Introduction
Cholangiocellular carcinoma (CCC) is the second most
frequent malignant primary liver tumor accounting for ~15% of all
reported cases (1). CCC is
classified into intrahepatic, perihilar and extrahepatic carcinoma,
accounting for 6–8%, 50–67% and 27–42% of cases, respectively
(2). The 5-year survival of newly
diagnosed CCC was only 18–19% in 2010 (3). CCC is usually diagnosed at advanced
stages and only a small portion of patients are eligible for
curative surgery. Even after R0 resection, the 5-year survival is
still very low, ranging from 0 to 40% of cases (4). For the majority of patients,
chemotherapy with gemcitabine, cisplatin or 5-fluorouracil remains
the only therapeutic option. However, even then the average
survival is ~6 months (5).
Cisplatin is one of the most effective
chemotherapeutic drugs that has been used in cancer treatment for
over 30 years. Cisplatin and other platinum-based drugs have been
used in monotherapy or in combination for treating various types of
cancers, including ovarian carcinomas, lung cancer, various
lymphomas, sarcomas and CCC (4,6–9). The
main mechanism of action of cisplatin is based on forming DNA
adducts and triggering double-strand breaks (10). Although, cisplatin is a very potent
anticancer drug, its effectiveness is diminished by the development
of increasing resistance to cisplatin (11). Thus, many researchers have focused
on identifying strategies to increase the sensitivity of
chemotherapeutic agents.
The bioactive agent, sulforaphane (SFN), is an
isothiocyanate cleavage product of glucoraphanin that has been
transformed by the plant enzyme myrosinase. It is obtained from
damaged cruciferous vegetables such as broccoli, cauliflower,
cabbage and Brussels sprouts (12,13).
In the past 20 years, SFN was proven to be a potent
anti-inflammatory, anti-carcinogenic, and chemopreventative agent
in many cancers (14). Recent
findings suggest that SFN is able to modulate the response to
various chemotherapeutic drugs either by increasing sensitivity or
reducing resistance of cancer cells to them (15–18).
In the present study, we demonstrated for the first time, that SFN
not only reduced the viability of human CCC cell lines, but also
reduces the resistance to cisplatin and synergistically increases
apoptosis, a process which was reflected by the modulation of the
expression of proteins involved in apoptosis.
Materials and methods
Cell culture and treatment
The CCC cell lines HuCCT-1 (19) and TFK-1 (20) represent intrahepatic and
extrahepatic cell origin and different grades of advancement [Riken
BRC Cell Bank (Tsukuba, Ibaraki, Japan); German Collection of
Microorganisms and Cell cultures (DSMZ; Braunschweig, Germany)],
respectively. HuCCT-1 and TFK-1 cells were cultured in RPMI medium
which was supplemented with 10% fetal calf serum (FCS), penicillin
(100 U/ml)/streptomycin (100 µg/ml) (both from Biochrom AG, Berlin,
Germany), and stable L-glutamine. Cells were incubated at 37°C in a
5% CO2 humidified atmosphere and harvested once a week
at full confluence. L-SFN (Sigma-Aldrich, St. Louis, MO, USA) was
diluted in dimethyl sulfoxide (DMSO) to a concentration of 100 mM
and stored in aliquots. We used cisplatin (CisPt) (Teva GmbH,
Radebeul, Germany) at a concentration of 1 mg/ml. Adequate
dilutions were made prior to treatment.
Cell viability assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Carl Roth, Karlsruhe, Germany) assay was used to test the viability
of the CCC cells. Approximately 3×103 cancer cells were
seeded in each well into 96-well plates. Cells were treated after
24 h pre-incubation with CisPt (5 µM), SFN (10 µM) and their
combination (CisPt 5 µM+SFN 10 µM). At the end of each treatment,
10 µl of 1 mg/ml MTT was added to each well and incubated for 4 h
at 37°C. After a 4-h incubation period, the medium was aspirated
and plates were left to dry for 30 min. The precipitated formazan
crystals were dissolved using 2-propanol (VWR International,
Darmstadt, Germany) and absorbance at 570 nm was measured using a
spectrophotometer (Anthos Mikrosysteme GmbH, Krefeld, Germany).
Drug combination index
To calculate the combination index (CI), we used the
following equation: CI = AB/A × B, where AB is the percentage of
viable cells in the group receiving combination treatment using
drugs A and B for different cell lines, and A and B represent the
percentage of viable cells for the treatment groups using drug A or
B alone, respectively. A CI value >1 indicates an antagonistic
effect, a CI value equivalent to 1 indicates an additive effect, a
CI value <1 indicates a synergistic effect and a CI value
<0.7 is indicative of significant synergy (21).
Flow cytometric analysis of
apoptosis
Cells (2×105) were seeded into 12-well
plates and left to adhere overnight. On the next day, medium was
replaced with treatment solution for the following 36 h. Apoptotic
cells were detected using Annexin V-FITC apoptosis detection kit
(BioVision, Mountain View, CA, USA). Briefly, cells were harvested
and centrifuged at 1,800 rpm for 5 min before being washed twice
with binding buffer. Then, the cells were incubated in binding
buffer containing Annexin V and propidium iodide. For flow
cytometric analysis, we used FACSCalibur (Becton-Dickinson
Biosciences, Franklin Lakes, NJ, USA). At least 10,000 cells were
gated for each experiment. The total numbers of cells in the right
upper and lower quadrants were counted as apoptotic cells.
Platinated DNA content analysis
To assess how much DNA was platinated, we seeded
2×105 cells into 12-well plates. After 24 h, we added
the treatment solution and incubated for an additional 48 h. Cells
were then harvested, centrifuged, and fixed with 4%
paraformaldehyde on ice, followed by permeabilization with 0.1%
Triton X-100 on ice. After permeabilization, primary anti-cisplatin
modified DNA (ab103261; ratio 1:200; Abcam, Cambridge, MA, USA)
antibody was added before being dissolved in phosphate-buffered
saline (PBS) containing 1% FCS. Cells were then washed twice after
a 1-h incubation and secondary Alexa Flour 488-conjugated anti-rat
IgG (Cell Signaling Technology, Beverly, MA, USA) antibody was
added in a ratio of 1:1,000. After 30 min of incubation in the
dark, the cells were analyzed using FACSCalibur
(Becton-Dickinson).
Western blot analysis
After 48 h of cell culture incubation with
treatment, cell lysates were prepared in RIPA buffer
(Sigma-Aldrich) using a proteinase inhibitor cocktail (Roche
Diagnostics GmbH, Mannheim, Germany). NuPAGE 4–12% Bis-Tris, NuPAGE
12% Bis-Tris and NuPAGE 4% Tris-Gly Gels (Novex, Carlsbad, CA, USA)
electrophoresis of 20 µg of each protein sample was performed using
XCell SureLock Mini-Cell Module (Invitrogen, Carlsbad, CA, USA).
Then, the cells were transferred to nitrocellulose membranes
(Bio-Rad Laboratories, Munich, Germany) using XCell II™ Blot Module
(Invitrogen). Membranes were blocked in TBS + 0.1% Tween with 5%
BSA (Serva Electrophoresis, Heidelberg, Germany) and incubated with
primary antibodies at 4°C overnight, followed by incubation with
secondary antibodies for 1 h at room temperature. All membranes
were scanned using LI-COR Odyssey CLx scanner (LI-COR
Biotechnology, Lincoln, NE, USA). All primary were purchased from
Cell Signaling Technology, unless otherwise indicated. Primary
antibodies were diluted to a ratio of 1:1,000 and secondary
antibodies to a ratio of 1:10,000 in 5% BSA solution. Western blot
quantification was performed with LI-COR Image Studio Software
(LI-COR Biotechnology).
Statistical analysis
All data are expressed as mean ± SD and were
evaluated with one-way ANOVA using GraphPad Prism 5 (GraphPad
Software Inc., San Diego, CA, USA). Each experiment was repeated at
least three times. Values were considered significant at
p<0.05.
Results
Impact of SFN and CisPt on cell
viability
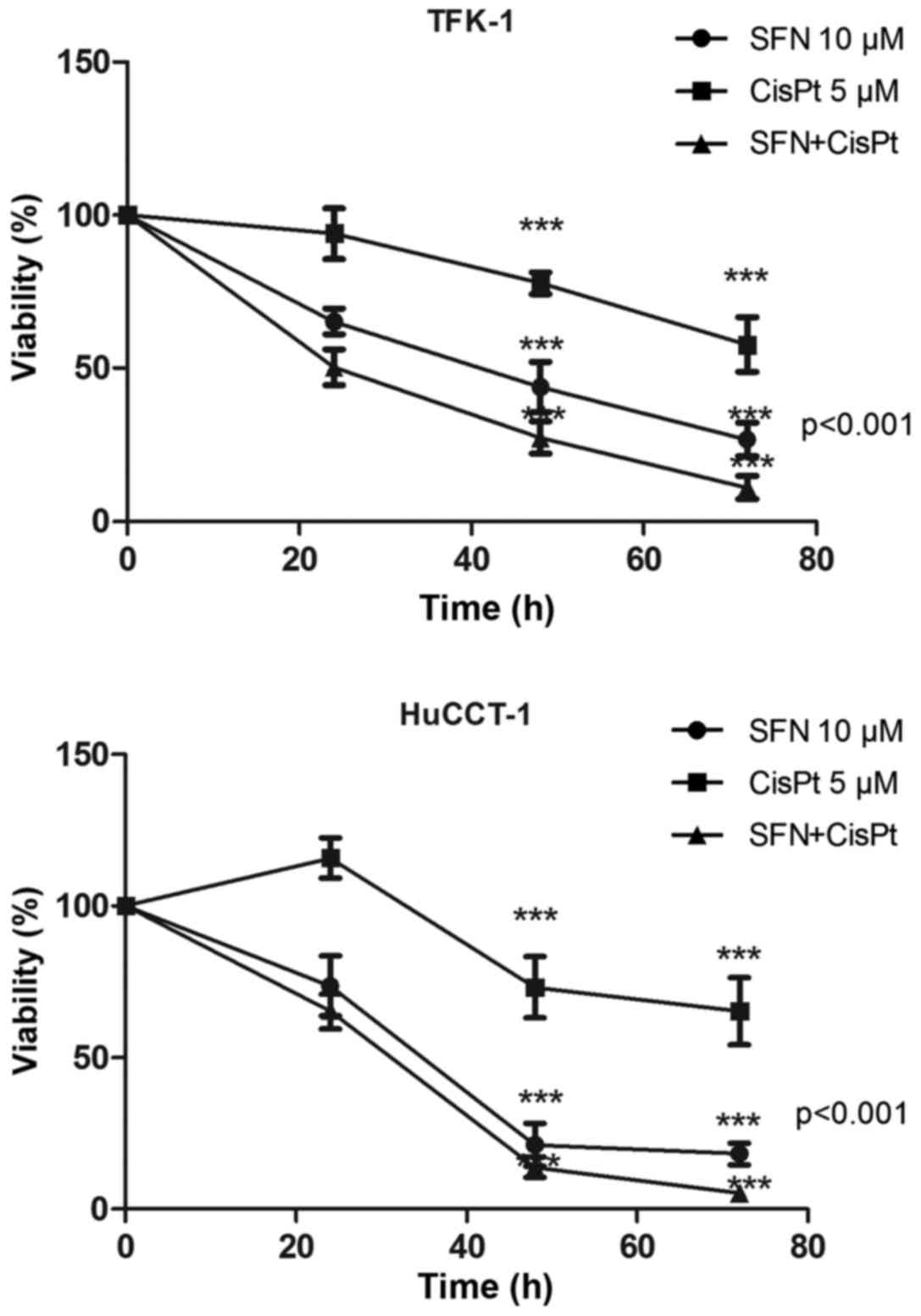
MTT viability assay was used to assess the effect of
SFN, CisPt and their combination therapy on the CCC cell lines,
TFK-1 and HuCCT-1 (Table I).
Treatment was shown to reduce the viability of TFK-1/HuCCT-1 cells
in a time-dependent manner to 26.7±5.53/18.13±3.56,
57.72±8.92/65.25±11.12 and 11.04±3.78/5.11±2.00%, respectively
after 72 h. The CI was 0.72 and 0.43 in the TFK-1 and HuCCT-1
cells, respectively (Fig. 1).
 | Table I.Drug combination layout indicating the
combination indices (CI). |
Table I.
Drug combination layout indicating the
combination indices (CI).
|
| CisPt (µM) |
|---|
|
|
|
|---|
| CCC cell line | 2.5 | 5 |
|---|
| TFK-1 cells |
|
|
| SFN
(µM) |
|
|
|
5 | 1.188 | 0.767 |
| 10 | 0.94 | 0.722 |
| HuCCT-1 cells |
|
|
| SFN (µM) |
|
|
|
5 | 1.692 | 1.284 |
| 10 | 0.834 | 0.4355 |
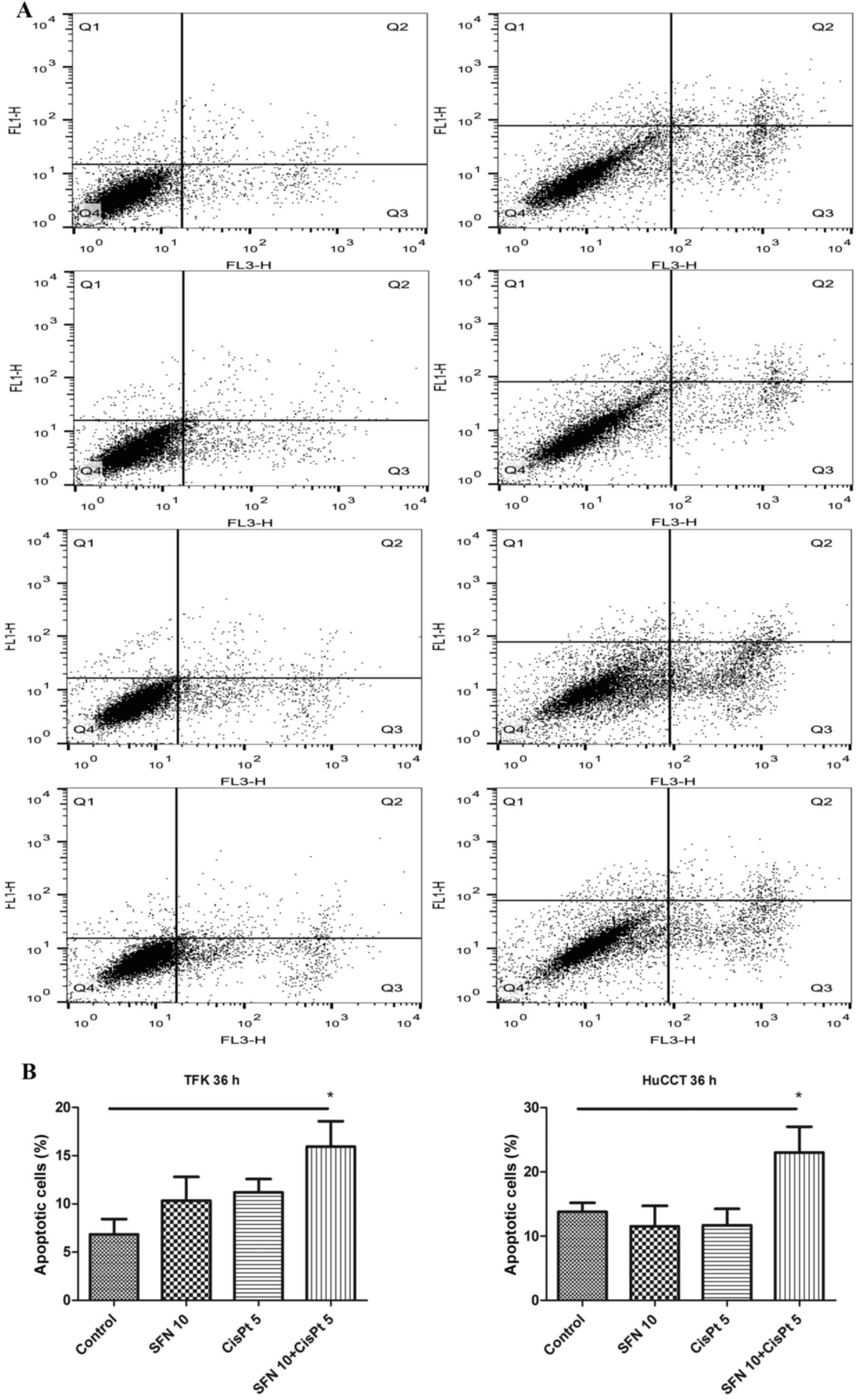
Apoptosis
To examine the effect on apoptosis, the cells were
treated, followed by staining with Annexin V, propidium iodide and
FACS analysis 36 h later. While the treatment with either SFN or
CisPt alone did not induce significant apoptosis at this early time
point, the combination treatment significantly increased the
percentage of apoptotic cells from 7 to 16%, and from 13 to 23% in
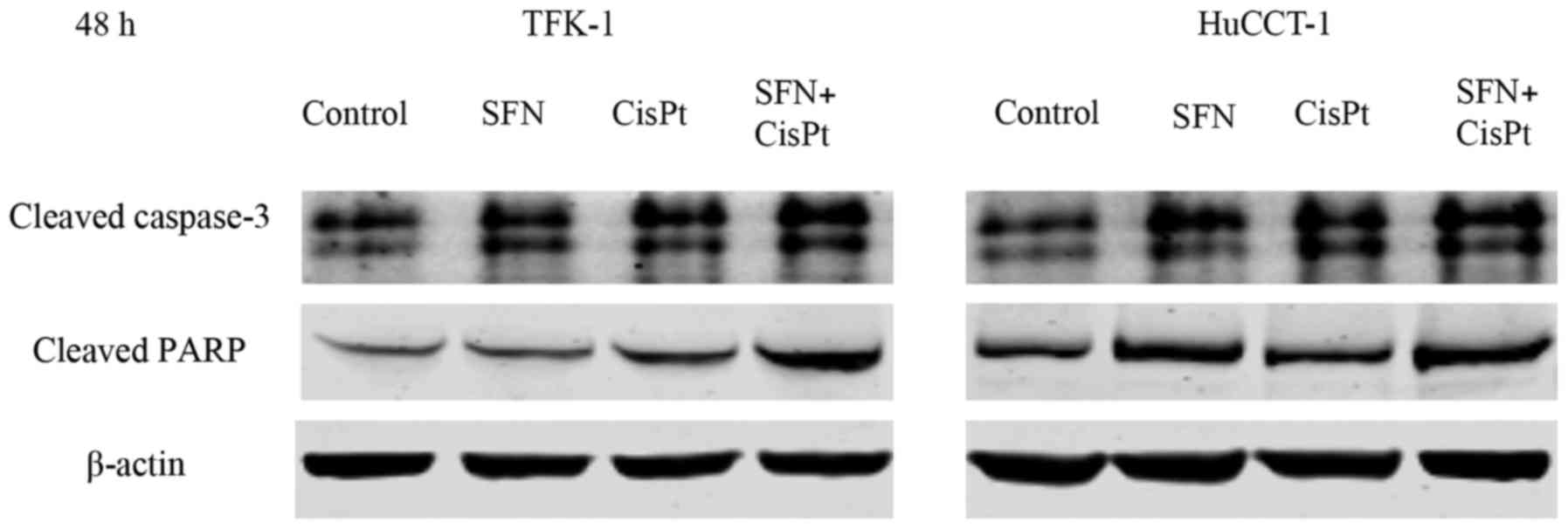
the TFK-1 and HuCCT-1 cell lines, respectively (Fig. 2). Correspondingly, the expression of
cleaved caspase-3 and cleaved PARP was greater after the
combination therapy compared to the expression levels noted in the
controls and single substances 48 h after treatment (Fig. 3).
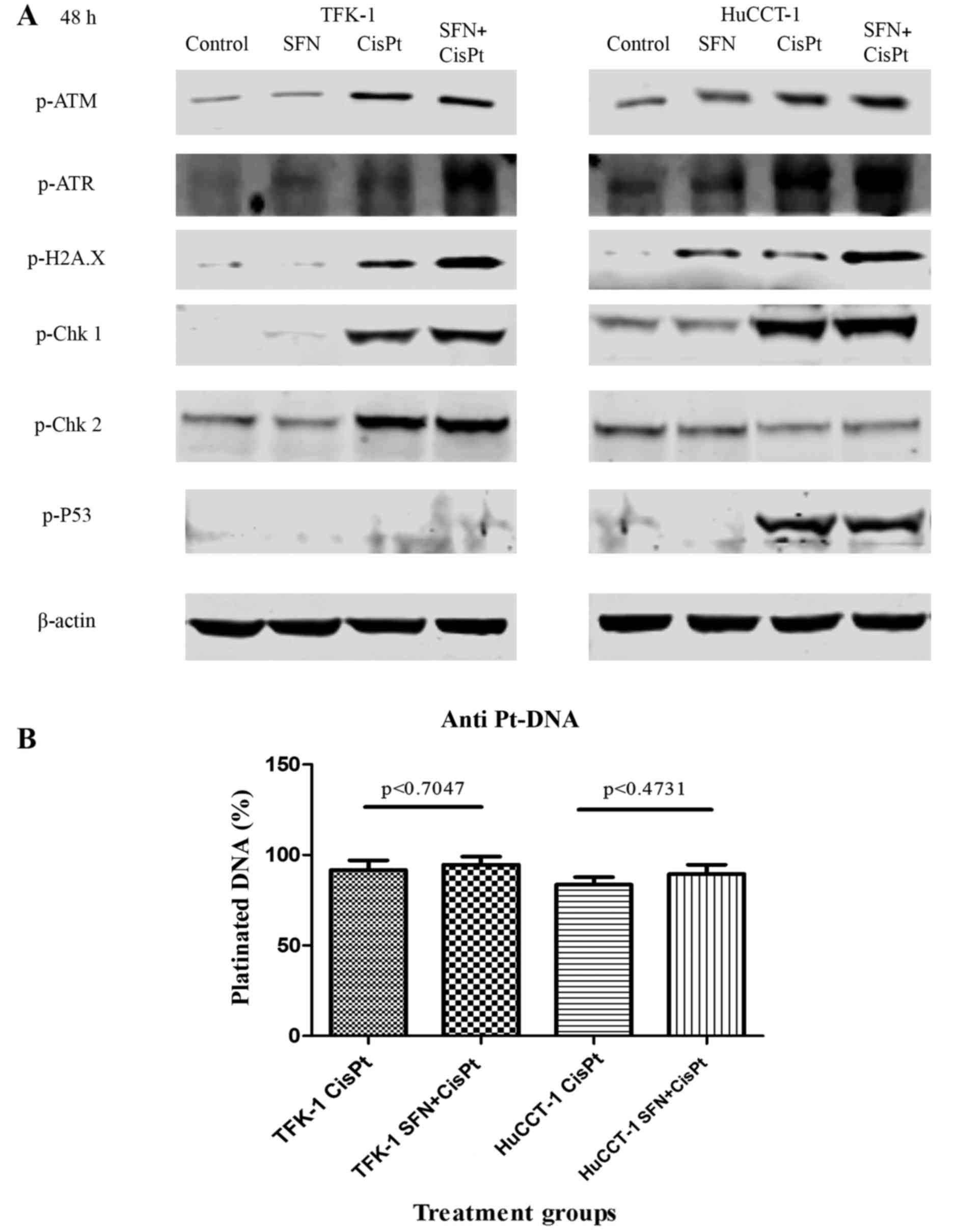
DNA damage quantification
To determine whether SFN mediated the observed
effect by modulating the DNA damage response, we examined the
expression of a panel of proteins known to be involved in DNA
damage response. In most cases, only CisPt led to a pronounced
induction in the phosphorylated forms of ATM, ATR, H2A.X (a genuine
marker of DNA damage) (22) Chk1,
Chk2 and p53, whereas the combination treatment largely did not
increase the protein levels that were already enhanced by the
presence of CisPt alone (Fig. 4A).
In order to elucidate whether SFN may have increased the percentage
of DNA, platinated by CisPt, we detected platinated DNA by staining
with a specific antibody and FACS analysis. However, we did not
detect a statistically significant increase in the amount of
platinated DNA upon the combination of CisPt and SFN (Fig. 4B), thereby suggesting that SFN may
sensitize the cells by influencing apoptosis mechanisms.
Downregulation of anti-apoptotic
proteins can overcome induced resistance
To further explain the assumption that SFN targets
apoptosis signaling, we examined the expression of the
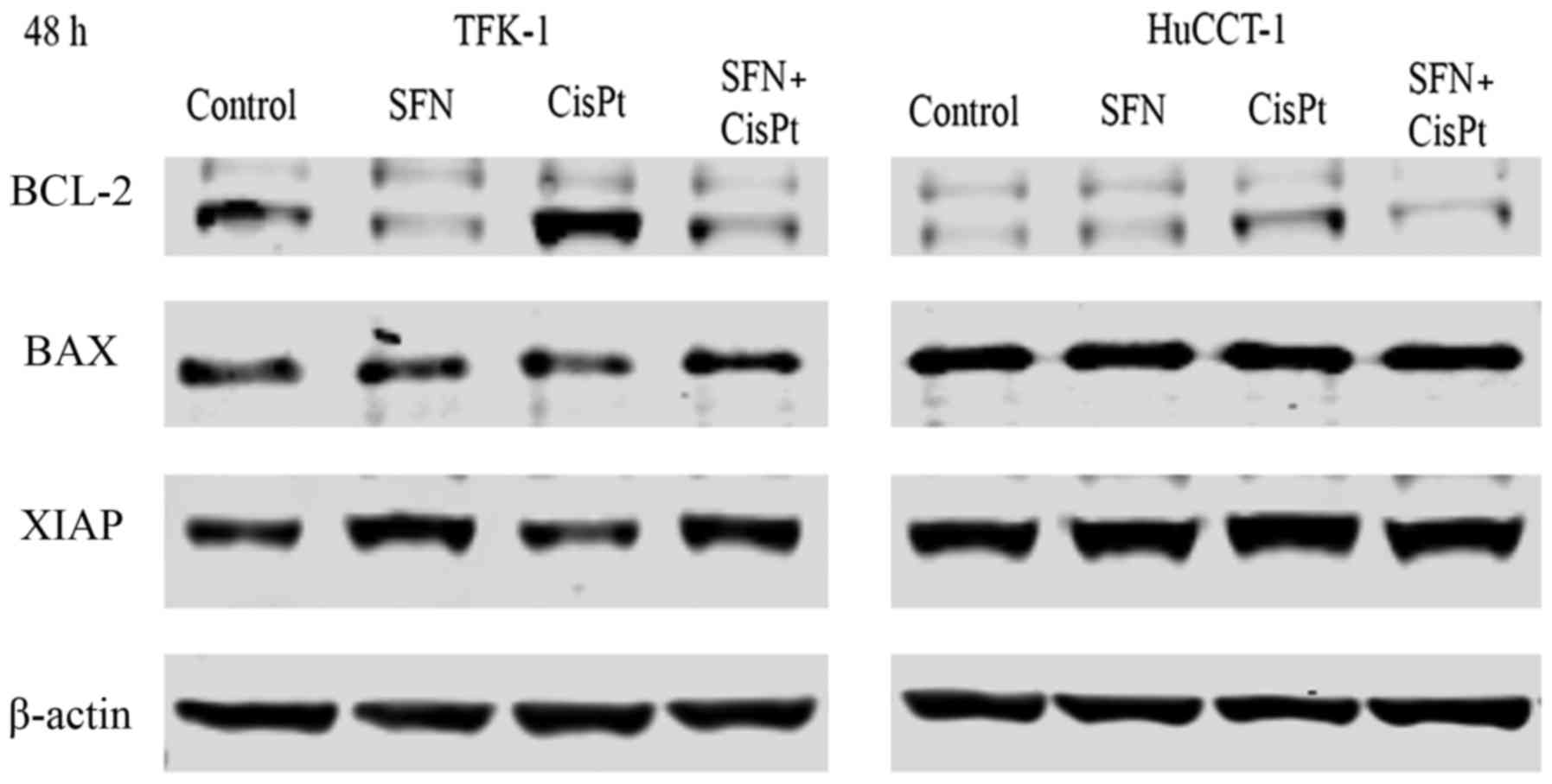
anti-apoptotic genes Bcl-2 and XIAP and pro-apoptotic Bax via
western blot analysis. CisPt induced the expression of Bcl-2 and
this induction was inhibited by the combination with SFN (Fig. 5). A similar tendency was observed
for the expression of XIAP. Bax expression was not altered by any
of the treatment groups.
Discussion
Sulforaphane has been studied for its antioxidant,
anti-carcinogenic, chemopreventative and chemosensitizing abilities
for over 20 years. It has been shown that sulforaphane enhances the
effect of various chemotherapeutic agents in a variety of cancers
(16–18,24–26).
Data presented in the present study indicate, for the first time,
that sulforaphane itself induced apoptosis in two human CCC cell
lines which represent intrahepatic and extrahepatic origins of CCC,
and enhanced cisplatin-mediated cytotoxicity. However, more
detailed analysis in drug synergism is required in future
studies.
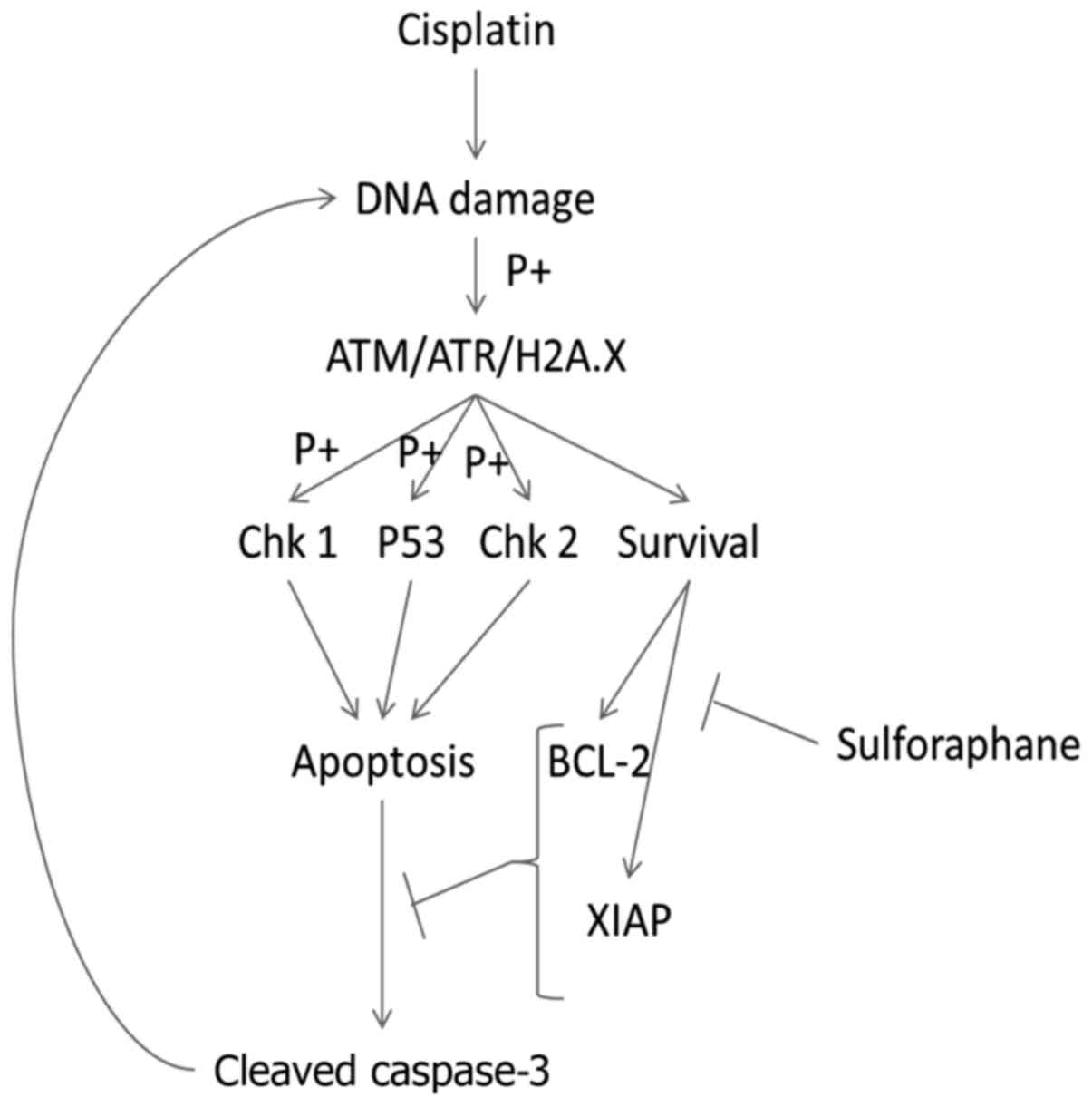
ATM, ATR and H2A.X play important roles in DNA
damage recognition and activation. ATM and ATR are automatically
phosphorylated upon DNA damage and this event is followed by
phosphorylation of H2A.X (27,28).
Phosphorylated H2A.X is considered the hallmark of DNA damage
(22,29). The data of the present study
indicated that cisplatin increased the expression of the DNA
damage-associated proteins, H2A.X, ATM and ATR. Activation of Chk
and P53 is the next step in the DNA damage recognition cascade,
which is usually followed by cell apoptosis when DNA damage is too
extensive (30,31). Indeed, we observed that activation
of Chk1 was increased after the combination treatment compared to
cisplatin only therapy, but the overall expression of pChk1 was
higher in the HuCCT-1 cells. Notably, pChk2 was only upregulated in
the TFK-1 cells. From these results, we can speculate that there
may be a balance in checkpoint kinase activation. This means, when
one kinase is downregulated, the other is upregulated; however,
further research is needed to confirm this assumption. We also
found that P53 phosphorylation was only present in the HuCCT-1
cells. These findings suggest that the combined treatment of
sulforaphane and cisplatin caused extensive DNA damage which may be
followed by increased P53 checkpoint activation and apoptosis,
thereby causing DNA damage (32).
Several studies indicate that DNA damage correlates
with the amount of cisplatin DNA adducts (11,23).
To investigate whether increased DNA damage is due to increased DNA
adducts, we performed flow cytometry on platinated DNA. The
quantities of platinated DNA detected after cisplatin only or the
combination therapy were comparable. Thus, other mechanisms may be
responsible for the increased DNA damage and apoptosis found after
the combination treatment. It has been reported that one of the
many mechanisms of resistance to cisplatin involves the
overexpression of anti-apoptotic proteins and the Bax/Bcl-2 ratio
(11,33). To investigate this hypothesis, we
examined the anti-apoptotic proteins Bcl-2 and XIAP by western blot
analysis. Cisplatin was found to induce the expression of Bcl-2 in
both cell lines, although the expression was higher in TFK-1 cells.
However, Bcl-2 expression was downregulated after sulforaphane only
or combination therapy. There was no difference in XIAP expression
in the TFK-1 cells, whereas it was downregulated in the HuCCT-1
cells after sulforaphane only or in the combination therapy.
Expression of pro-apoptotic protein Bax was not altered, which
leads to believe that only anti-apoptotic proteins influenced the
resistance and the Bax/Bcl-2 ratio. These results suggest that one
of the contributing factors in the increased apoptosis and DNA
damage was the downregulation of anti-apoptotic proteins, thus
enabling the activity of the apoptosis signaling cascade to proceed
without interruption (Fig. 6).
In conclusion, data shown in the present study
clearly indicate, for the first time, that sulforaphane decreased
the drug resistance of human CCC cells to cisplatin in vitro
via various mechanisms, including a decrease in the levels of
anti-apoptotic proteins and increased DNA damage. If the effect of
sulforaphane is confirmed in humans, chemotherapy against CCC could
be more effective by dietary supplementation and may ultimately
increase patient survival.
Acknowledgements
We would like to thank E. Mohr for laboratory and
technical advice, and Dr M. Zoeller for technical support. The
present study was supported by a research grant of the Heidelberg
Surgical Foundation.
References
|
1
|
Macias RI: Cholangiocarcinoma: Biology,
clinical management, and pharmacological perspectives. ISRN
Hepatol. 2014:8280742014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakeeb A, Pitt HA, Sohn TA, Coleman J,
Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ and Cameron
JL: Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and
distal tumors. Ann Surg. 224:463–475. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogel A, Wege H, Caca K, Nashan B and
Neumann U: The diagnosis and treatment of cholangiocarcinoma. Dtsch
Arztebl Int. 111:748–754. 2014.PubMed/NCBI
|
|
4
|
DeOliveira ML, Cunningham SC, Cameron JL,
Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ and Schulick
RD: Cholangiocarcinoma: Thirty-one-year experience with 564
patients at a single institution. Ann Surg. 245:755–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eckel F and Schmid RM: Chemotherapy in
advanced biliary tract carcinoma: A pooled analysis of clinical
trials. Br J Cancer. 96:896–902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Y, Hu Y, Hu X, Li X, Lin L and Han X:
Cisplatin combined with irinotecan or etoposide for untreated
extensive-stage small cell lung cancer: A multicenter randomized
controlled clinical trial. Thorac Cancer. 6:785–791. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Velasquez WS, Cabanillas F, Salvador P,
McLaughlin P, Fridrik M, Tucker S, Jagannath S, Hagemeister FB,
Redman JR, Swan F, et al: Effective salvage therapy for lymphoma
with cisplatin in combination with high-dose Ara-C and
dexamethasone (DHAP). Blood. 71:117–122. 1988.PubMed/NCBI
|
|
8
|
O'Kane GM, Cadoo KA, Walsh EM, Emerson R,
Dervan P, O'Keane C, Hurson B, O'Toole G, Dudeney S, Kavanagh E, et
al: Perioperative chemotherapy in the treatment of osteosarcoma: A
26-year single institution review. Clin Sarcoma Res. 5:172015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stein A, Arnold D, Bridgewater J,
Goldstein D, Jensen LH, Klümpen HJ, Lohse AW, Nashan B, Primrose J,
Schrum S, et al: Adjuvant chemotherapy with gemcitabine and
cisplatin compared to observation after curative intent resection
of cholangiocarcinoma and muscle invasive gallbladder carcinoma
(ACTICCA-1 trial) - a randomized, multidisciplinary, multinational
phase III trial. BMC Cancer. 15:5642015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Talalay P, Cho CG and Posner GH:
A major inducer of anticarcinogenic protective enzymes from
broccoli: Isolation and elucidation of structure. Proc Natl Acad
Sci USA. 89:pp. 2399–2403. 1992; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fahey JW, Zalcmann AT and Talalay P: The
chemical diversity and distribution of glucosinolates and
isothiocyanates among plants. Phytochemistry. 56:5–51. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Juge N, Mithen RF and Traka M: Molecular
basis for chemoprevention by sulforaphane: A comprehensive review.
Cell Mol Life Sci. 64:1105–1127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin CY, Moon DO, Lee JD, Heo MS, Choi YH,
Lee CM, Park YM and Kim GY: Sulforaphane sensitizes tumor necrosis
factor-related apoptosis-inducing ligand-mediated apoptosis through
downregulation of ERK and Akt in lung adenocarcinoma A549 cells.
Carcinogenesis. 28:1058–1066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hunakova L, Gronesova P, Horvathova E,
Chalupa I, Cholujova D, Duraj J and Sedlak J: Modulation of
cisplatin sensitivity in human ovarian carcinoma A2780 and SKOV3
cell lines by sulforaphane. Toxicol Lett. 230:479–486. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rausch V, Liu L, Kallifatidis G, Baumann
B, Mattern J, Gladkich J, Wirth T, Schemmer P, Büchler MW, Zöller
M, et al: Synergistic activity of sorafenib and sulforaphane
abolishes pancreatic cancer stem cell characteristics. Cancer Res.
70:5004–5013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Landen CN, Li Y, Alvarez RD and
Tollefsbol TO: Enhancement of cisplatin-mediated apoptosis in
ovarian cancer cells through potentiating G2/M arrest and p21
upregulation by combinatorial epigallocatechin gallate and
sulforaphane. J Oncol. 2013:8729572013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyagiwa M, Ichida T, Tokiwa T, Sato J and
Sasaki H: A new human cholangiocellular carcinoma cell line
(HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium.
In Vitro Cell Dev Biol. 25:503–510. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saijyo S, Kudo T, Suzuki M, Katayose Y,
Shinoda M, Muto T, Fukuhara K, Suzuki T and Matsuno S:
Establishment of a new extrahepatic bile duct carcinoma cell line,
TFK-1. Tohoku J Exp Med. 177:61–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong ZF, Zhao WX, Yin ZY, Xie CR, Xu YP,
Chi XQ, Zhang S and Wang XM: Capsaicin enhances the drug
sensitivity of cholangiocarcinoma through the inhibition of
chemotherapeutic-induced autophagy. PLoS One. 10:e01215382015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mah LJ, El-Osta A and Karagiannis TC:
gammaH2AX: A sensitive molecular marker of DNA damage and repair.
Leukemia. 24:679–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roberts JJ and Fraval HN: Cisplatin:
Current Status and New Developments. Prestayko AW, Crooke ST and
Carter SK: Academic Press; Orlando: pp. 57–77. 1980, View Article : Google Scholar
|
|
24
|
Kallifatidis G, Labsch S, Rausch V,
Mattern J, Gladkich J, Moldenhauer G, Büchler MW, Salnikov AV and
Herr I: Sulforaphane increases drug-mediated cytotoxicity toward
cancer stem-like cells of pancreas and prostate. Mol Ther.
19:188–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma C, Sadrieh L, Priyani A, Ahmed M,
Hassan AH and Hussain A: Anti-carcinogenic effects of sulforaphane
in association with its apoptosis-inducing and anti-inflammatory
properties in human cervical cancer cells. Cancer Epidemiol.
35:272–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fimognari C, Lenzi M, Sciuscio D,
Cantelli-Forti G and Hrelia P: Combination of doxorubicin and
sulforaphane for reversing doxorubicin-resistant phenotype in mouse
fibroblasts with p53Ser220 mutation. Ann NY Acad Sci.
1095:62–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Helt CE, Cliby WA, Keng PC, Bambara RA and
O'Reilly MA: Ataxia telangiectasia mutated (ATM) and ATM and
Rad3-related protein exhibit selective target specificities in
response to different forms of DNA damage. J Biol Chem.
280:1186–1192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Podhorecka M, Skladanowski A and Bozko P:
H2AX phosphorylation: Its role in DNA damage response and cancer
therapy. J Nucleic Acids. 2010:9201612010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doerks T, Copley RR, Schultz J, Ponting CP
and Bork P: Systematic identification of novel protein domain
families associated with nuclear functions. Genome Res. 12:47–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khalil HS, Tummala H and Zhelev N: ATM in
focus: A damage sensor and cancer target. Biodiscovery.
5(1)2012.doi: 10.7750/BioDiscovery.2012.5.1.
|
|
32
|
Liu X, He Y, Li F, Huang Q, Kato TA, Hall
RP and Li CY: Caspase-3 promotes genetic instability and
carcinogenesis. Mol Cell. 58:284–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|