Introduction
Primary effusion lymphoma (PEL, also termed body
cavity lymphoma) is a highly aggressive non-Hodgkin's B cell
lymphoma associated with poor prognosis that arises frequently in
immune-compromised individuals, such as organ transplantation
recipients and the HIV-infected population (1,2). PEL
is strictly associated with Kaposi's sarcoma-associated herpesvirus
(KSHV) and characterized by lymphomatous effusions of pleural,
pericardial and abdominal cavities in the absence of tumor masses
(1,2). It is generally resistant to
conventional chemotherapy with a short median survival of less than
6 months. Furthermore, the myelosuppressive effects of systemic
cytotoxic chemotherapy synergize with those caused by
antiretroviral therapy or immune suppression (3,4).
Therefore, new therapeutic strategies are needed for PEL.
PEL cell survival relays on the constitutive
activation of several pathways. These include nuclear factor-κB
(NF-κB) and activator protein-1 (AP-1) (5–8). The
KSHV-encoded viral FLICE-inhibitory protein and G protein-coupled
receptor mediate activation of NF-κB and AP-1 pathways (6–8). It
has been reported that inhibition of NF-κB significantly decreases
PEL cell survival (5).
Fucoidan, a sulfated polysaccharide, is abundant in
brown seaweeds. It is composed of L-fucose as well as other sugars,
such as D-xylose, D-galactose, D-mannose and glucuronic acid
(9). Several studies have reported
that fucoidan possesses many desired biological effects, such as
anticancer and antiviral activities (9,10).
Moreover, it is a well-tolerated agent (11,12).
The present study was designed to determine the
anti-PEL activity of fucoidan both in vitro and in
vivo. The results revealed that fucoidan inhibited
constitutively active NF-κB, AP-1 and lymphokine-activated killer
T-cell-originated protein kinase (TOPK), leading to G1
cell cycle arrest and apoptosis of PEL cells. Oral administration
of fucoidan suppressed PEL progression in a xenograft murine
model.
Materials and methods
Reagents
Fucoidan was prepared from the brown algae
Cladosiphon okamuranus Tokida cultivated in Okinawa as
previously described in detail (13). Nanoparticle fucoidan was also
previously described (14).
Fucoidan was dissolved in RPMI-1640 medium (cat. no. 30264-56,
Nacalai Tesque, Inc., Kyoto, Japan). The primary antibodies against
cleaved caspase-3 (cat. no. 9664), caspase-8 (cat. no. 9446),
caspase-9 (cat. no. 9501) and poly(ADP-ribose) polymerase (PARP)
(cat. no. 9541), Bcl-xL (cat. no. 2762), phospho-IκBα (Ser32 and
36) (cat. no. 9246), RelA (cat. no. 8242), TOPK (cat. no. 4942) and
phospho-TOPK (Thr9) (cat. no. 4941) were obtained from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Antibodies against
CDK4 (cat. no. MS-299), CDK6 (cat. no. MS-398), cyclin E (cat. no.
MS-870), Bcl-2 (cat. no. MS-597) and actin (cat. no. MS-1295) were
purchased from Neomarkers, Inc. (Fremont, CA, USA). An antibody
against XIAP (cat. no. M044-3) was purchased from Medical &
Biological Laboratories, Co. (Aichi, Japan). Antibodies against
cyclin D2 (cat. no. sc-593), Mcl-1 (cat. no. sc-819), c-IAP2 (cat.
no. sc-7944), IκBα (cat. no. sc-371), lamin B (cat. no. sc-6216),
JunB (cat. no. sc-46) and JunD (cat. no. sc-74), and NF-κB subunits
p50 (cat. no. sc-114X), p52 (cat. no. sc-298X), RelA (cat. no.
sc-109X), c-Rel (cat. no. sc-70X) and RelB (cat. no. sc-226X), and
AP-1 subunits c-Fos (cat. no. sc-52X), FosB (cat. no. sc-48X),
Fra-1 (cat. no. sc-605X), Fra-2 (cat. no. sc-604X), c-Jun (cat. no.
sc-45X), JunB (cat. no. sc-46X) and JunD (cat. no. sc-74X) for
supershift assays were obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
Cells
The human B-cell lines derived from PEL, carrying
KSHV, BCBL-1 (15) and TY-1
(16), were maintained in RPMI-1640
medium supplemented with 10% heat-inactivated fetal bovine serum
(Biological Industries, Kibbutz Beit Haemek, Israel) and 1%
penicillin/streptomycin (cat. no. 09367-34, Nacalai Tesque, Inc.)
in a 5% CO2 humidified incubator at 37°C.
Cell proliferation and cytotoxic
assay
PEL cells were seeded into 96-wells plates in
triplicate at a density of 104 cells/well and treated
with various concentrations of fucoidan for 72 h. The effects of
fucoidan on cell proliferation and cytotoxicity were determined by
the water-soluble tetrazolium (WST)-8 uptake method. After
incubation, 10 µl of the WST-8 reagent (cat. no. 07553-44, Nacalai
Tesque, Inc.) were added to each well. After 4 h, WST-8 reduction
was measured at 450 nm using a 680 XR microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The percentage of cells was
calculated by normalizing the optical density values of
fucoidan-treated samples vs. the untreated control samples.
Apoptosis assay
PEL cells were treated with various doses of
fucoidan for 24–72 h and then permeabilized by incubation on ice
for 20 min with 100 µg/ml of digitonin, and treated with the
phycoerythrin-conjugated APO2.7 antibody (cat. no. IM2088, Beckman
Coulter, Inc., Marseille, France) for 15 min at room temperature.
After staining with the APO2.7 antibody, apoptosis was determined
using an Epics XL flow cytometer (Beckman Coulter, Inc., Brea, CA,
USA).
In vitro assessment of caspase
activity
Caspase activity was assessed using Colorimetric
Caspase Assay kits (cat. nos. 4800, 4805 and 4810; Medical &
Biological Laboratories, Co.). Briefly, cell extracts were
recovered using the cell lysis buffer supplied with the kits and
assessed for caspase-3, −8 and −9 activities using colorimetric
probes. The assay kits are based on the detection of chromophore
ρ-nitroanilide after cleavage from caspase-specific labeled
substrates. Colorimetric readings were performed in an automated
microplate reader.
Cell cycle analysis
Cells were stained with the CycleTEST Plus DNA
Reagent kit (cat. no. 340242; Becton-Dickinson Immunocytometry
Systems, San Jose, CA, USA). The cell cycle distribution was
analyzed for 10,000 collected cells by an Epics XL flow cytometer
equipped with MultiCycle software (version 3.0; Phoenix Flow
Systems, San Diego, CA, USA). The population of nuclei in each
phase of the cell cycle was determined.
Western blot analysis
Whole cell extracts were prepared by subjecting
fucoidan-treated cells to lysis in lysis buffer [62.5 mM Tris-HCl
(pH 6.8) (cat. no. 35434-21), 2% sodium dodecyl sulfate (cat. no.
31607-65), 10% glycerol (cat. no. 17045-65), 6% 2-mercaptoethanol
(cat. no. 21438-82; all from Nacalai Tesque, Inc.) and 0.01%
bromophenol blue (cat. no. 021-02911; Wako Pure Chemical
Industries, Osaka, Japan)]. Lysates were spun to remove insoluble
material. Supernatants were collected and protein concentrations
were assessed. Protein lysates (20 µg) were resolved by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Then, the proteins were transferred onto polyvinylidene difluoride
membranes (cat. no. IPVH00010EMD; Millipore, Darmstadt, Germany)
and immunoblotted with relevant specific antibodies. Immunoreactive
bands were identified by an enhanced chemiluminescence reagent
(cat. no. RPN2232; Amersham Biosciences Corp., Piscataway, NJ,
USA).
Electrophoretic mobility shift assay
(EMSA)
To determine NF-κB and AP-1 activation, we prepared
nuclear extracts from fucoidan-treated cells and performed EMSA as
previously described (17). The
obtained nuclear extracts were also subjected to SDS-PAGE. The top
strand sequences of the oligonucleotide probes or competitors were
as follows: for a consensus NF-κB element of the interleukin-2
receptor α chain (IL-2Rα) gene,
5′-GATCCGGCAGGGGAATCTCCCTCTC-3′ and for the typical AP-1 element of
the IL-8 gene, 5′-GATCGTGATGACTCAGGTT-3′. The above
underlined sequences are the NF-κB and AP-1 binding sites,
respectively. To determine the specificity of the binding, nuclear
extracts were preincubated with 100-fold excess of unlabeled
oligonucleotides for 15 min. For supershift assays, different
antibodies against NF-κB or AP-1 subunits were preincubated with
nuclear protein for 45 min at room temperature before the addition
of probes. The dried gels were visualized.
PEL xenograft model
Aliquots of 5×106 TY-1 cells or
8×107 BCBL-1 cells were suspended in sterile RPMI-1640
medium (200 µl), and 5-week-old female C.B-17/Icr-severe combined
immune deficiency (SCID) mice (Kyudo, Co., Tosu, Japan) received
intraperitoneal injections with a single-cell aliquot. Fucoidan
(200 mg/kg for TY-1 and 150 mg/kg for BCBL-1), or vehicle alone,
was administered using oral gavage once daily for 5 days per week,
and the treatment was continued for 50 days (TY-1) and 56 days
(BCBL-1), beginning the day of injection (TY-1) or one day after
injection (BCBL-1). PEL expansion in vivo was confirmed by
testing the expression of cell surface markers, including cluster
of differentiation 30 (CD30) in ascites tumor cells, using flow
cytometry. Body weight was recorded weekly as a surrogate measure
of tumor progression. All experiments were performed in compliance
with the Guidelines for Animal Experimentation of the University of
the Ryukyus (Nishihara, Japan) and approved by the Animal Care and
Use Committee of the University of the Ryukyus (reference nos. 5885
and A2016097).
Biomarker analysis
Serum and ascitic concentrations of human soluble
CD30 (sCD30) (cat. no. RAF091R; BioVendor Inc., Brno, Czech
Republic) were assessed by enzyme-linked immunosorbent assay
(ELISA), according to the protocol supplied by the
manufacturer.
Statistical analysis. Data are expressed as the mean
± standard deviation (SD), unless otherwise stated. Data of two
groups were compared with the Student's t-test. Differences were
considered significant at the 95% confidence level when
P<0.05.
Results
Effects of fucoidan on PEL cell
proliferation and apoptosis
The two different PEL cell lines, BCBL-1 and TY-1,
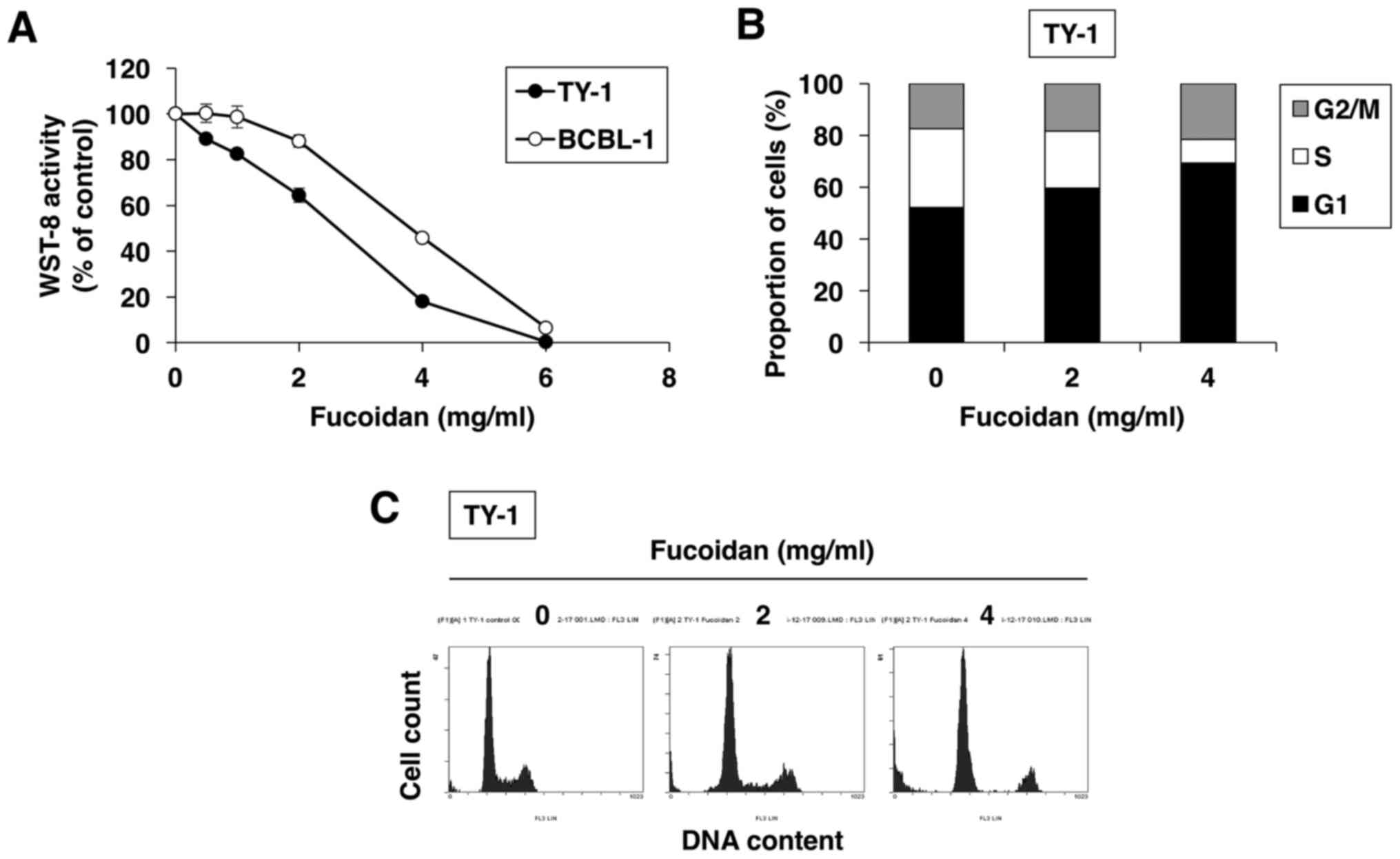
were treated with increasing doses of fucoidan for 72 h. Fucoidan
decreased cell proliferation and survival in a dose-dependent
fashion in both PEL cell lines, as assessed by WST-8 assay
(Fig. 1A).
We also evaluated the effect of fucoidan on cell
cycle regulation using flow cytometry. As shown in Fig. 1B and C, upon 24-h culture of
fucoidan-treated TY-1 cells, the percentage of cells in the
G1 phase increased from 52.1% (in vehicle-treated cells)
to 59.6% (in 2 mg/ml fucoidan-treated cells) and 69.3% (in 4 mg/ml
fucoidan-treated cells), whereas the percentage of cells in the S
phase decreased from 30.5% (in vehicle-treated cells) to 22.1% (in
2 mg/ml fucoidan-treated cells) and 7.5% (in 4 mg/ml
fucoidan-treated cells). Thus, fucoidan enhanced accumulation in
the G1 phase of the cell cycle in a dose-dependent
manner.
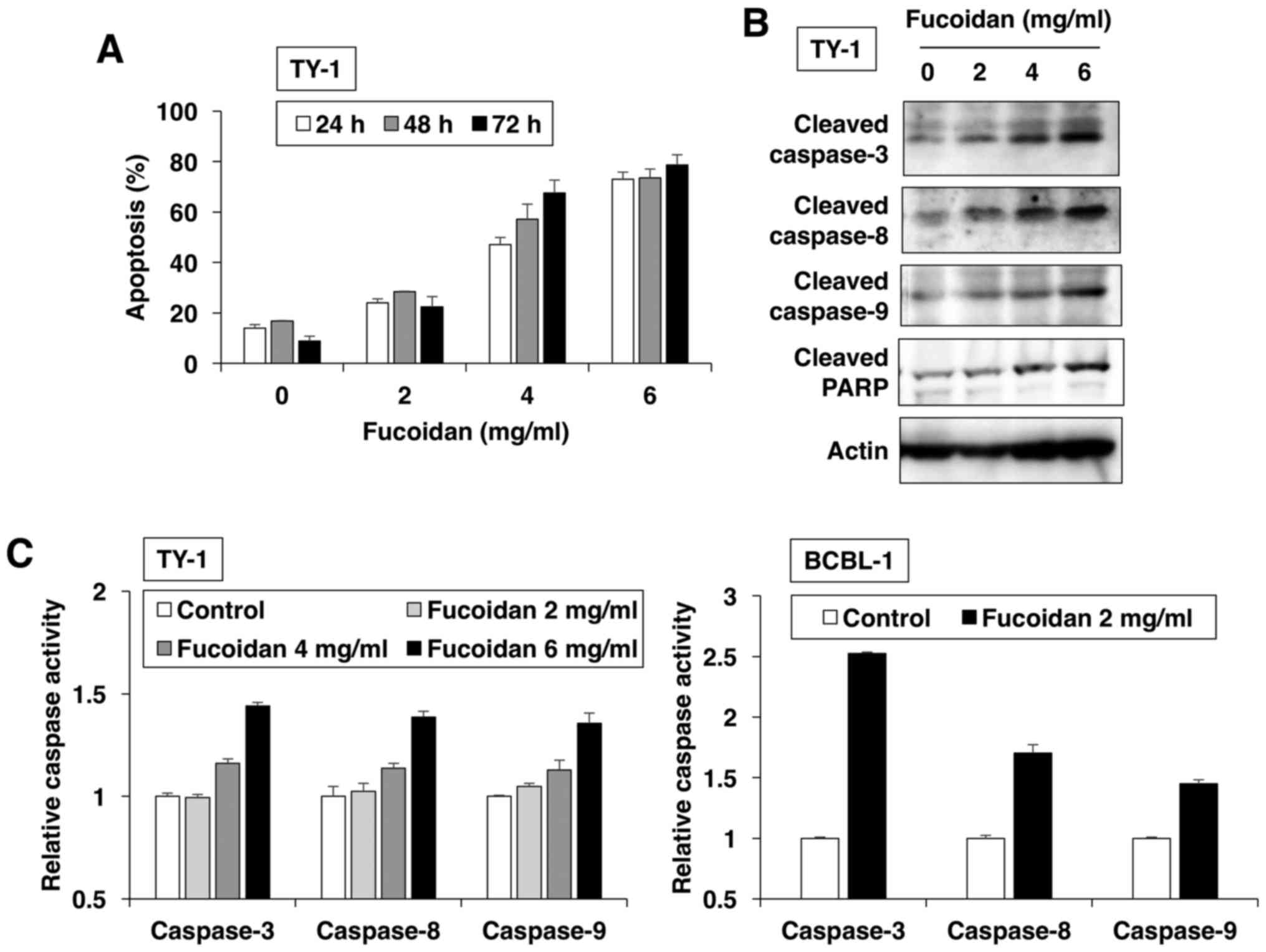
In the next step, we investigated the type of cell
death induced by fucoidan and found that it increased the number of
APO2.7-positive cells in dose- and time-dependent manners (Fig. 2A). APO2.7 antibody reacted with a
38-kDa mitochondrial membrane protein (named 7A6 antigen), which is
detected in late apoptotic cells (18). These results revealed that fucoidan
induced apoptotic cell death. The apoptotic process was executed by
a member of the highly conserved caspase family (19). Western blot analysis revealed that
fucoidan dose-dependently induced the cleavage of initiators
caspase-8 and −9, and the executioner caspase-3 and its substrate
PARP (Fig. 2B). To confirm caspase
activation by fucoidan for induction of apoptosis, ELISA was
performed with various caspase substrates. PEL cells treated with
fucoidan exhibited enhanced activation of caspase-8 and −9; thereby
activating effector caspase-3 (Fig.
2C).
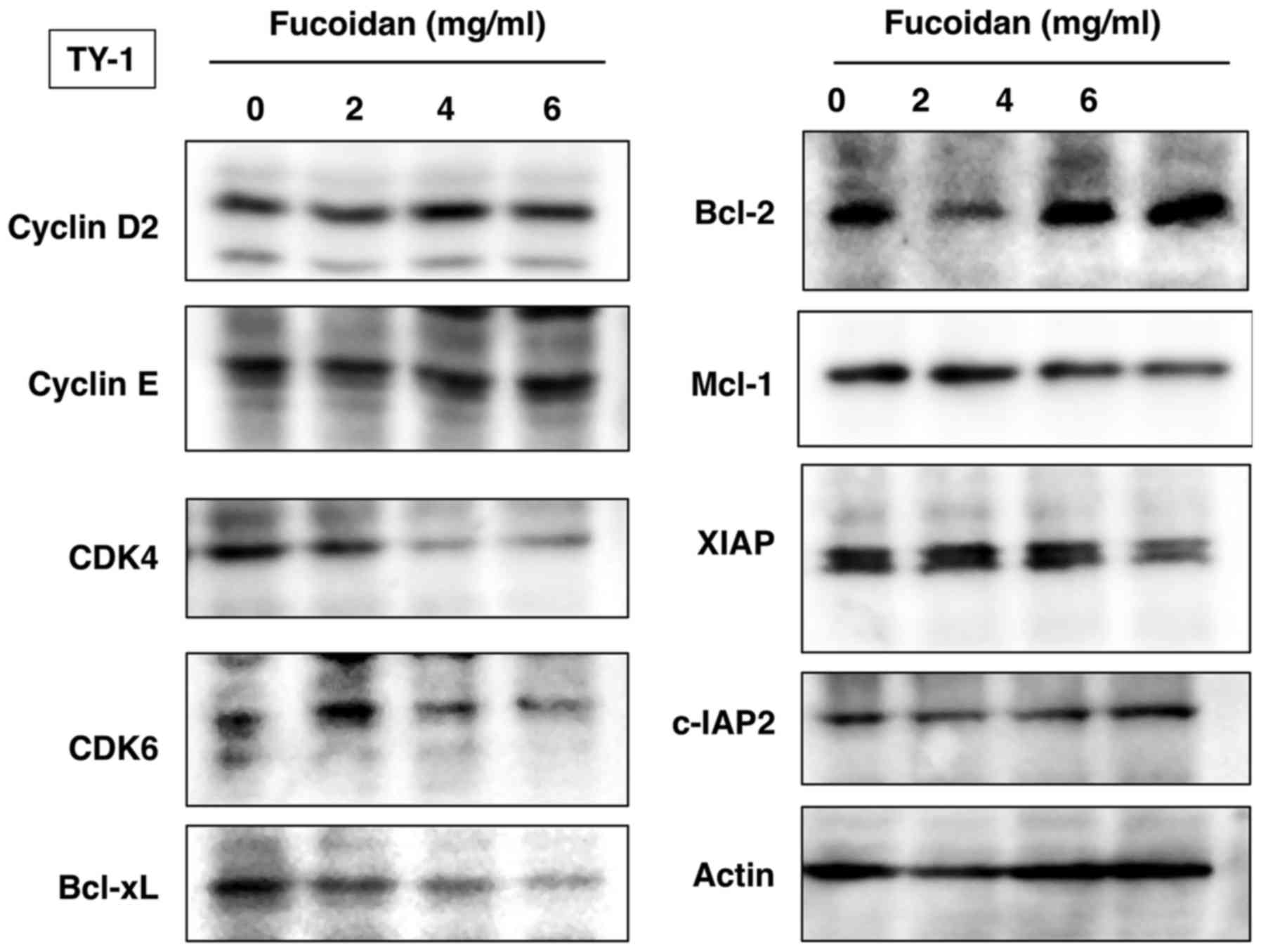
Fucoidan induces downregulation of
CDK4, CDK6, Bcl-xL, Mcl-1 and XIAP
We further investigated the levels of various
molecules involved in cellular proliferation and survival. Western
blot analysis revealed dose-dependent downregulation of
cyclin-dependent kinases (CDK4 and CDK6) and anti-apoptotic
proteins Bcl-xL, Mcl-1 and XIAP in TY-1 cells treated with fucoidan
(Fig. 3).
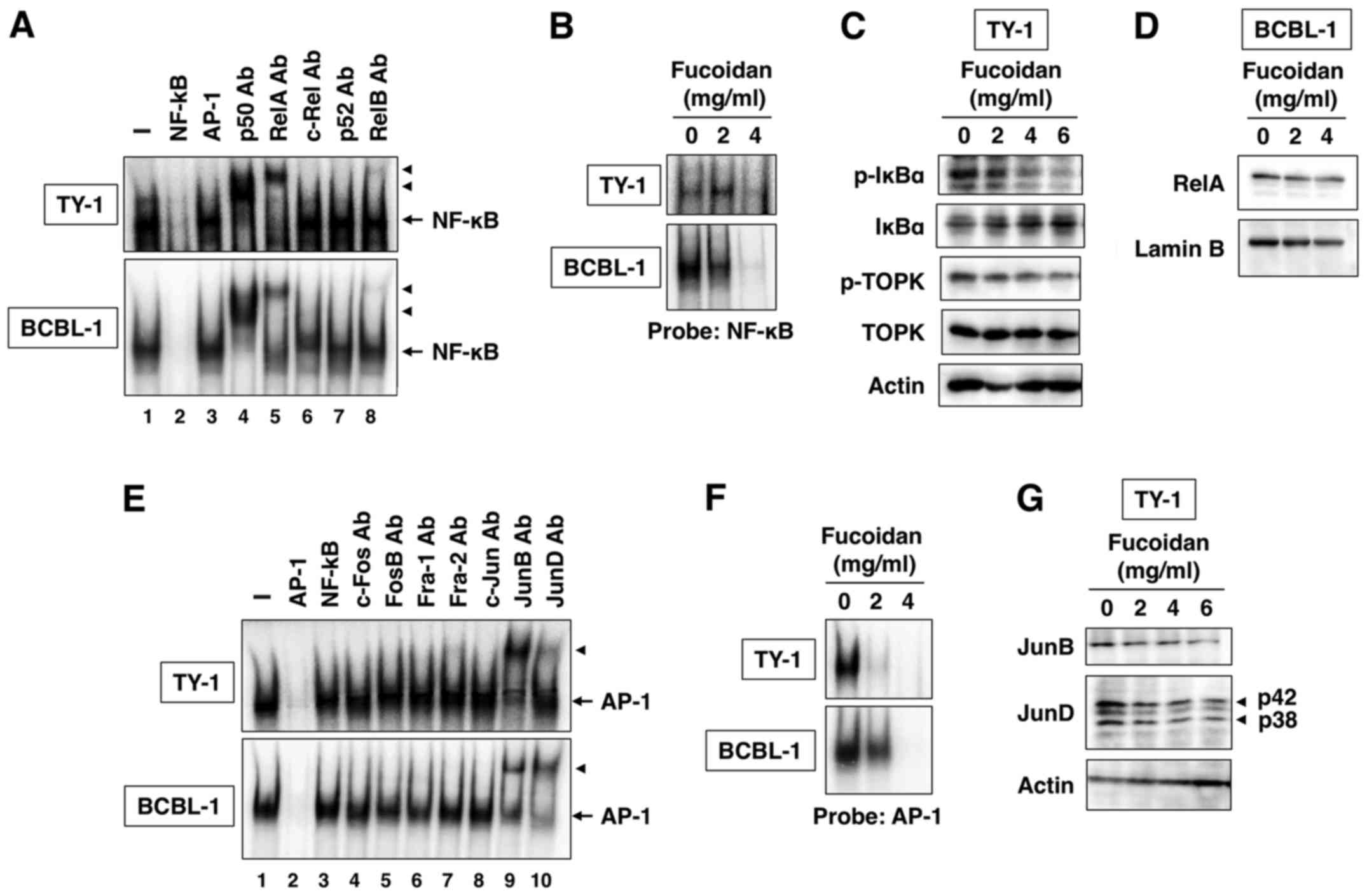
Effect of fucoidan on NF-κB
activation
In Fig. 4A, we
revealed constitutive NF-κB DNA-binding activity in TY-1 and BCBL-1
cells. This binding reaction was specific because NF-κB, but not
AP-1 oligonucleotides, competed with the NF-κB DNA-binding activity
(Fig. 4A, lanes 2 and 3). The
components of NF-κB DNA-binding complex were analyzed with specific
antibodies against five NF-κB family proteins. Preincubation of
nuclear extracts of PEL cells with anti-p50, anti-RelA or anti-RelB
antibody caused slow migration of the complex (Fig. 4A, lanes 4, 5 and 8). The results
indicate the presence of p50, RelA and RelB in the DNA-binding
complex in PEL cells.
To examine the effect of fucoidan on NF-κB
DNA-binding activity, we incubated PEL cells with fucoidan (2 and 4
mg/ml) for 48 h. The prepared nuclear extracts were analyzed for
NF-κB DNA-binding activity. The results revealed that fucoidan
decreased NF-κB DNA-binding activity in PEL cells (Fig. 4B). NF-κB is maintained in an
inactive status in the cytoplasm of nonstimulated cells through
interaction with specific inhibitor, IκBα (20). In response to stimuli, IκBα is
phosphorylated and degraded through ubiquitin-dependent
proteolysis, resulting in the release of free NF-κB dimers, which
translocate to the nucleus to induce transcription of the target
genes (20). That fucoidan
inhibited NF-κB DNA-binding activity suggests it may act on
NF-κB-associated inhibitory protein IκBα. We treated TY-1 cells
with fucoidan (2, 4 and 6 mg/ml) for 48 h and IκBα was determined
by western blot. Fucoidan dose-dependently inhibited the
phosphorylation and degradation of IκBα (Fig. 4C). We also examined the suppression
of NF-κB nuclear translocation. The amount of RelA in the nucleus
decreased in PEL cells treated with fucoidan for 48 h (Fig. 4D). Lamin B was used as a quality
control to assess nuclear fraction purity and loading levels.
Fucoidan did not alter the amount of lamin B in PEL cells (Fig. 4D).
Effect of fucoidan on AP-1
activation
We also investigated the DNA-binding activity of
AP-1, another transcription factor, in PEL cells. EMSA demonstrated
the constitutive formation of an AP-1 protein complex that retarded
the electrophoretic mobility of the AP-1 probe (Fig. 4E). An excess of the cold AP-1 probe
abrogated the band, whereas excess of the NF-κB probe had no effect
(Fig. 4E, lanes 2 and 3).
Preincubation of nuclear extracts of PEL cells with anti-JunB or
anti-JunD antibody caused slow migration of the complex (Fig. 4E, lanes 9 and 10), suggesting that
the AP-1 protein complex in PEL cells is composed of JunB and JunD.
Fucoidan-treated PEL cells revealed decreased AP-1 DNA-binding
activity (Fig. 4F). Furthermore,
fucoidan decreased JunB and JunD protein expression in a
dose-dependent manner (Fig.
4G).
Effect of fucoidan on TOPK
activation
TOPK, a serine-threonine kinase is known to be
upregulated in many types of cancer, including lymphoma and
leukemia (21). TOPK is a mitotic
kinase activated by the CDK1/cyclin B1 complex to promote
cytokinesis, and contributes to oncogenic cellular functions
(22). Recently, fucoidan was
reported to directly interact with TOPK kinase and inhibit its
kinase activity (23). Furthermore,
TOPK directly interacted with and phosphorylated IκBα (24). Therefore, we investigated whether
fucoidan influenced the activity of TOPK, an upstream kinase of
IκBα. Fucoidan inhibited the phosphorylation of TOPK but not total
TOPK protein expression level (Fig.
4C). TOPK is a mitogen-activated protein kinase (MAPK)
kinase-like protein kinase (22).
However, fucoidan did not alter the phosphorylation of MAPK,
including p38, JNK and ERK (data not shown). These results revealed
that TOPK may act as an upstream kinase for IκBα but not MAPK in
PEL cells.
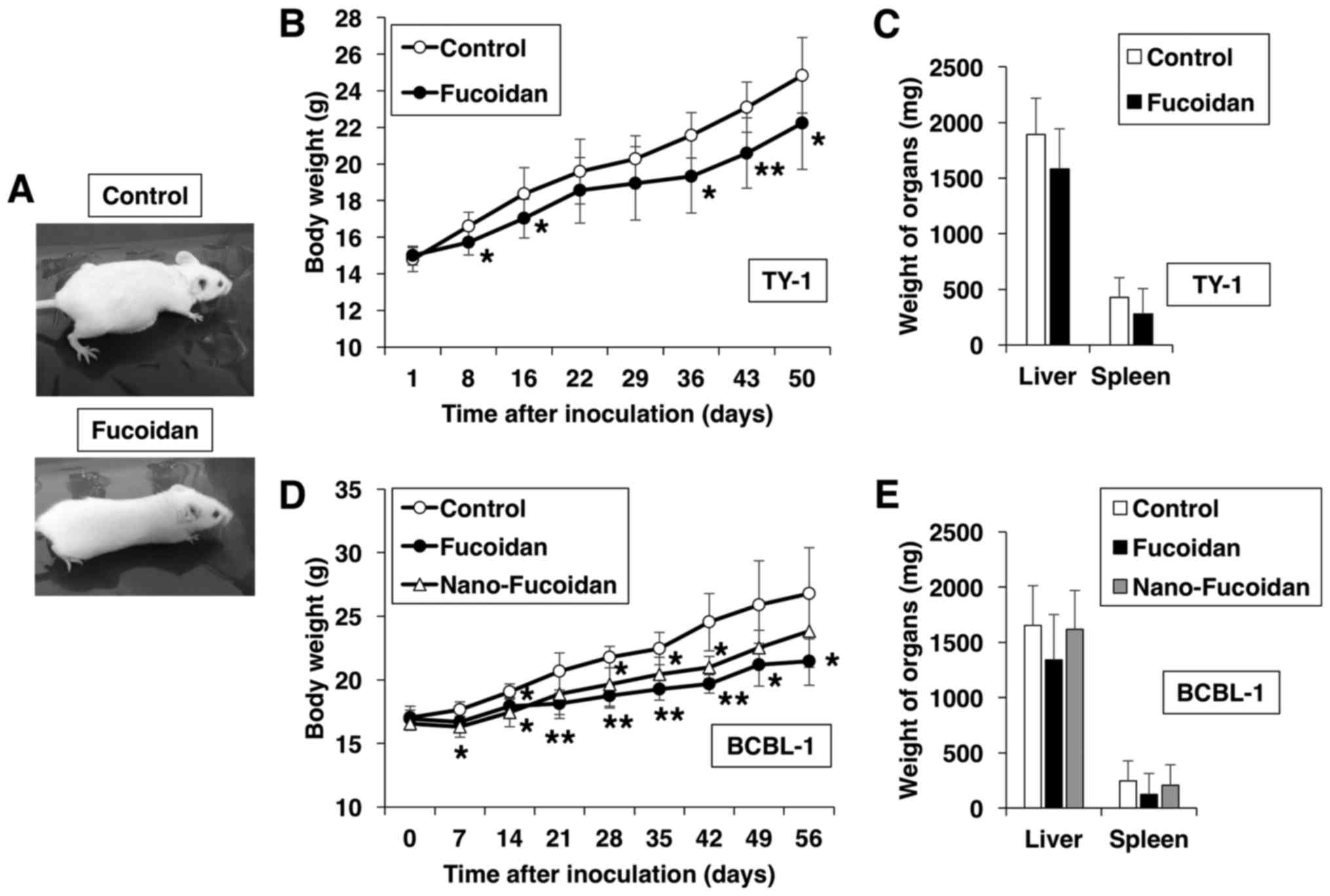
Treatment with fucoidan suppresses PEL
development in vivo
Finally, we investigated whether fucoidan can
suppress PEL development in vivo using an established
xenograft murine model. TY-1 cells were injected intraperitoneally
into SCID mice followed by oral administration of fucoidan or
vehicle for 50 days. Fucoidan-treated mice were slimmer than the
control, the latter exhibited massive ascites and expansion of the
abdomen (Fig. 5A). Furthermore, the
body weight increase of the fucoidan-treated mice throughout the
50-day experiment was less marked than that of the control mice
(Fig. 5B). PEL cells express CD30
(2), and sCD30 is produced by PEL
cells (25). The ascitic levels of
sCD30 revealed a 66% decrease in fucoidan-treated mice, compared
with the control group, albeit statistically insignificant. In
addition, fucoidan treatment tended to suppress the increase in
liver and spleen weight, albeit insignificantly (Fig. 5C). It has been suggested that
different antitumor effects of fucoidan are probably related to the
molecular weight of the different preparations (26,27).
Therefore, we analyzed the size-dependent bioactivities of fucoidan
by comparing the anti-PEL effects of native fucoidan to those of
fucoidan lipid nanoparticles in vivo. Relative to the
control mice, treatment with native fucoidan and nanoparticle
fucoidan resulted in a significant decrease in the rate of body
weight increase, although the effect of the former was greater than
that of the latter (P<0.05 at 42 days after inoculation;
Fig. 5D). The weights of the liver
and spleen in native fucoidan-treated mice were less than those of
either the control or the nanoparticle fucoidan-treated mice
(Fig. 5E). The serum levels of
sCD30 appear to be a useful biological tumor marker for the
diagnosis and management of PEL (25). ELISA used to determine the
circulating levels of sCD30 secreted by BCBL-1 cells revealed an 85
and 45% decrease, respectively, in native fucoidan- and
nanofucoidan-treated mice, compared with the control group, albeit
statistically insignificant. These results indicate that
intraperitoneal inoculation of PEL cells resulted in the
development of ascites in SCID mice, and that treatment with
fucoidan modulated this process. Furthermore, these effects were
dependent on the molecular weight of fucoidan.
Discussion
Fucoidan, a sulfated polysaccharide, is a
constituent of brown algae. It has been extensively studied based
on its numerous biological activities, including anticoagulation,
antiviral, antitumor, immunomodulatory, anti-inflammatory and
antioxidant activities (9,10,28).
We reported previously the antitumor and antiviral activities of
fucoidan extracted from Cladosiphon okamuranus Tokida
cultivated in Okinawa (11–14,29).
PEL, an aggressive neoplasm caused by KSHV, presents as a
lymphomatous effusion in body cavities. To address the potential
clinical use of fucoidan, we evaluated the cytotoxic effects of
fucoidan on PEL cell lines and determined the molecular mechanism
of the anti-PEL effect of fucoidan both in vitro and in
vivo.
Our results revealed that fucoidan was cytotoxic
against PEL. The cytotoxicity of fucoidan was mediated through cell
cycle arrest and apoptosis, as demonstrated by the results of cell
cycle analysis, apoptosis and determination of expression levels of
cell cycle- and apoptosis-related proteins. Fucoidan inhibited
NF-κB signaling through dephosphorylation of TOPK and IκBα, and
suppressed AP-1 signaling by decreasing JunB and JunD proteins.
CDK4, CDK6, Bcl-xL, XIAP and JunB are NF-κB-regulatory gene
products (30,31). Thus, the results revealed that the
effects of fucoidan on PEL cells were mediated by dysregulation of
various signaling pathways, including NF-κB, AP-1, and TOPK
signaling. TOPK directly interacted with and phosphorylated IκBα,
leading to NF-κB activation (24),
and NF-κB elements contributed to JunB inducibility (32). These findings demonstrated that
fucoidan targeted TOPK, which led to inactivation of NF-κB that is
required for AP-1 activation. In addition, the in vivo study
revealed that fucoidan exerted anti-PEL activity in SCID mice. In
toxicology studies, fucoidan derived from Laminaria japonica
and Undaria pinnatifida was found to be safe in rats
administered orally at 300 mg/kg per day for 180 days and at
250–1,000 mg/kg per day for 28 days, respectively (33,34).
In our animal model, fucoidan from Cladosiphon okamuranus
Tokida also exhibited little toxicity at 150 mg/kg per day for 56
days and at 200 mg/kg per day for 50 days, respectively.
The effects of fucoidan when used in preparations of
different molecular weights, remain unknown. We previously
demonstrated that the in vivo antitumor activity of fucoidan
was significantly higher for nanoparticle fucoidan than for native
fucoidan in a xenograft osteosarcoma model (14). However, in the present study,
nanoparticle fucoidan had lower anti-PEL activity compared to
native fucoidan in the SCID mouse model. High-molecular weight
fucoidan has been reported to promote a greater increase in the
proportion of cytotoxic T cells than middle- or low-molecular
weight fucoidan in mice which were fed an experimental diet
(35). SCID mice have a genetic
defect that prevents the functional development of T and B
lymphocytes, resulting in lack of both T and B lymphocytes
(36), but have certain residual
immunity, such as natural killer (NK) cells, that somehow limits
post-transplantation growth and metastasis of human xenografts
(37). NK cells play an important
role in the control of growth and infiltration of PEL cells
(37). It has been reported that
oral administration of high-molecular weight fucoidan increased the
size of the NK cell population in splenic tissue compared to that
observed in either control or intermediate-molecular weight
fucoidan (26). Native fucoidan may
induce a larger increase in NK cell activity compared with
nanoparticle fucoidan in SCID mice. The cell proliferation and
survival were decreased in PEL cells treated with relatively higher
concentrations of fucoidan in vitro. Fucoidan may exert
anti-PEL effects indirectly via enhancing immunity in
vivo.
Collectively, our results demonstrated that fucoidan
has potent anti-PEL activity both in vitro and in
vivo. Fucoidan directly inhibited the growth of PEL cells by
inducing cell cycle arrest and apoptosis. Future studies should
explore the mechanisms of action of fucoidan when used at various
molecular weights on PEL cells or immune cells. The results
presented here suggest that fucoidan could be potentially useful
for the treatment of PEL.
Acknowledgements
We thank Dr Harutaka Katano for providing TY-1 and
BCBL-1, and Kanehide Bio Co. (Okinawa, Japan) for providing native
and nanoparticle fucoidan. This study was supported in part by JSPS
KAKENHI (grant nos: 15K18414, 25461428 and 17K07175). This study
was funded by Kanehide Bio Co. (Okinawa, Japan).
References
|
1
|
Okada S, Goto H and Yotsumoto M: Current
status of treatment for primary effusion lymphoma. Intractable Rare
Dis Res. 3:65–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nador RG, Cesarman E, Chadburn A, Dawson
DB, Ansari MQ, Sald J and Knowles DM: Primary effusion lymphoma: A
distinct clinicopathologic entity associated with the Kaposi's
sarcoma-associated herpes virus. Blood. 88:645–656. 1996.PubMed/NCBI
|
|
3
|
Chen YB, Rahemtullah A and Hochberg E:
Primary effusion lymphoma. Oncologist. 12:569–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carbone A and Gloghini A:
KSHV/HHV8-associated lymphomas. Br J Haematol. 140:13–24.
2008.PubMed/NCBI
|
|
5
|
Keller SA, Schattner EJ and Cesarman E:
Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary
effusion lymphoma cells. Blood. 96:2537–2542. 2000.PubMed/NCBI
|
|
6
|
Gopalakrishnan R, Matta H and Chaudhary
PM: A purine scaffold HSP90 inhibitor BIIB021 has selective
activity against KSHV-associated primary effusion lymphoma and
blocks vFLIP K13-induced NF-κB. Clin Cancer Res. 19:5016–5026.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cannon ML and Cesarman E: The KSHV G
protein-coupled receptor signals via multiple pathways to induce
transcription factor activation in primary effusion lymphoma cells.
Oncogene. 23:514–523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
An J, Sun Y, Sun R and Rettig MB: Kaposi's
sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6
expression: The role of the NF-kappaB and JNK/AP1 pathways.
Oncogene. 22:3371–3385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwak J-Y: Fucoidan as a marine anticancer
agent in preclinical development. Mar Drugs. 12:851–870. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Wang S-X and Guan H-S: The
antiviral activities and mechanisms of marine polysaccharides: An
overview. Mar Drugs. 10:2795–2816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mori N, Nakasone K, Tomimori K and
Ishikawa C: Beneficial effects of fucoidan in patients with chronic
hepatitis C virus infection. World J Gastroenterol. 18:2225–2230.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Araya N, Takahashi K, Sato T, Nakamura T,
Sawa C, Hasegawa D, Ando H, Aratani S, Yagishita N, Fujii R, et al:
Fucoidan therapy decreases the proviral load in patients with human
T-lymphotropic virus type-1-associated neurological disease.
Antivir Ther. 16:89–98. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeda K, Tomimori K, Kimura R, Ishikawa
C, Nowling TK and Mori N: Anti-tumor activity of fucoidan is
mediated by nitric oxide released from macrophages. Int J Oncol.
40:251–260. 2012.PubMed/NCBI
|
|
14
|
Kimura R, Rokkaku T, Takeda S, Senba M and
Mori N: Cytotoxic effects of fucoidan nanoparticles against
osteosarcoma. Mar Drugs. 11:4267–4278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Renne R, Zhong W, Herndier B, McGrath M,
Abbey N, Kedes D and Ganem D: Lytic growth of Kaposi's
sarcoma-associated herpesvirus (human herpesvirus 8) in culture.
Nat Med. 2:342–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katano H, Hoshino Y, Morishita Y, Nakamura
T, Satoh H, Iwamoto A, Herndier B and Mori S: Establishing and
characterizing a CD30-positive cell line harboring HHV-8 from a
primary effusion lymphoma. J Med Virol. 58:394–401. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori N and Prager D: Transactivation of
the interleukin-1alpha promoter by human T-cell leukemia virus type
I and type II Tax proteins. Blood. 87:3410–3417. 1996.PubMed/NCBI
|
|
18
|
Zhang C, Ao Z, Seth A and Schlossman SF: A
mitochondrial membrane protein defined by a novel monoclonal
antibody is preferentially detected in apoptotic cells. J Immunol.
157:3980–3987. 1996.PubMed/NCBI
|
|
19
|
Khan N, Afaq F and Mukhtar H: Apoptosis by
dietary factors: The suicide solution for delaying cancer growth.
Carcinogenesis. 28:233–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simons-Evelyn M, Bailey-Dell K, Toretsky
JA, Ross DD, Fenton R, Kalvakolanu D and Rapoport AP: PBK/TOPK is a
novel mitotic kinase which is upregulated in Burkitt's lymphoma and
other highly proliferative malignant cells. Blood Cells Mol Dis.
27:825–829. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abe Y, Takeuchi T, Kagawa-Miki L, Ueda N,
Shigemoto K, Yasukawa M and Kito K: A mitotic kinase TOPK enhances
Cdk1/cyclin B1-dependent phosphorylation of PRC1 and promotes
cytokinesis. J Mol Biol. 370:231–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vishchuk OS, Sun H, Wang Z, Ermakova SP,
Xiao J, Lu T, Xue P, Zvyagintseva TN, Xiong H, Shao C, et al:
PDZ-binding kinase/T-LAK cell-originated protein kinase is a target
of the fucoidan from brown alga Fucus evanescens in the prevention
of EGF-induced neoplastic cell transformation and colon cancer
growth. Oncotarget. 7:18763–18773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park J-H, Yoon D-S, Choi H-J, Hahm D-H and
Oh S-M: Phosphorylation of IκBα at serine 32 by
T-lymphokine-activated killer cell-originated protein kinase is
essential for chemoresistance against doxorubicin in cervical
cancer cells. J Biol Chem. 288:3585–3593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michai M, Goto H, Hattori S,
Vaeteewoottacharn K, Wongkham C, Wongkham S and Okada S: Soluble
CD30: A possible serum tumor marker for primary effusion lymphoma.
Asian Pac J Cancer Prev. 13:4939–4941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang C, Chung D, Shin IS, Lee H, Kim J,
Lee Y and You S: Effects of molecular weight and hydrolysis
conditions on anticancer activity of fucoidans from sporophyll of
Undaria pinnatifida. Int J Biol Macromol. 43:433–437. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Azuma K, Ishihara T, Nakamoto H, Amaha T,
Osaki T, Tsuka T, Imagawa T, Minami S, Takashima O, Ifuku S, et al:
Effects of oral administration of fucoidan extracted from
Cladosiphon okamuranus on tumor growth and survival time in a
tumor-bearing mouse model. Mar Drugs. 10:2337–2348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiao G, Yu G, Zhang J and Ewart HS:
Chemical structures and bioactivities of sulfated polysaccharides
from marine algae. Mar Drugs. 9:196–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haneji K, Matsuda T, Tomita M, Kawakami H,
Ohshiro K, Uchihara JN, Masuda M, Takasu N, Tanaka Y, Ohta T, et
al: Fucoidan extracted from Cladosiphon okamuranus Tokida induces
apoptosis of human T-cell leukemia virus type 1-infected T-cell
lines and primary adult T-cell leukemia cells. Nutr Cancer.
52:189–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwanaga R, Ohtani K, Hayashi T and
Nakamura M: Molecular mechanism of cell cycle progression induced
by the oncogene product Tax of human T-cell leukemia virus type I.
Oncogene. 20:2055–2067. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brown RT, Ades IZ and Nordan RP: An acute
phase response factor/NF-kappa B site downstream of the junB gene
that mediates responsiveness to interleukin-6 in a murine
plasmacytoma. J Biol Chem. 270:31129–31135. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li N, Zhang Q and Song J: Toxicological
evaluation of fucoidan extracted from Laminaria japonica in Wistar
rats. Food Chem Toxicol. 43:421–426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chung H-J, Jeun J, Houng S-J, Jun H-J,
Kweon D-K and Lee S-J: Toxicological evaluation of fucoidan from
Undaria pinnatifida in vitro and in vivo. Phytother Res.
24:1078–1083. 2010.PubMed/NCBI
|
|
35
|
Shimizu J, Wada-Funada U, Mano H, Matahira
Y, Kawaguchi M and Wada M: Proportion of murine cytotoxic T cells
is increased by high molecular-weight fucoidan extracted from
Okinawa Mozuku (Cladosiphon okamuranus). J Health Sci. 51:394–397.
2005. View Article : Google Scholar
|
|
36
|
Bosma GC, Custer RP and Bosma MJ: A severe
combined immunodeficiency mutation in the mouse. Nature.
301:527–530. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dewan MZ, Terunuma H, Toi M, Tanaka Y,
Katano H, Deng X, Abe H, Nakasone T, Mori N, Sata T, et al:
Potential role of natural killer cells in controlling growth and
infiltration of AIDS-associated primary effusion lymphoma cells.
Cancer Sci. 97:1381–1387. 2006. View Article : Google Scholar : PubMed/NCBI
|