Introduction
Approximately 50% of all patients with malignant
melanoma harbor an activating mutation (V600E) in the BRAF kinase
(1,2). This leads to an activation of the MAPK
pathway and thereby to uncontrolled tumor growth (3). Vemurafenib and dabrafenib are two
approved inhibitors of mutated BRAF and have shown efficacy in
patients with BRAF V600E mutated melanoma (4,5).
In brain tumors BRAF V600E mutations have only been
reported for rare entities and at low frequency (6). Gangliogliomas, dysembryoblastic
neuroepithelial tumours (DNT) and supratentorial pilocytic
astrocytomas are mostly benign tumors that harbor BRAF V600E
mutations in 20–60, 30 and 5% of the examined cases, respectively
(7–12). Clinically relevant are BRAF V600E
mutations in pleomorphic xanthoastrocytomas (PXA) (including
anaplastic variants) with a frequency of 50–78% and epitheloid
glioblastomas with 50% (8,9,13–18).
Due to the low incidence of these tumors BRAF V600E mutations are
rare in everyday clinical practice (6,19).
Based on the results for BRAF inhibition in
malignant melanoma, vemurafenib and dabrafenib have been used on an
individual treatment basis in a few reported brain tumor patients.
These are predominantly pediatric patients with ganglioglioma
treated with vemurafenib (20–23).
Furthermore, there are two case reports on children treated with
vemurafenib for glioblastoma and pilomyxoid astrocytoma (24,25).
There are three reports on pediatric patients with BRAF mutated
brain tumors treated with dabrafenib. Two children suffered from a
ganglioglioma and one from a low-grade glioma (26–28).
Finally, there are three reports on adult patients treated with
vemurafenib for pleomorphic xanthoastrocytoma (29–31).
The largest case series was reported by Chamberlain with 4 patients
receiving vemurafenib for pleomorphic xanthoastrocytoma (31). Radiographic response assessment
showed progressive disease in one, stable disease in two and
partial response in one patient. Median progression-free survival
was reported with 5 months (range, 2 10 months) and median overall
survival with 8 months (range, 4–14 months). We are not aware of
reports on dabrafenib in adult patients.
In this study, we report on the first case series of
3 patients with BRAF V600E mutated, malignant glioma and
leptomeningeal disease successfully treated with dabrafenib.
Materials and methods
Patients
We included three consecutive patients with
malignant glioma and known BRAF V600E mutation treated with
dabrafenib for progressive disease. All patients have been
diagnosed and treated at the Brain Tumor Center of the University
Hospital Frankfurt.
Ethics and approval
The study was performed in accordance with all
ethical standards laid down in the 1964 Declaration of Helsinki and
its later amendments. All patient data were anonymized prior to
analysis. Approval of our local ethics committee was obtained for
this study (No. 4/09, University Hospital Frankfurt, Germany). All
patients in this study gave their written informed consent for
scientific evaluation.
Generation and treatment of ex vivo
cell culture
After centrifugation of 2 ml of cerebrospinal fluid
(CSF), the resulting pellet was resuspended in Dulbeccos modified
Eagles medium (DMEM) containing 10% fetal calf serum (FCS), 100
IU/ml penicillin and 100 mg/ml streptomycin. Subsequently, the
primary cell cultures were passaged for three passages; 100,000
cells/well of 24-well plates were incubated in the presence of
vehicle dimethyl sulfoxide (DMSO) or 100 nM dabrafenib for 72 h.
Life cell microscopy was done with a Biozero Keyence
microscope.
Western blot analysis
Glioma cells derived from CSF of patient 3 were
incubated for 1 h in DMEM containing 10% FCS in the presence of
vehicle or 100 nM dabrafenib. After the incubation cells were
washed with ice-cold phosphate-buffered saline (PBS) and
immediately frozen by placing the dishes in fluid nitrogen. Lysates
were prepared as described using lysis buffer P and subjected to
SDS-PAGE analysis (32). Membranes
were probed with antibodies for P-AKT (Ser473), P-Erk1/2
(Thr202/Tyr204), P-S6RP (Ser235/235) or Rab11 (#7100; Cell
Signaling Technology, Danvers, MA, USA). The secondary anti-rabbit
antibody [Peroxidase AffiniPure Goat Anti-Rabbit IgG (H+L)] was
purchased from Jackson ImmunoReseach Laboratories (West Grove, PA,
USA). Chemiluminescence solution was used for detection.
Results
Histopathology and molecular
markers
All 3 patients presented with highly pleomorphic
astroglial tumors (Fig. 1). Besides
anaplasia, the tumors showed vascular proliferations and partly
necroses, as well as a strong immunohistochemical expression of
BRAF V600E (Fig. 1). In all
patients we performed sequencing of the BRAF gene and confirmed the
BRAF V600E mutation. We profiled all three tumors for DNA
methylation patterns using the Illumina HumanMethylation450
BeadChip. Methylation patterns did not reveal a precise diagnosis.
Therefore, diagnoses were based on morphological characteristics.
Patients 1 and 2 showed an anaplastic pleomorphic
xanthoastrocytoma, while the tumor in patient 3 fulfilled the
criteria for glioblastoma, but without signs for epithelioid
differentiation.
Previous treatment and response to
dabrafenib
Patient 1
The first patient (male) presented at the age of 24
with blurred vision, headache and papilledema. MRI scan showed a
large, contrast enhancing tumor (maximum diameter of 6 cm) with
minor hemorrhage in the right temporal lobe. A gross total
resection was feasible and histopathology showed an anaplastic
pleomorphic xanthoastrocytoma. The MGMT promotor was unmethylated
and IDH1 R132H was not detectable. Immunohistochemistry as well as
DNA-sequencing revealed BRAF V600E mutation. After gross total
resection of the right temporal tumor all symptoms resolved and he
was treated with combined radiochemotherapy according to the EORTC
22981/26981 study (33,34). After 2 cycles of temozolomide
chemotherapy he developed lower back pain and sciatica in both
legs. Furthermore, he reported on incontinence and constipation.
MRI showed disseminated leptomeningeal spreading with several
contrast enhancing lesions in the brain and major contrast
enhancement mainly around the lumbar spinal cord and the nerve
fibers. CSF analysis showed a cell count of 28/µl, elevated lactate
(5.12 mmol/l), elevated albumin (3960 mg/l) and clearly detectable
tumor cells. He then received palliative radiotherapy for the
lumbar spine as this was the symptomatic region. Despite
radiotherapy, all symptoms and all MRI lesions progressed shortly
after radiotherapy. Two months after radiotherapy we initiated oral
dabrafenib treatment with 150 mg twice daily. Dabrafenib was well
tolerated, but the patient developed acne inversa as a side-effect
of dabrafenib. MRI scans and clinical symptoms improved markedly.
After 2 months of treatment all symptoms had resolved completely.
Fig. 2 shows brain MRI and lumbar
MRI before and one year after the initiation of dabrafenib
treatment. All lesions fully regressed and all CSF parameters
normalized. To date, he is on dabrafenib for 27 months and MRI
still shows complete remission without contrast enhancing
lesions.
Patient 2
The second patient (male) was diagnosed at the age
of 50. Notably, an MRI scan showed changes on T2 sequences in the
left temporal lobe 6 years before (incidental finding). Because of
a first epileptic seizure and progressive MRI (maximum diameter of
4 cm) with faint contrast enhancement, a gross total resection was
performed at another University hospital. Histopathology suggested
a glioblastoma with methylated MGMT promoter and wild-type IDH.
Retrospective analyses of the initial tumor tissue at our
institution and by an external reference neuropathologist revealed
an anaplastic pleomorphic xanthoastrocytoma. Immunohistochemistry
as well as DNA-sequencing revealed BRAF V600E mutation. After gross
total resection of the left temporal tumor he was treated with
combined radiochemotherapy according to the EORTC 22981/26981 study
(Stupp regimen) with 6 cycles of temozolomide chemotherapy
(33,34). Three and a half years after the end
of the treatment MRI showed progressive tumor and the patient,
suffering mild aphasia, underwent another gross total resection.
Thereafter, he received 6 cycles of chemotherapy with lomustine and
procarbazine. Six months after the end of the last cycle MRI again
showed progressive disease and the patient suffered minimal aphasia
and minimal visual deficit. Another gross total resection was
achieved. Afterwards, he received a second radiotherapy with 10 ×
3.5 Gy. MRI again showed progressive disease 5 months later with
contrast enhancement along all ventricles (Fig. 3). Aphasia had slightly progressed
and he now showed a latent hemiparesis and mild cognitive
impairment. Furthermore, MRI showed a large contrast enhancing mass
in the left parietal lobe (Fig. 3).
Notably, this mass did not show elevated relative cerebral blood
volume (rCBV) and no relevant
O-(2-(18F)Fluoroethyl)-L-tyrosine (FET) uptake in PET
(35). In contrast, the
disseminated enhancement in the ventricles showed both, increased
rCBV (Fig. 3, second row) and
marked FET uptake (Fig. 3, third
row), suggesting tumor recurrence. After one month of treatment
with dabrafenib at 150 mg twice daily (Fig. 3, fourth row) the disseminated tumor
markedly decreased (white arrows) while the radiation necrosis
increased (white star). During further follow-up (2 and 4 months)
the tumor further regressed and the radiation necrosis resolved as
well. All symptoms improved and 8 months after the initiation of
dabrafenib MRI was stable and patient doing well. Dabrafenib was
well tolerated but the patient developed several small
fibroepithelial polyps.
Patient 3
The third patient (male) was diagnosed at the age of
25. He first noticed headache and then developed mild aphasia and
impairment of fine motor skills of his right hand. MRI scan showed
a contrast enhancing, cystic mass in the left temporal lobe with a
maximum diameter of 5 cm. First, the tumor was biopsied. As
histopathology did reveal a malignant astrocytic tumor and because
of the tumor size a gross total resection was done. Histopathology
showed a glioblastoma without epithelioid cells. Analysis of MGMT
promoter status was inconclusive, the tumor showed regular
expression of ATRX and was negative for IDH1 R132H on
immunohistochemistry. Immunohistochemistry as well as
DNA-sequencing revealed BRAF V600E mutation.
After gross total resection he still suffered mild
aphasia and latent hemiparesis and was treated with combined
radiochemotherapy according to the EORTC 22981/26981 study (Stupp
regimen) with 6 cycles of temozolomide chemotherapy (33,34).
MRI follow-up two months after the last cycle showed local tumor
progression and another gross total resection was performed. He
then received another irradiation with 10 × 3.5 Gy. Second
follow-up MRI showed disseminated tumor spreading suggestive for
leptomeningeal gliomatosis. At that time-point, the health
insurance refused our application for treatment with dabrafenib and
chemotherapy with lomustine was initiated. After two cycles all
lesions had progressed. The patient now suffered massive headache,
nausea and vomiting due to hydrocephalus. Therefore, he received a
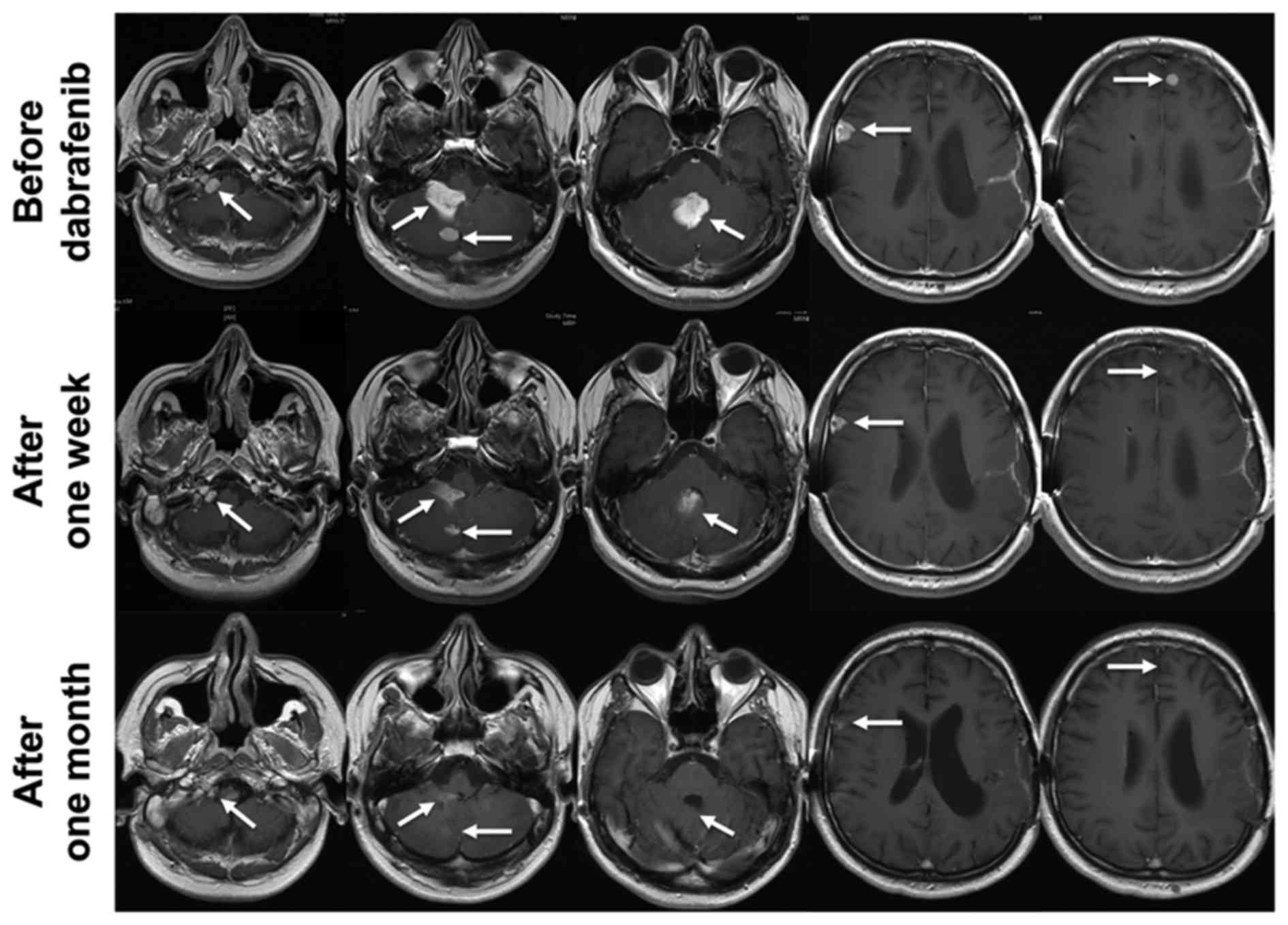
ventriculoperitoneal shunt. The first row of Fig. 4 shows contrast enhancing lesions in
the right foramen jugulare, the fourth ventricle, the right
cerebellum, the right frontal lobe and the left frontal lobe. Now
the health insurance decided to cover the costs for dabrafenib.
Dabrafenib was started with 150 mg twice daily, was well tolerated
and no drug related side-effects have been reported. As early as
one week after the initiation of dabrafenib MRI showed a partial
remission and the patient improved dramatically (Fig. 4). After one month he reached a
nearly complete response (Fig. 4).
All symptoms had resolved completely. Three months after the
initiation of dabrafenib MRI was stable.
Ex vivo tumor cell culture
Fig. 5A shows CSF of
our first patient with microscopic CSF analysis after Pappenheim
staining (left) and immunohistochemistry for mutant (V600E) BRAF
(right). CSF showed pleomorphic, partly multinucleated tumor cells
with basophilic cytoplasms, cytoplasmic blebbing (Fig. 5A, left) and an expression of BRAF
V600E (Fig. 5A, right). We were
able to generate an ex vivo tumor cell culture as described
in Materials and methods. During culture the cells remained
positive for BRAF V600E but exhibited slow growth kinetics. Cells
were treated with dabrafenib, survival was analyzed by light
microscopy and protein lysates were obtained for western blotting.
Fig. 5B shows microscopic
photographs of the cells 72 h after treatment with DMSO control
(left) and after treatment with dabrafenib at a concentration of
100 nM (right). This nicely shows that these cells not only harbor
a BRAF V600E mutation, but also are susceptible to kinase
inhibition with dabrafenib. Furthermore, our western blot analysis
shows that phosphorylation of ERK, a major downstream target of
BRAF, is inhibited in these cells by dabrafenib.
Discussion
In this study we present a retrospective series of 3
patients with BRAF V600E mutated recurrent glioma and disseminated
leptomeningeal disease treated with dabrafenib at 150 mg twice
daily. Despite their poor prognosis, all patients showed an
impressive response and one patient is stable for as long as 27
months. Furthermore, we were able to generate an ex vivo
cell culture. In these cells treatment with dabrafenib resulted in
a reduced cell density and inhibition of ERK phosphorylation.
These encouraging results corroborate the need for
molecular screening of rare mutations in brain tumors. Furthermore,
our results add to the existing data that BRAF V600E is a driver
mutation in malignant glioma. All our patients suffered from
markedly disseminated leptomeningeal disease. At this stage life
expectancy is usually a few weeks, further confirming the clinical
relevance of our results. As all patients suffered leptomeningeal
disease and responded to dabrafenib this confirms that dabrafenib
crosses the blood-brain barrier and reaches effective
concentrations in the CSF.
As this is a retrospective study of only 3 patients
we are not able to reliably compare our results to other case
reports or the case series on pleomorphic xanthoastrocytoma treated
with vemurafenib by Chamberlain et al (31). Since BRAF mutations are rare in
brain tumors, studies on other treatment approaches are also
lacking. To the best of our knowledge this is the first series in
adult patients with BRAF mutated gliomas treated with dabrafenib.
Furthermore, all of our patients showed leptomeningeal disease.
Whether this represents a relevant bias and might select for tumors
that are particularly vulnerable to dabrafenib treatment remains
unclear. Nonetheless, our results are favorable and support the use
of dabrafenib in BRAF V600E mutated malignant glioma and
leptomeningeal disease should not be an impediment.
In BRAF mutated malignant melanoma dabrafenib is
usually combined with a MEK inhibitor such as trametinib (36). We are not aware of published cases
of patients with BRAF mutated glioma that have been treated with
combined BRAF and MEK inhibition. There is one study on dabrafenib
and trametinib in a syngeneic murine glioma model (37). In this study, combined treatment was
more effective in reducing tumor growth and extending animal
survival (37). There are ongoing
trials evaluating the combined treatment with dabrafenib and
trametinib in patients with brain tumors.
In conclusion, this small retrospective study
suggests that dabrafenib has activity in BRAF V600E mutated
malignant glioma progressing after radiotherapy and
chemotherapy.
Acknowledgements
The Dr. Senckenberg Institute of Neurooncology is
supported by the Dr. Senckenberg Foundation.
References
|
1
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pollock PM, Harper UL, Hansen KS, Yudt LM,
Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J,
et al: High frequency of BRAF mutations in nevi. Nat Genet.
33:19–20. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Long GV, Menzies AM, Nagrial AM, Haydu LE,
Hamilton AL, Mann GJ, Hughes TM, Thompson JF, Scolyer RA and
Kefford RF: Prognostic and clinicopathologic associations of
oncogenic BRAF in metastatic melanoma. J Clin Oncol. 29:1239–1246.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: BRIM-3 Study Group: Improved survival with vemurafenib in
melanoma with BRAF V600E mutation. N Engl J Med. 364:2507–2516.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hauschild A, Grob JJ, Demidov LV, Jouary
T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr,
Kaempgen E, et al: Dabrafenib in BRAF-mutated metastatic melanoma:
A multicentre, open-label, phase 3 randomised controlled trial.
Lancet. 380:358–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chappé C, Padovani L, Scavarda D, Forest
F, Nanni-Metellus I, Loundou A, Mercurio S, Fina F, Lena G, Colin
C, et al: Dysembryoplastic neuroepithelial tumors share with
pleomorphic xanthoastrocytomas and gangliogliomas
BRAFV600E mutation and expression. Brain Pathol.
23:574–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dougherty MJ, Santi M, Brose MS, Ma C,
Resnick AC, Sievert AJ, Storm PB and Biegel JA: Activating
mutations in BRAF characterize a spectrum of pediatric low-grade
gliomas. Neuro Oncol. 12:621–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schindler G, Capper D, Meyer J, Janzarik
W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C,
Hasselblatt M, et al: Analysis of BRAF V600E mutation in 1,320
nervous system tumors reveals high mutation frequencies in
pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar
pilocytic astrocytoma. Acta Neuropathol. 121:397–405. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prabowo AS, Iyer AM, Veersema TJ, Anink
JJ, Schouten-van Meeteren AY, Spliet WG, van Rijen PC, Ferrier CH,
Capper D, Thom M, et al: BRAF V600E mutation is associated with
mTOR signaling activation in glioneuronal tumors. Brain Pathol.
24:52–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones DTW, Hutter B, Jäger N, Korshunov A,
Kool M, Warnatz HJ, Zichner T, Lambert SR, Ryzhova M, Quang DA, et
al: International Cancer Genome Consortium PedBrain Tumor Project:
Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic
astrocytoma. Nat Genet. 45:927–932. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Wu G, Miller CP, Tatevossian RG,
Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, et
al: St. Jude Children's Research Hospital-Washington University
Pediatric Cancer Genome Project: Whole-genome sequencing identifies
genetic alterations in pediatric low-grade gliomas. Nat Genet.
45:602–612. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dias-Santagata D, Lam Q, Vernovsky K, Vena
N, Lennerz JK, Borger DR, Batchelor TT, Ligon KL, Iafrate AJ, Ligon
AH, et al: BRAF V600E mutations are common in pleomorphic
xanthoastrocytoma: Diagnostic and therapeutic implications. PLoS
One. 6:e179482011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ida CM, Vrana JA, Rodriguez FJ, Jentoft
ME, Caron AA, Jenkins SM and Giannini C: Immunohistochemistry is
highly sensitive and specific for detection of BRAF V600E mutation
in pleomorphic xanthoastrocytoma. Acta Neuropathol Commun.
1:202013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koelsche C, Sahm F, Wöhrer A, Jeibmann A,
Schittenhelm J, Kohlhof P, Preusser M, Romeike B, Dohmen-Scheufler
H, Hartmann C, et al: BRAF-mutated pleomorphic xanthoastrocytoma is
associated with temporal location, reticulin fiber deposition and
CD34 expression. Brain Pathol. 24:221–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schiffman JD, Hodgson JG, VandenBerg SR,
Flaherty P, Polley MY, Yu M, Fisher PG, Rowitch DH, Ford JM, Berger
MS, et al: Oncogenic BRAF mutation with CDKN2A inactivation is
characteristic of a subset of pediatric malignant astrocytomas.
Cancer Res. 70:512–519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Broniscer A, Tatevossian RG, Sabin ND,
Klimo P Jr, Dalton J, Lee R, Gajjar A and Ellison DW: Clinical,
radiological, histological and molecular characteristics of
paediatric epithelioid glioblastoma. Neuropathol Appl Neurobiol.
40:327–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kleinschmidt-DeMasters BK, Aisner DL,
Birks DK and Foreman NK: Epithelioid GBMs show a high percentage of
BRAF V600E mutation. Am J Surg Pathol. 37:685–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Behling F, Barrantes-Freer A, Skardelly M,
Nieser M, Christians A, Stockhammer F, Rohde V, Tatagiba M,
Hartmann C, Stadelmann C, et al: Frequency of BRAF V600E mutations
in 969 central nervous system neoplasms. Diagn Pathol. 11:552016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aguilera D, Janss A, Mazewski C,
Castellino RC, Schniederjan M, Hayes L, Brahma B, Fogelgren L and
MacDonald TJ: Successful retreatment of a child with a refractory
brainstem ganglioglioma with vemurafenib. Pediatr Blood Cancer.
63:541–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bautista F, Paci A, Minard-Colin V, Dufour
C, Grill J, Lacroix L, Varlet P, Valteau-Couanet D and Geoerger B:
Vemurafenib in pediatric patients with BRAFV600E mutated high-grade
gliomas. Pediatr Blood Cancer. 61:1101–1103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
del Bufalo F, Carai A, Figà-Talamanca L,
Pettorini B, Mallucci C, Giangaspero F, Antonelli M, Badiali M, Moi
L, Bianco G, et al: Response of recurrent BRAFV600E mutated
ganglioglioma to Vemurafenib as single agent. J Transl Med.
12:3562014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rush S, Foreman N and Liu A: Brainstem
ganglioglioma successfully treated with vemurafenib. J Clin Oncol.
31:e159–e160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robinson GW, Orr BA and Gajjar A: Complete
clinical regression of a BRAF V600E-mutant pediatric glioblastoma
multiforme after BRAF inhibitor therapy. BMC Cancer. 14:2582014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skrypek M, Foreman N, Guillaume D and
Moertel C: Pilomyxoid astrocytoma treated successfully with
vemurafenib. Pediatr Blood Cancer. 61:2099–2100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shih KC, Shastry M, Williams JT, Jelsma
PF, Abram SR, Ayyanar K, Burris HA III and Infante JR: Successful
treatment with dabrafenib (GSK2118436) in a patient with
ganglioglioma. J Clin Oncol. 32:e98–e100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lassaletta A, Guerreiro Stucklin A,
Ramaswamy V, Zapotocky M, McKeown T, Hawkins C, Bouffet E and
Tabori U: Profound clinical and radiological response to BRAF
inhibition in a 2-month-old diencephalic child with
hypothalamic/chiasmatic glioma. Pediatr Blood Cancer. 63:20382016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meletath SK, Pavlick D, Brennan T,
Hamilton R, Chmielecki J, Elvin JA, Palma N, Ross JS, Miller VA,
Stephens PJ, et al: Personalized treatment for a patient with a
BRAF V600E mutation using gabrafenib and a tumor treatment fields
device in a high-grade glioma arising from ganglioglioma. J Natl
Compr Canc Netw. 14:1345–1350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee EQ, Ruland S, LeBoeuf NR, Wen PY and
Santagata S: Successful treatment of a progressive BRAF
V600E-mutated anaplastic pleomorphic xanthoastrocytoma with
vemurafenib monotherapy. J Clin Oncol. 34:e87–e89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Usubalieva A, Pierson CR, Kavran CA,
Huntoon K, Kryvenko ON, Mayer TG, Zhao W, Rock J, Ammirati M,
Puduvalli VK, et al: Primary meningeal pleomorphic
xanthoastrocytoma with anaplastic features: A report of 2 cases,
one with BRAF(V600E) mutation and clinical response to the BRAF
inhibitor dabrafenib. J Neuropathol Exp Neurol. 74:960–969. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chamberlain MC: Salvage therapy with BRAF
inhibitors for recurrent pleomorphic xanthoastrocytoma: A
retrospective case series. J Neurooncol. 114:237–240. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steinbach JP, Wolburg H, Klumpp A, Probst
H and Weller M: Hypoxia-induced cell death in human malignant
glioma cells: Energy deprivation promotes decoupling of
mitochondrial cytochrome c release from caspase processing and
necrotic cell death. Cell Death Differ. 10:823–832. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group: Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group: Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Langen KJ, Galldiks N, Hattingen E and
Shah NJ: Advances in neuro-oncology imaging. Nat Rev Neurol.
13:279–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Flaherty KT, Infante JR, Daud A, Gonzalez
R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N,
et al: Combined BRAF and MEK inhibition in melanoma with BRAF V600
mutations. N Engl J Med. 367:1694–1703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grossauer S, Koeck K, Murphy NE, Meyers
ID, Daynac M, Truffaux N, Truong AY, Nicolaides TP, McMahon M,
Berger MS, et al: Concurrent MEK targeted therapy prevents MAPK
pathway reactivation during BRAFV600E targeted inhibition in a
novel syngeneic murine glioma model. Oncotarget. 7:75839–75853.
2016. View Article : Google Scholar : PubMed/NCBI
|