Introduction
Gestational trophoblastic disease is a spectrum of
trophoblast abnormal proliferation (1), among which choriocarcinoma is the most
malignant one tending to spread to lung, liver and/or brain. Though
most choriocarcinoma occurs after a complete mole, it may also be
secondary to any normal or abnormal pregnancy, including partial
mole, term pregnancy, induced/spontaneous abortion, premature
delivery and stillbirth (2).
However, ~25% of gestational trophoblastic tumors will be resistant
to, or will relapse after initial chemotherapy (3,4). Also,
in development of gestational trophoblastic disease, excess
proliferation is an important clue and is related with outcome of
treatment (5,6). Therefore, we are wondering if there is
an alternative medicine which may inhibit trophoblastic cell
proliferation and improve the curative rate of choriocarcinoma.
Cell proliferation is regulated by positive and
negative cell cycle regulatory factors. Cyclin-dependent kinases
(CDKs) are the key factors forming CDKs/cyclins complexes (such as
CDK4/cyclin D1), while CDK inhibitors (CKIs), such as p21 and p15,
are negative regulators acting as the brakes of the cell cycle
transition by obstructing the formation of CDK/cyclin complexes
(7,8). Activation of CDK/cyclins is required
for cell cycle progression through the G1/S checkpoint.
Proliferation nuclear antigen (PCNA), associated with DNA
synthesis, functions in the cell cycle progression from early G1 to
S phase, and its function is inhibited when p21 binds to it
(9). c-myc positively regulates the
expression and/or activity of cyclins (D1, D2, E and A), CDK2/CDK4,
and additionally suppresses CDKIs such as p15, p21 and p27
(10). Therefore, these proteins
are often used to indicate the interference on the cell cycle.
Also, arresting cell cycle in G1 phase or G2/M phase is one of the
mechanisms used by anticancer medicines.
Cellular proliferation is strongly regulated by
mitogen-activated protein kinase (MAPK), including extracellular
signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase
(JNK), and p38 MAPK, in a variety of cell types. The ERK1/2
signaling pathway, activated by phosphorylation and subsequent
nuclear translocation, has been implicated as a regulator of cell
cycle arrest (11,12). Also, ERK signal pathway is involved
in trophoblast cell proliferation and invasion (13,14).
Recently, the antitumor effects of ingredients from
diet have been paid attention to because of their accessibility and
security. Daidzein belongs to the isoflavones family, one of the
most commonly ingested and most intensely studied type of
phytoestrogen, often found in nuts, fruits, soybeans, and soy-based
products (15). Previously,
daidzein garnered interest in its antitumor activity especially in
proliferation inhibition and apoptosis induction. Daidzein induces
apoptosis in prostate cancer cells (LnCap and PC3) (16), and its metabolite inhibits prostate
cancer growth in vitro and in vivo (17). In breast cancer, daidzein shows
antiproliferation activity, causing cell cycle arrest at the G1 and
G2/M phases and its metabolite enhance tamoxifen's caspase-mediate
apoptosis induction (18,19). Daidzein also plays its antitumor
role in colon cancer, arresting cell cycle at G0/G1 phase (20), as well as other cancers such as
cervical cancer, melanoma, hepatic cancer and gastric carcinoma
effecting proliferation and/or apoptosis (21–24).
As to trophoblast cells, Jeschke et al found a significant
decrease of hCG production in daidzein-treated trophoblast cells in
a concentration-dependent way (25). However, scarce information on
daidzein effect in growth of choriocarcinoma is available.
Moreover, daidzein has a close relationship with
ERK. Soy isoflavones decreases the expression causing cell cycle
arrest and upregulation of p21 in colon adenocarcinoma (26). Daidzein suppresses phosphorylation
levels of ERK when exerting antitumor activity against bladder
cancer cells (27). Also, its
metabolites are able to inhibit the activation of ERK (28,29).
We hypothesized that daidzein can inhibit
choriocarcinoma cell proliferation by arresting the cell cycle, and
this may due to the suppression of expression of ERK pathway.
Materials and methods
Cell culture
Choriocarcinoma cell lines JAR and JEG-3 obtained
from American Type Culture Collection (Manassas, VA, USA) were
cultured in DMEM medium with 10% fetal bovine serum at 37°C with 5%
CO2. ERK was activated by 10 µM concentration of
ceramide C6 (C-C6; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
added 24 h after daidzein treatment for another 24 h.
MTT assay
Growth rates of cells were measured by 3-
(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Sigma, St. Louis, MO, USA) assay. The absorbance was measured at
490 nm using a universal microplate reader (Model ELx800; BioTek
Instruments, Inc., Winooski, VT, USA).
Colony formation assay
JAR and JEG-3 cells were seeded onto 6-well plates
at a density of 1,000 cells per well. After 14 days of culture,
cells were washed with PBS, fixed with 4% paraformaldehyde, and
subsequently stained with 0.1% crystal violet solutions.
Cell cycle analysis
Cells with 60–80% confluence were trypsinized,
washed with cold PBS, resuspended with cold 70% ethanol then stored
at −20°C overnight. Before subjected to flow cytometry analysis,
ethanol was removed and pellet cells were washed with cold PBS
twice, cells were resuspended in PBS with 0.5 µg/ml RNase and 50
µg/ml propidium iodide and incubated at room temperature in the
dark for 30 min. Then cell samples were analyzed in a FACSCalibur
flow cytometer (Becton-Dickinson, San Jose, CA, USA) and CellQuest
software.
Western blot analysis
Cells were washed once with cold PBS and lysed in
RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 0.1% SDS, 1% NP-40,
and 0.5% sodium deoxycholate) containing protease inhibitors.
Approximately 20 µg of protein was separated with 8–12% SDS-PAGE
gel and blotted onto nitrocellulose membranes. Then membranes were
blocked with 5% skim milk at room temperature for 1 h and then
incubated with primary antibodies against β-actin (Abcam,
Cambridge, UK), c-myc (Abcam), PCNA (Santa Cruz), cyclin D1 (Santa
Cruz), p21 (Cell Signaling Technology, MA, USA), ERK (Abcam) and
p-ERK (Abcam) at 4°C overnight, followed by TBST wash and 1-h
incubation with HRP-conjugated secondary antibodies at room
temperature. Protein bands were visualized by a Molecular Imager
ChemiDoc XRS System (Bio-Rad Laboratories, Hercules, CA, USA).
Immunofluorescence microscopy
Cells were fixed in 4% paraformaldehyde in PBS for
15 min, and then permeabilized with 0.1% Triton X-100 for 15 min
and blocked in 1% BSA for 1 h. Cells were sequentially incubated
with primary antibody (p-ERK; 1:100 in 1% BSA) at 4°C overnight,
followed by Alexa Fluor 488 second antibody (1:150 in 1% BSA) for 1
h and DAPI for 5 min. Slides were analyzed and photographed by
Olympus BX51 Microscope (Olympus, Tokyo, Japan).
Xenograft tumor model
Fifteen mice were randomly divided into three groups
depending on different gavage components: negative control group
(edible oil only), low dose group (10 mg/kg body weight/day of
daidzein dissolved in oil) and high dose group (20 mg/kg body
weight/day of daidzein dissolved in oil). For tumorigenesis assay
in nude mice, 5×106 JEG-3 (considering JEG-3 more easily
formed subcutaneous xenografts than JAR when performing
pre-experiment) were injected subcutaneously into left sides of the
flank region after 1 week daily gavage feeding. Three weeks after
subcutaneous injection with JEG-3 (4 weeks after daily gavage
with/without different concentration of daidzein), the mice were
sacrificed, then xenograft tumors were harvested, weighed and fixed
with 4% paraformaldehyde. Animal care and protocols were in
accordance with the guidelines of the Institutional Animal Care and
Use Committee of Xi'an Jiaotong University.
Immunohistochemistry
Tumor sections of nude mouse xenografts were studied
by immunohistochemistry (IHC) assay using EnVision™ System (Dako,
Carpinteria, CA, USA). Primary antibodies used in IHC were c-myc
(Abcam), PCNA (Santa Cruz), p-ERK (Abcam). Staining signals were
photographed using an Olympus BX51 microscope (Olympus). Average
intensity score of the positive cells (0, none; 1, weak; 2,
intermediate; 3, strong) and percentage score of stained cells (1,
0–25%; 2, 25–50%; 3, 50–75%; 4, 75–100%) were multiplied to get the
total staining score, ranging from 0 to 12.
Statistical analysis
All experiments were repeated at least three times.
Statistical analysis was carried out using the SPSS 19.0
statistical software package (SPSS, Inc., Chicago, IL, USA) was
used to analyze differences between two groups (Student's t-test)
and p-values <0.05 were regarded as statistically
significant.
Results
Daidzein reduces cell viability in
choriocarcinoma cell lines
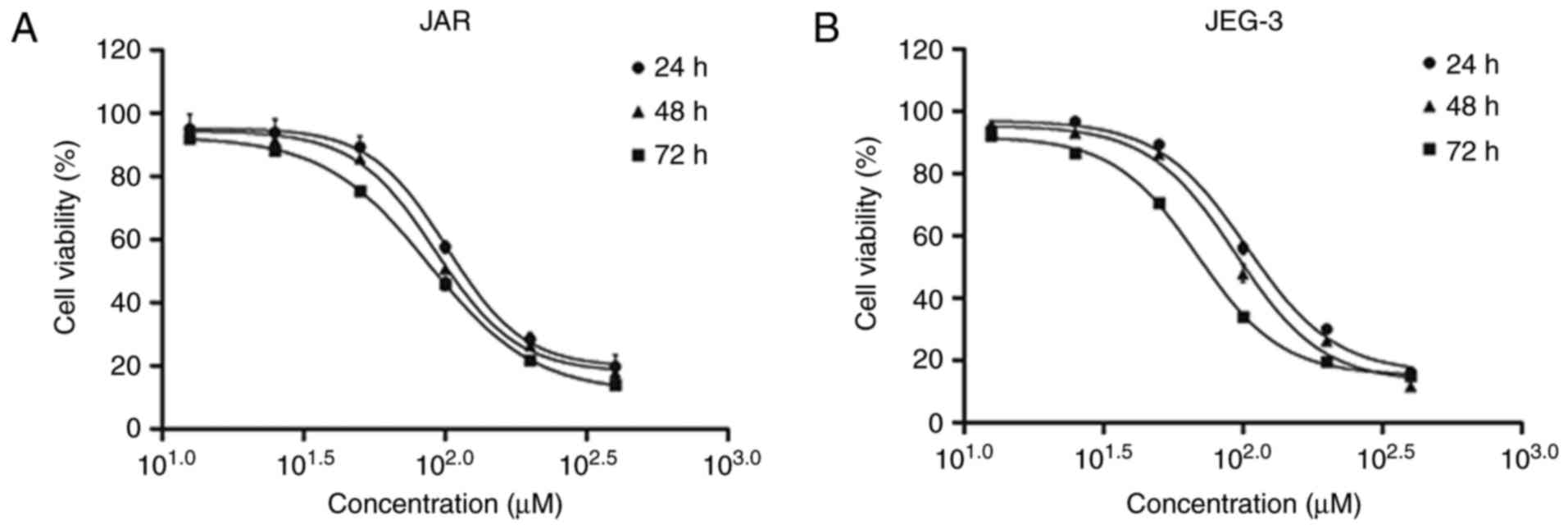
Using MTT assay, we investigated the viability
inhibition effect of daidzein in CC cell lines JAR and JEG-3. Both
cell lines were treated with daidzein at concentration of 12.5, 25,
50, 100, 200 and 400 µM. The results showed that daidzein had a
concentration- and time-dependent effect on JAR cell viability.
With the increasing of concentration or time of treatment with
daidzein, the viability decreased. Also, the IC50 at 24,
48 and 72 h were 101.60, 92.56 and 87.73 µM, respectively (Fig. 1A). Similar effect were seen in
JEG-3, while the IC50 was 103.10, 94.14 and 68.82 µM,
respectively (Fig. 1B).
Daidzein treatment inhibits
proliferation of choriocarcinoma cells
In order to determine whether the cell viability
reduction is due to proliferation inhibition, we performed colony
formation assay on both cell types with daidzein treatment. The
colony-forming capacity of JAR and JEG-3 decreased as the
concentration of daidzein increased. While 25 µM daidzein seemed
not to change much, treatment with 50 and 100 µM daidzein markedly
suppressed proliferation. However, when the concentration reached
200 µM, colonies hardly formed (Fig. 2A
and B).
Cell cycle is arrested in G1 phase
when treated with daidzein
Cell proliferation inhibition is usually associated
with cell cycle arresting at a specific stage. To examine if
daidzein causes cell cycle arrest, we performed
fluorescence-activated cell sorting (FACS) analysis. In the
asynchronized steady state, the percentage of proportion of G1
phase cells was greater after treated with daidzein. In JAR cells,
G1 phase percentage of proportion was 61.66, 65.38 and 72.64%
treated with 25, 50 and 100 µM, respectively, while it was only
48.02 without (Fig. 2C). As to
JEG-3 cells, daidzein increased G1 phase percentage from 50.85% (0
µM) to 56.34, 60.00 and 61.07% (25, 50 and 100 µM, respectively,
Fig. 2D). Then we detected the
expression of G1 phase related proteins (c-myc, PCNA and cyclin D1)
and p21, one of CDKIs family, by western blot analysis. The
expression levels of c-myc, PCNA and cyclin D1 were decreased
significantly, and the decrease was greater with the increase of
concentration (p<0.05, respectively). On the contrary, the
expression level of p21 increased, and the differences were also
significant (p<0.05, respectively, Fig. 2E and F). These results suggest that
choriocarcinoma cells are arrested at the G1 phase by daidzein.
Daidzein suppresses ERK1/2
phosphorylation and p-ERK1/2 nuclear translocation
When detecting expression of cell cycle associated
protein, we also found that daidzein suppressed ERK1/2 by reducing
phosphorylation, which suggested ERK1/2 might participate in
daidzein proliferation inhibition capacity (p<0.05,
respectively, Fig. 3A and B). As
known, ERK1/2 is involved in phosphorylation and subsequent nuclear
translocation, so we wondered whether daidzein might also effect
nuclear translocation of ERK1/2. Therefore, we undertook to examine
whether daidzein influenced this process by using
immunofluorescence staining (IF), and the results showed that
compared with DMSO, phospho-ERK1/2 translocated into the nucleus
less after daidzein treatment (Fig. 3C
and D) in both cell-lines. Taken together, our results
suggested daidzein could inhibit ERK1/2 phosphorylation and
p-ERK1/2 nuclear translocation.
Daidzein-caused proliferation
inhibition was abolished by C-C6
The changes of phosphorylation and translocation of
ERK1/2 were obtained during daidzein's proliferation inhibition as
described above, thus we hypothesized ERK1/2 involved in
daidzein-caused choriocarcinoma cell proliferation suppression.
Ceramide C6 is ERK pathway agonist, and could significantly
increase phosphorylation of ERK which was inhibited by daidzein in
both cell lines (p<0.05, respectively, Fig. 4A and B). At the same time, colony
formation assay showed that C-C6 obviously increased the amount of
cell colonies which were decreased by daidzein (Fig. 4C and D). Compared to daidzein only,
C-C6 decreased G1 phase percentage from 74.56 to 63.02 and 60.75 to
54.02 in JAR and JEG-3, respectively on the basis of daidzein
(Fig. 4E and F). Besides, C-C6 also
abolished daidzein's increase on protein expressions of c-myc, PCNA
and cyclin D1 and decreased p21 (Fig.
4G and H). In summary, daidzein's proliferation inhibition on
choriocarcinoma cells can be abolished by ERK pathway agonist C-C6,
therefore, we consider that ERK participated in this process.
Daidzein inhibits choriocarcinoma
growth in vivo
To determine whether daidzein's anti-proliferation
activity in vitro could be extended in vivo,
subcutaneous xenograft model was introduced in this study. Average
weights of tumors in three groups showed negative correlation with
daidzein concentration (Fig. 5A).
The low dose group was lighter than that of control group, and
weight in high dose group was even lighter than low dose group
(p<0.05, Fig. 5B). We then
stained c-myc, PCNA and p-ERK expression in these JEG-3 xenograft
tissues using IHC assays (Fig. 5C).
The reduced c-myc and PCNA expression was accompanied with less
p-ERK staining in low dose group and even less in high dose group
than control. The IHC scores of c-myc, PCNA and p-ERK in control
group were 10.60±1.95, 8.20±2.49 and 7.40±3.13, those in low dose
group were 5.60±1.67, 4.20±1.10 and 3.60±1.82, while those in high
dose group were 2.60±1.95, 2.20±1.30 and 0.80±0.84, respectively,
with significant differences, and the scores of c-myc and PCNA
showed positive correlation with p-ERK (Fig. 5D). These data suggested that
daidzein could also inhibit choriocarcinoma growth in vivo
by downregulating c-myc and PCNA, and this process was probably
mediated by p-ERK.
Discussion
Choriocarcinoma is a highly malignant tumor arising
from abnormal gestational trophoblasts proliferation. The use of
chemotherapy has dramatically improved the prognosis of these
lesions. However, despite serious side effect of chemotherapy,
there are still some patients who become drug-resistant or relapse,
and may finally die of liver or brain metastasis (30). Thus, it is meaningful to investigate
inhibition of choriocarcinoma cell proliferation without increasing
the patients side-effect burden.
Daidzein is a member of isoflavones, and is easily
gained from food especially soybeans or soy-based product (31–33).
Daidzein has been studied in many tumors due to its
anti-proliferation action (16,21,24).
In choriocarcinoma, daidzein shows its regulatory function in hCG
production (25). In this study, we
performed in vitro and in vivo experiments to examine
the effects of daidzein on proliferation of choriocarcinoma and
explore the underlying mechanism. Also, our results demonstrate an
antiproliferation action of daidzein on choriocarcinoma.
Daidzein has shown its antiproliferation function in
various cells by cell cycle arrest. Daidzein can induce
accumulation of cells in G1 phase in human melanoma cells (34), and cause cell cycle arrest at G1 and
G2/M phase in human breast cancer MCF-7 and MDA-MB-453 cells
(19). Daidzein can also block G1
phase cell cycle progression of Swiss 3T3, an immortal line of
fibroblast-like cells (35). In
this study, we found that daidzein inhibited choriocarcinoma
cell-lines JAR and JEG-3 growth rate and clone formation in a time-
and concentration-dependent manner, during which cell cycle was
arrested at G1 phase in both cell types detected by flow cytometer.
At the same time, the expression of cyclin D1 was reduced by
daidzein, as well as c-myc and PCNA, which can also help cell cycle
progression. While the expression of p21, one of CKDIs, increased.
These results strongly support that daidzein can inhibit
proliferation by cell cycle arrest at G1 phase.
ERK1/2 is one of MAPK family members involved in
proliferation and other cell activities (36–38).
Daidzein has been reported to suppress ERK1/2 activation during its
antiproliferation function in tumors (26,27).
In this study, during daidzein treatment, the expression of
phospho-ERK1/2 was reduced, determined by western blotting.
Immunofluorescence staining showed that p-ERK translocation into
the nucleus was obstructed as more p-ERK stayed in the cytoplasm
suggesting that daidzein suppress ERK activation by inhibiting
phosphorylation and translocation into the nucleus. Then we used
ERK agonist C-C6 to confirm whether ERK was involved in daidzein
proliferation in JAR and JEG-3. Unsurprisingly, compared with
daidzein treatment only, C-C6 increased colony formation of both
cells, and also decreased G1 phase percentage. At the same time,
cyclin D1, c-myc and PCNA reduction and p21 increase were weakened
or offset. Collectively, daidzein inhibits choriocarcinoma cells
proliferation by cell cycle arrest at G1 phase via ERK pathway
in vitro.
Furthermore, we performed experiments in vivo
to see if this proliferation inhibition could be still effective
in vivo. It has been reported that daidzein and its
metabolite can cause tumor growth inhibition in other tumors in
vivo (39–41). Similar effect was also obtained in
our study, as daidzein also inhibited subcutaneous xenograft
growth. Average tumor weight in control group was much higher than
that of low dose group, while weight of high dose group was even
lower. Then IHC scores of c-myc, PCNA and p-ERK in these tumor
tissue slides showed similar tendency with western blotting in
vitro experiment: daidzein addition decrease p-ERK expression
in xenografts as well as c-myc and PCNA, and the higher dose we
gave, the more effect we gained.
In conclusion, we demonstrated that daidzein can
inhibit choriocarcinoma cell proliferation in vitro and
in vivo, and underlying mechanism behind the inhibitory
effects may probably be suppressing ERK pathway and afterwards
arresting cell cycle at G1 phase. Our findings provide new insight
into the application of daidzein in treatment of choriocarcinoma to
improve therapy efficiency.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81172489).
References
|
1
|
Bolze PA, Attia J, Massardier J, Seckl MJ,
Massuger L, van Trommel N, Niemann I, Hajri T, Schott AM and
Golfier F: EOTTD group: Formalised consensus of the European
Organisation for Treatment of Trophoblastic Diseases on management
of gestational trophoblastic diseases. Eur J Cancer. 51:1725–1731.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryu N, Ogawa M, Matsui H, Usui H and Shozu
M: The clinical characteristics and early detection of postpartum
choriocarcinoma. Int J Gynecol Cancer. 25:926–930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ngu SF and Chan KK: Management of
chemoresistant and quiescent gestational trophoblastic disease.
Curr Obstet Gynecol Rep. 3:84–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alazzam M, Tidy J, Osborne R, Coleman R,
Hancock BW and Lawrie TA: Chemotherapy for resistant or recurrent
gestational trophoblastic neoplasia. Cochrane Database Syst Rev.
1:CD0088912016.
|
|
5
|
Rumer KK, Post MD, Larivee RS, Zink M,
Uyenishi J, Kramer A, Teoh D, Bogart K and Winn VD: Siglec-6 is
expressed in gestational trophoblastic disease and affects
proliferation, apoptosis and invasion. Endocr Relat Cancer.
19:827–840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siu MK, Yeung MC, Zhang H, Kong DS, Ho JW,
Ngan HY, Chan DC and Cheung AN: p21-activated kinase-1 promotes
aggressive phenotype, cell proliferation, and invasion in
gestational trophoblastic disease. Am J Pathol. 176:3015–3022.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Shen M, Yue Z, Yang Z, Wang M, Li
C, Xin C, Wang Y, Mei Q and Wang Z: Triptolide inhibits
colon-rectal cancer cells proliferation by induction of G1 phase
arrest through upregulation of p21. Phytomedicine. 19:756–762.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju SM, Lee J, Kang JG, Jeong SO, Park JH,
Pae HO, Lee GS, Kim WS, Lyu YS and Jeon BH: Nardostachys chinensis
induces granulocytic differentiation with the suppression of cell
growth through p27 (Kip1) protein-related G0/G1 phase arrest in
human promyelocytic leukemic cells. Pharm Biol. 53:1002–1009. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gehen SC, Vitiello PF, Bambara RA, Keng PC
and O'Reilly MA: Downregulation of PCNA potentiates p21-mediated
growth inhibition in response to hyperoxia. Am J Physiol Lung Cell
Mol Physiol. 292:L716–L724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Faouzi M, Kischel P, Hague F, Ahidouch A,
Benzerdjeb N, Sevestre H, Penner R and Ouadid-Ahidouch H: ORAI3
silencing alters cell proliferation and cell cycle progression via
c-myc pathway in breast cancer cells. Biochim Biophys Acta.
1833:752–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang F, Kong DS, Zhang ZL, Lei N, Zhu XJ,
Zhang XP, Chen L, Lu Y and Zheng SZ: Tetramethylpyrazine induces
G0/G1 cell cycle arrest and stimulates mitochondrial-mediated and
caspase-dependent apoptosis through modulating ERK/p53 signaling in
hepatic stellate cells in vitro. Apoptosis. 18:135–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu E, Li J, Shi S, Wang X, Liang T, Wu B
and Li Q: Sustained ERK activation-mediated proliferation
inhibition of farrerol on human gastric carcinoma cell line by
G0/G1-phase cell-cycle arrest. Eur J Cancer Prev. 25:490–499. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao HB, Tang CL, Hou YL, Xue LR, Li MQ,
Du MR and Li DJ: CXCL12/CXCR4 axis triggers the activation of EGF
receptor and ERK signaling pathway in CsA-induced proliferation of
human trophoblast cells. PLoS One. 7:e383752012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie Y, Cui D, Sui L, Xu Y, Zhang N, Ma Y,
Li Y and Kong Y: Induction of forkhead box M1 (FoxM1) by EGF
through ERK signaling pathway promotes trophoblast cell invasion.
Cell Tissue Res. 362:421–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liggins J, Mulligan A, Runswick S and
Bingham SA: Daidzein and genistein content of cereals. Eur J Clin
Nutr. 56:961–966. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu A, Bray TM, Helferich WG, Doerge DR
and Ho E: Differential effects of whole soy extract and soy
isoflavones on apoptosis in prostate cancer cells. Exp Biol Med
(Maywood). 235:90–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu Z, Zhou R, Kong Y, Wang J, Xia W, Guo
J, Liu J, Sun H, Liu K, Yang J, et al: S-equol, a secondary
metabolite of natural anticancer isoflavone daidzein, inhibits
prostate cancer growth in vitro and in vivo, Though activating the
Akt/FOXO3a pathway. Curr Cancer Drug Targets. 16:455–465. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Charalambous C, Pitta CA and Constantinou
AI: Equol enhances tamoxifen's anti-tumor activity by induction of
caspase-mediated apoptosis in MCF-7 breast cancer cells. BMC
Cancer. 13:2382013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi EJ and Kim GH: Daidzein causes cell
cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7
and MDA-MB-453 cells. Phytomedicine. 15:683–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo JM, Xiao BX, Liu DH, Grant M, Zhang S,
Lai YF, Guo YB and Liu Q: Biphasic effect of daidzein on cell
growth of human colon cancer cells. Food Chem Toxicol.
42:1641–1646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iwashita K, Kobori M, Yamaki K and
Tsushida T: Flavonoids inhibit cell growth and induce apoptosis in
B16 melanoma 4A5 cells. Biosci Biotechnol Biochem. 64:1813–1820.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park HJ, Jeon YK, You DH and Nam MJ:
Daidzein causes cytochrome c-mediated apoptosis via the
Bcl-2 family in human hepatic cancer cells. Food Chem Toxicol.
60:542–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang S, Hu J, Meng Q, Dong X, Wang K, Qi
Y, Chu C, Zhang X and Hou L: Daidzein induced apoptosis via
down-regulation of Bcl-2/Bax and triggering of the mitochondrial
pathway in BGC-823 cells. Cell Biochem Biophys. 65:197–202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo JM, Kang GZ, Xiao BX, Liu DH and Zhang
S: Effect of daidzein on cell growth, cell cycle, and telomerase
activity of human cervical cancer in vitro. Int J Gynecol Cancer.
14:882–888. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeschke U, Briese V, Richter DU, Bruer G,
Plessow D, Waldschläger J, Mylonas I and Friese K: Effects of
phytoestrogens genistein and daidzein on production of human
chorionic gonadotropin in term trophoblast cells in vitro. Gynecolo
Endocrinol. 21:180–184. 2005. View Article : Google Scholar
|
|
26
|
Bielecki A, Roberts J, Mehta R and Raju J:
Estrogen receptor-β mediates the inhibition of DLD-1 human colon
adenocarcinoma cells by soy isoflavones. Nutr Cancer. 63:139–150.
2011.PubMed/NCBI
|
|
27
|
He Y, Wu X, Cao Y, Hou Y, Chen H, Wu L, Lu
L, Zhu W and Gu Y: Daidzein exerts anti-tumor activity against
bladder cancer cells via inhibition of FGFR3 pathway. Neoplasma.
63:523–531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lim TG, Kim JE, Lee SY, Park JS, Yeom MH,
Chen H, Bode AM, Dong Z and Lee KW: The daidzein metabolite,
6,7,4′-Trihydroxyisoflavone, is a novel inhibitor of PKCα in
suppressing solar UV-induced matrix metalloproteinase 1. Int J Mol
Sci. 15:21419–21432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang NJ, Lee KW, Rogozin EA, Cho YY, Heo
YS, Bode AM, Lee HJ and Dong Z: Equol, a metabolite of the soybean
isoflavone daidzein, inhibits neoplastic cell transformation by
targeting the MEK/ERK/p90RSK/activator protein-1 pathway. J Biol
Chem. 282:32856–32866. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seckl MJ, Sebire NJ and Berkowitz RS:
Gestational trophoblastic disease. Lancet. 376:717–729. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bao C, Namgung H, Lee J, Park HC, Ko J,
Moon H, Ko HW and Lee HJ: Daidzein suppresses tumor necrosis
factor-α induced migration and invasion by inhibiting hedgehog/Gli1
signaling in human breast cancer cells. J Agric Food Chem.
62:3759–3767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi EJ and Kim GH: Antiproliferative
activity of daidzein and genistein may be related to ERα/c-erbB-2
expression in human breast cancer cells. Mol Med Rep. 7:781–784.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Suzuki N, Laxmi Santosh YR, Okamoto
Y and Shibutani S: Anti-breast cancer potential of daidzein in
rodents. Life Sci. 91:415–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Casagrande F and Darbon JM: Effects of
structurally related flavonoids on cell cycle progression of human
melanoma cells: Regulation of cyclin-dependent kinases CDK2 and
CDK1. Biochem Pharmacol. 61:1205–1215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Higashi K and Ogawara H: Daidzein inhibits
insulin- or insulin-like growth factor-1-mediated signaling in cell
cycle progression of Swiss 3T3 cells. Biochim Biophys Acta.
1221:29–35. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ito T, Yamada S, Tanaka C, Ito S, Murai T,
Kobayashi D, Fujii T, Nakayama G, Sugimoto H, Koike M, et al:
Overexpression of L1CAM is associated with tumor progression and
prognosis via ERK signaling in gastric cancer. Ann Surg Oncol.
21:560–568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sadaria MR, Yu JA, Meng X, Fullerton DA,
Reece TB and Weyant MJ: Secretory phospholipase A2 mediates human
esophageal adenocarcinoma cell growth and proliferation via ERK 1/2
pathway. Anticancer Res. 33:1337–1342. 2013.PubMed/NCBI
|
|
38
|
Han S, Li Z, Master LM, Master ZW and Wu
A: Exogenous IGFBP-2 promotes proliferation, invasion, and
chemoresistance to temozolomide in glioma cells via the integrin
β1-ERK pathway. Br J Cancer. 111:1400–1409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh-Gupta V, Zhang H, Yunker CK, Ahmad
Z, Zwier D, Sarkar FH and Hillman GG: Daidzein effect on hormone
refractory prostate cancer in vitro and in vivo compared to
genistein and soy extract: Potentiation of radiotherapy. Pharm Res.
27:1115–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee DE, Lee KW, Jung SK, Lee EJ, Hwang JA,
Lim TG, Kim BY, Bode AM, Lee HJ and Dong Z:
6,7,4′-trihydroxyisoflavone inhibits HCT-116 human colon cancer
cell proliferation by targeting CDK1 and CDK2. Carcinogenesis.
32:629–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Somjen D, Grafi-Cohen M, Katzburg S,
Weisinger G, Izkhakov E, Nevo N, Sharon O, Kraiem Z, Kohen F and
Stern N: Anti-thyroid cancer properties of a novel isoflavone
derivative, 7-(O)-carboxymethyl daidzein conjugated to
N-t-Boc-hexylenediamine in vitro and in vivo. J Steroid Biochem Mol
Biol. 126:95–103. 2011. View Article : Google Scholar : PubMed/NCBI
|