:226–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghorai A, Sarma A, Bhattacharyya NP and
Ghosh U: Carbon ion beam triggers both caspase-dependent and
caspase-independent pathway of apoptosis in HeLa and status of
PARP-1 controls intensity of apoptosis. Apoptosis. 20:562–580.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joza N, Susin SA, Daugas E, Stanford WL,
Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, et al:
Essential role of the mitochondrial apoptosis-inducing factor in
programmed cell death. Nature. 410:549–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Candé C, Vahsen N, Garrido C and Kroemer
G: Apoptosis-inducing factor (AIF): Caspase-independent after all.
Cell Death Differ. 11:591–595. 2004.PubMed/NCBI
|
|
11
|
Zhang J, Li XX, Bian HJ, Liu XB, Ji XP and
Zhang Y: Inhibition of the activity of Rho-kinase reduces
cardiomyocyte apoptosis in heart ischemia/reperfusion via
suppressing JNK-mediated AIF translocation. Clin Chim Acta.
401:76–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferrand-Drake M, Zhu C, Gidö G, Hansen AJ,
Karlsson JO, Bahr BA, Zamzami N, Kroemer G, Chan PH, Wieloch T, et
al: Cyclosporin A prevents calpain activation despite increased
intracellular calcium concentrations, as well as translocation of
apoptosis-inducing factor, cytochrome c and caspase-3 activation in
neurons exposed to transient hypoglycemia. J Neurochem.
85:1431–1442. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Chen J, Graham SH, Du L, Kochanek
PM, Draviam R, Guo F, Nathaniel PD, Szabó C, Watkins SC, et al:
Intranuclear localization of apoptosis-inducing factor (AIF) and
large scale DNA fragmentation after traumatic brain injury in rats
and in neuronal cultures exposed to peroxynitrite. J Neurochem.
82:181–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hangen E, Blomgren K, Bénit P, Kroemer G
and Modjtahedi N: Life with or without AIF. Trends Biochem Sci.
35:278–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oláh G, Szczesny B, Brunyánszki A,
López-García IA, Gerö D, Radák Z and Szabo C:
Differentiation-associated downregulation of poly(ADP-ribose)
polymerase-1 expression in myoblasts serves to increase their
resistance to oxidative stress. PLoS One. 10:e01342272015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kolthur-Seetharam U, Dantzer F, McBurney
MW, de Murcia G and Sassone-Corsi P: Control of AIF-mediated cell
death by the functional interplay of SIRT1 and PARP-1 in response
to DNA damage. Cell Cycle. 5:873–877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong SJ, Dawson TM and Dawson VL: Nuclear
and mitochondrial conversations in cell death: PARP-1 and AIF
signaling. Trends Pharmacol Sci. 25:259–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu SW, Wang H, Poitras MF, Coombs C,
Bowers WJ, Federoff HJ, Poirier GG, Dawson TM and Dawson VL:
Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by
apoptosis-inducing factor. Science. 297:259–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carruthers R and Chalmers AJ: Combination
of PARP inhibitors with clinical radiotherapy. Cancer Drug Discov
Dev. 83:533–551. 2015. View Article : Google Scholar
|
|
20
|
Wieler S, Gagné JP, Vaziri H, Poirier GG
and Benchimol S: Poly(ADP-ribose) polymerase-1 is a positive
regulator of the p53-mediated G1 arrest response following ionizing
radiation. J Biol Chem. 278:18914–18921. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen ZT, Zhao W, Qu S, Li L, Lu XD, Su F,
Liang ZG, Guo SY and Zhu XD: PARP-1 promotes autophagy via the
AMPK/mTOR pathway in CNE-2 human nasopharyngeal carcinoma cells
following ionizing radiation, while inhibition of autophagy
contributes to the radiation sensitization of CNE-2 cells. Mol Med
Rep. 12:1868–1876. 2015.PubMed/NCBI
|
|
22
|
Cummings BS, Kinsey GR, Bolchoz LJ and
Schnellmann RG: Identification of caspase-independent apoptosis in
epithelial and cancer cells. J Pharmacol Exp Ther. 310:126–134.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jo GH, Bögler O, Chwae YJ, Yoo H, Lee SH,
Park JB, Kim YJ, Kim JH and Gwak HS: Radiation-induced autophagy
contributes to cell death and induces apoptosis partly in malignant
glioma cells. Cancer Res Treat. 47:221–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghorai A, Sarma A, Bhattacharyya NP and
Ghosh U: Carbon ion beam triggers both caspase-dependent and
caspase-independent pathway of apoptosis in HeLa and status of
PARP-1 controls intensity of apoptosis. Apoptosis. 20:562–580.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cregan SP, Fortin A, MacLaurin JG,
Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS,
Kroemer G, et al: Apoptosis-inducing factor is involved in the
regulation of caspase-independent neuronal cell death. J Cell Biol.
158:507–517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kondo K, Obitsu S, Ohta S, Matsunami K,
Otsuka H and Teshima R: Poly(ADP-ribose) polymerase
(PARP)-1-independent apoptosis-inducing factor (AIF) release and
cell death are induced by eleostearic acid and blocked by
α-tocopherol and MEK inhibition. J Biol Chem. 285:13079–13091.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Kim NS, Haince JF, Kang HC, David
KK, Andrabi SA, Poirier GG, Dawson VL and Dawson TM:
Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is
critical for PAR polymerase-1-dependent cell death (parthanatos).
Sci Signal. 4:ra202011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prabhakaran K, Li L, Borowitz JL and Isom
GE: Caspase inhibition switches the mode of cell death induced by
cyanide by enhancing reactive oxygen species generation and PARP-1
activation. Toxicol Appl Pharmacol. 195:194–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang N, Chen Y, Jiang R, Li E, Chen X, Xi
Z, Guo Y, Liu X, Zhou Y, Che Y, et al: PARP and RIP 1 are required
for autophagy induced by 11′-deoxyverticillin A, which precedes
caspase-dependent apoptosis. Autophagy. 7:598–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin W, Xu W, Chen J, Zhang X, Shi L and
Ren C: Remote limb preconditioning protects against
ischemia-induced neuronal death through ameliorating neuronal
oxidative DNA damage and parthanatos. J Neurol Sci. 366:8–17. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Linsenbardt AJ, Breckenridge JM, Wilken GH
and Macarthur H: Dopaminochrome induces caspase-independent
apoptosis in the mesencephalic cell line, MN9D. J Neurochem.
122:175–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding W, Liu W, Cooper KL, Qin XJ, de Souza
Bergo PL, Hudson LG and Liu KJ: Inhibition of poly(ADP-ribose)
polymerase-1 by arsenite interferes with repair of oxidative DNA
damage. J Biol Chem. 284:6809–6817. 2009. View Article : Google Scholar : PubMed/NCBI
|