Introduction
BC (BC) is the most common malignant disease among
women worldwide, and is associated with high morbidity and
mortality (1). Surgery combined
with adjuvant therapy was the main treatment strategy in BC
patients. In addition, endocrine and targeted therapies were
gradually applied to clinical therapy (2,3).
However, in the treatment of aggressive BC cases, chemoresistance
and toxicity are the leading causes for failure. Thus, it is
important to define and develop new therapeutic agents, which may
bind to BC cells specifically and effectively.
Frankincense, the gum resin derived from
Boswellia species, contains active ingredients. The oil has
been demonstrated to modulate critical biological activities
including anti-rheumatism, anti-inflammatory (4,5),
antibacterial, antifungal and anticancer activities (6–9).
Frankincense oil is prepared by the steam distillation of
frankincense gum resin. Based on the biological function of
frankincense, it possibly possesses anticancer characteristics.
Pine needle (Pinus densiflora Siebold & Zucc.), is
usually utilized as a herbal medicine, tea bag infusion and health
supplement in East Asian countries, such as Korea and China
(10). It is beneficial in the
therapy of patients with coronary heart disease (CHD),
neurodegenerative disorders and carcinoma. Moreover, it was also
reported that extracts from pine needle inhibited apoptosis of the
normal cells induced by a hydroxyl radical (11). As a central material, geranium
essential oil has been used in the cosmetic, perfume, aromatherapy
and food industries. In addition, the oil famous for its
antibacterial, antioxidative and anti-inflammatory properties, has
been used as a traditional drug for a long time (12–15).
However, whether frankincense, pine needle and
geranium essential oils have any effect on progression of BC in
MCF-7 cells remains unclear. The present study investigated the
anticancer properties of the prepared essential oils on the MCF-7
cells. Moreover, we elucidated the regulatory AMPK/mTOR pathway
involving essential oils in BC cell proliferation, invasion and
apoptosis development.
Materials and methods
Cell culture
MCF-7 cells were obtained from the Dalian Institute
of Chemical Physics, Chinese Academy of Sciences (Dalian, China).
Cells were seeded in RPMI-1640 medium (HyClone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island,
NY, USA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA,
USA). The cells were maintained in a humidified incubator at 37°C
with 5% CO2.
Materials
Stock solutions of frankincense, pine needle and
geranium essential oils were obtained from the Hualin Natural
Health Cosmetics Company (Beijing, China). Dimethyl sulfoxide
(DMSO) at a ratio of 1:2 (v/v) was used as a vehicle of the oils.
Subsequently, the oils were diluted with complete medium to a
series of different concentrations. The frankincense injection was
prepared by diluting the stock solution of frankincense by mixing
it with DMSO in a 1:2 ratio, and then diluting this mixture to
1:1,000 (v/v) with phosphate-buffered saline (PBS). The
frankincense smear was prepared by diluting the stock solution of
frankincense to 1% (v/v) with grape seed oil.
Western blotting
SDS buffer (60 mM Tris-HCl with a pH value at 6.8,
10% glycerol, 2% SDS, with 5% 2-mercaptoethanol) was utilized to
store the lysate of the cells and tissues. The 4–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels were
used to separate the cell lysate. Proteins were transferred to
polyvinylidene fluoride (PVDF) membranes (Invitrogen), and the
membranes were blocked with Tris-buffered saline plus 0.1% Tween-20
(TTBS) containing 5% skim milk for 2 h. The primary antibodies
specific to phospho-ERK, total-ERK, phospho-4E-BP1, phospho-mTOR,
total-mTOR, phospho-AMPK, total-AMPK, poly(ADP-ribose) polymerase
(PARP) (Asp214) (Cell Signaling Technology, Beverly, MA, USA),
Bcl-2 (Abgent, Inc., San Diego, CA, USA) were immunoblotted. All
bands were washed in TBS with Tween-20 (TBST) and immunoblotted
with peroxidase-conjugated anti-mouse or anti-rabbit secondary
antibodies, respectively. The bands were detected using an enhanced
chemiluminescence (ECL) Western Blotting kit and exposed to film.
The β-actin antibody (BIOSS, Beijing, China) was used as an
internal marker for control. All experiments were carried out
thrice.
Cell cycle analysis
For DNA content analysis, MCF-7 cells were treated
with different oils. The cells were gathered, washed and
resuspended after 48 h. Ethanol (75%) was used to fix cells
overnight. The cells were centrifugated and exposed to RNase (100
µl) for 30 min at 37°C. Propidium iodide (PI) (400 µl) was used to
stain DNA for 30 min without light. DNA contents were analyzed
using BD Biosciences Accuri C6 flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) and FlowJo software. DNA histograms in the
G0-G1, S and G2-M phases were defined and the ratio in each phase
was calculated.
Apoptosis assay
Cell apoptosis assays were carried out with the use
of an Annexin V-FITC apoptosis detection kit. Specifically, cells
(1×105 cells/well) were treated with or without oils for
48 h, collected, washed and resuspended. Then, the cells were
treated with a mixture of Annexin V-FITC and PI for 15 min in the
dark at room temperature. Subsequently, binding buffer (400 µl) was
added. The cells were then analyzed by the BD Biosciences Accuri C6
flow cytometer.
Cell Counting Kit-8 (CCK-8) assay
To evaluate cell proliferation, cells
(1×105 cells/well) were plated in 96-well plates
containing 100 µl full medium, and incubated with different oils at
various time-points. CCK-8 (10 µl) was added to the plates and
incubation followed at 37°C in 5% CO2 for 4 h. The
spectrometric absorbance was determined by microplate
spectrophotometer (Multiscan MK3; Thermo Fisher Scientific,
Waltham, MA, USA) at 450 nm.
Focus formation assay
Cells (5×102 cells/well) were trypsinized
to a single-cell suspension and seeded into 6-well plates. The
cultures were plated in the corresponding media with different oils
until the appearance of foci from transformed cells was evident (8
days after incubation). Crystal violet (0.2%) was utilized to stain
the colonies and images were captured with a Nikon digital camera
(Nikon Corporation, Tokyo, Japan).
Wound healing assay
Wound healing assay was performed to evaluate the
migratory ability of the tumor cells. Briefly, MCF-7
(1×104) cells were cultured into a 12-well plate with
various oil treatments and increased to 70–80% confluence. The
sterilized P200 pipette tip passed through the cell monolayer
causing a wound. The cells were observed to migrate to the wound at
different times. The migrated cells to the injured area were
photographed at different time-points under an inverted microscope.
In each well, at least 8 regions of each condition were captured
randomly at a magnification of ×100. The experiment was conducted
in triplicate.
Cell invasion assay
A cell invasion assay was performed using Transwell
inserts with polycarbonate membranes of 8.0-µm pore size (Corning
Inc., Corning, NY, USA) with ECMatrix gel (Chemicon, Temecula, CA,
USA) to form a continuous thin layer. In brief, 4×104
cells (with different oil treatments) and serum-free medium were
inoculated into the upper chamber. Culture medium containing 10%
FBS was used as a chemical attractant in the lower chamber. Cells
were incubated at 37°C in incubators for 24 h. Then, the invaded
cells under the membrane were fixed with methanol, stained with
Wright-Giemsa, photographed (magnification, ×400) and cells were
counted in 5 random areas. Each experiment was performed
thrice.
In vivo assays for tumor growth
To further determine whether frankincense regulated
tumor growth, in vivo tumorigenesis was performed. MCF-7
cells (2×105) were injected into the left and right
dorsal flank of 4–5 week-old female nude mice (purchased from the
Animal Center Dalian Medical University), respectively. When mice
exhibited a palpable tumor (~1 week after tumor cell inoculation),
they were randomly divided into 7 groups (n=5 animals/group): the
WT group, treated with nothing; the DMSO and negative group,
treated with DMSO subcutaneous injection; the base oil and negative
group, treated with grape seed oil smear; the injection group,
treated with frankincense subcutaneous injection (1:1,000 v/v, 0.1
ml); the external smear group, treated with frankincense smear (0.1
ml); the combination group, treated with frankincense subcutaneous
injection combined with frankincense smear; the PTX and positive
group, treated with PTX subcutaneous injection (10 mg/kg) (16). The treatment was administered every
4 days for 12 days. The tumor dimensions were gauged every 4 days
for 12 days (17). The estimated
tumor volume (mm3) was calculated using the following
formulas: Tumor volume = length × width2 × 0.52. All
mice were kept under specific pathogen-free conditions for air
filtration. All experiments with animals complied with the
standards in the guidelines of the University Animal Care and Use
Committee of Dalian Medical University. Finally, the tumors were
fixed in 10% formalin and embedded in paraffin for
immunohistochemistry.
Tunel assay
TUNEL analysis was used to determine the apoptosis
of tumor cells induced by essential oils. In short, the paraffin
section was cut into 5-µm of thickness, dewaxed and rehydrated.
Apoptotic cells were detected using an in situ cell death
assay kit. After the terminal deoxynucleotidyl transferase
reaction, the labeled end of the incision was identified by
alkaline phosphatase-based immunohistochemistry, with fast red as
the substrate. Stained slides were washed and sealed with an
aqueous mounting medium.
Statistical analysis
Student's t-test was applied to detect statistically
significant differences for non-paired replicates. One-way analysis
of variance (ANOVA) was used to compare replicate means. P-value
<0.05 was considered to indicate a statistically significant
result (P<0.05, P<0.01). Error bars represent the mean ±
standard deviation (SD) unless specified otherwise.
Results
Frankincense, pine needle and geranium
essential oils suppress MCF-7 cell viability and proliferation
To determine whether these three essential oils
affected MCF-7 cell viability, the number of viable MCF-7 cells was
determined following various dilutions (frankincense,
1:1,000-1:4,000; pine needle, 1:1,000-1:4,000; geranium,
1:1,000-1:8,000) of oil exposure. As shown in Fig. 1A-C, the cell viability was decreased
in a dose-dependent manner when MCF-7 cells were treated with oil.
In addition, no viable MCF-7 cells remained after 48 h treatment
with 1:1,000 dilution of oil. Based on the CCK-8 assay,
IC50 values (the 50% inhibitory concentration of
frankincense/pine needle/geranium essential oil) for MCF-7 cells
were 42.8 µg/ml, 90.2 µg/ml and 73.9 µg/ml, respectively (data not
shown). Moreover, to determine whether oil suppresses cell
viability, MCF-7 cells were subjected to morphological evaluation
assessment. As shown in Fig. 1D,
MCF-7 cells underwent significant morphological changes, such as
detaching from tissue culture plates and shrinking following oil
exposure (with IC50 dilution treatment for 48 h).
Furthermore, the colony formation assay revealed that the colony
size and colony forming capacity of MCF-7 cells were strongly
reduced in the presence of the oils compared to the controls
(Fig. 1E). These results indicated
that frankincense, pine needle and geranium essential oils played a
role in BC cell proliferation.
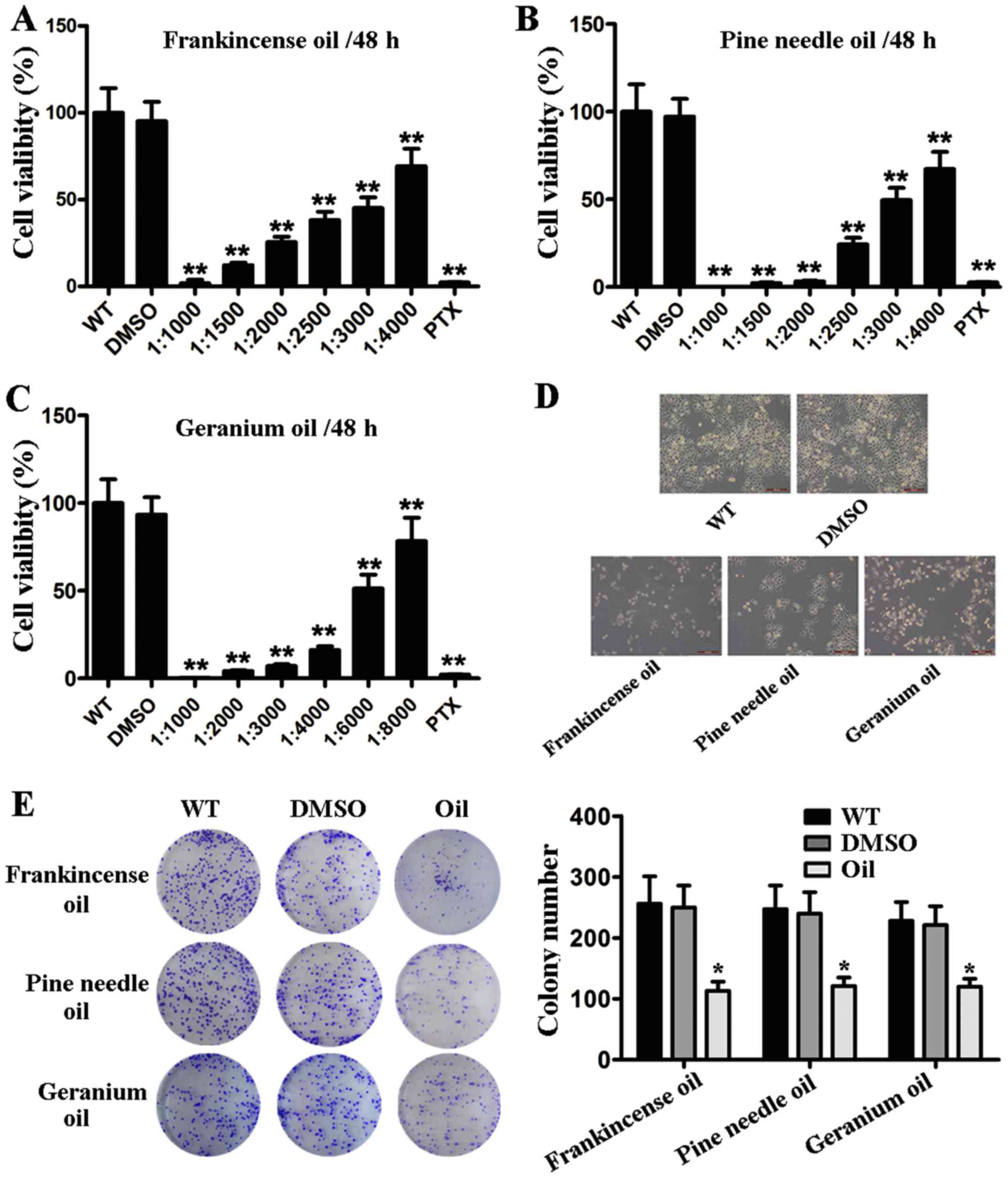 | Figure 1.Frankincense, pine needle and
geranium essential oils suppress the cell viability and
proliferation of MCF-7 cells. (A-C) MCF-7 cells were seeded in a
96-well tissue culture. The cells were subjected to serial
dilutions of frankincense, pine needle and geranium essential oils
treatment (WT, cells treated with nothing; DMSO, cells treated with
DMSO; oil, cells treated with oil; PTX, cells treated with
paclitaxel). Cell viability was determined at 48 h after essential
oil treatment by CCK-8 assay. (D) Morphological changes of MCF-7
cells following frankincense, pine needle and geranium essential
oil stimulation. MCF-7 cells were subjected to frankincense, pine
needle and geranium essential oil, respectively (1:3,000; 1:3,300;
and 1:6,000 v/v). Images were captured at 48 h after treatment. (E)
Cells were seeded in each well of a 6-well plate, subsequently
exposed to and incubated with frankincense/pine needle/geranium
essential oil (1:3,000; 1:3,300; and 1:6,000 v/v). After 8 days of
culture, the surviving colonies were counted with crystal violet
staining. Data are presented as the mean ± SD from 3 independent
experiments (*P<0.05, **P<0.01). |
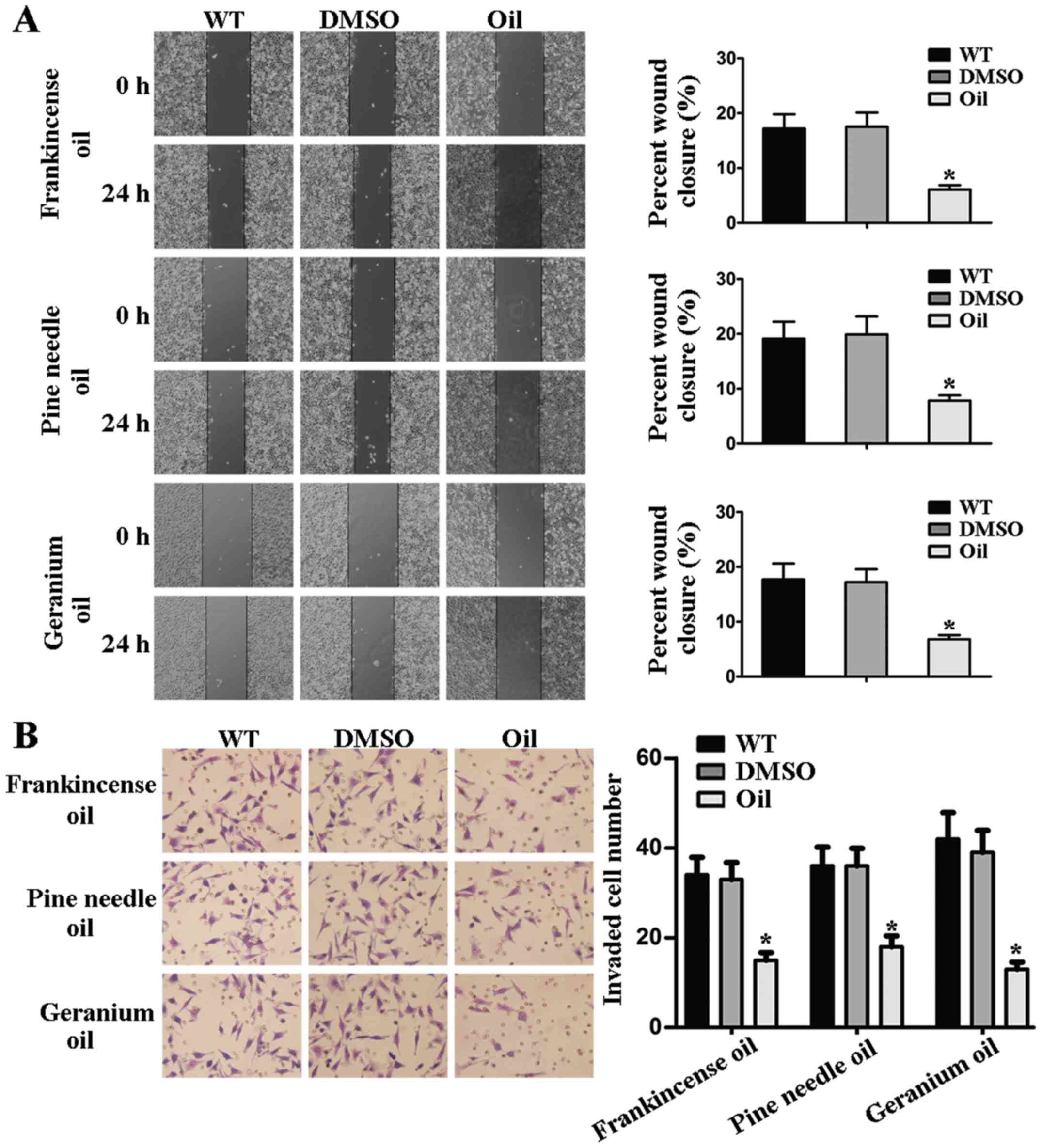
Frankincense, pine needle and geranium
essential oils regulate aggressiveness of MCF-7 cells
To examine whether these 3 essential oils exerted a
direct regulation on the response to BC aggressiveness, MCF-7 cells
were treated with different essential oils. Upon frankincense, pine
needle and geranium essential oil treatments, the migratory
capability was significantly decreased as determined by wound
healing assay (Fig. 2A).
Concomitantly to the aforementioned experiments, we investigated
the potential role of these 3 essential oils in mediating the
invasive abilities of MCF-7 cells. The results of the Transwell
invasion assay revealed that the invaded cell number/field was much
lower than that in the control group (Fig. 2B). Collectively, these results
demonstrated that frankincense, pine needle and geranium essential
oils regulated the aggressiveness of BC cells.
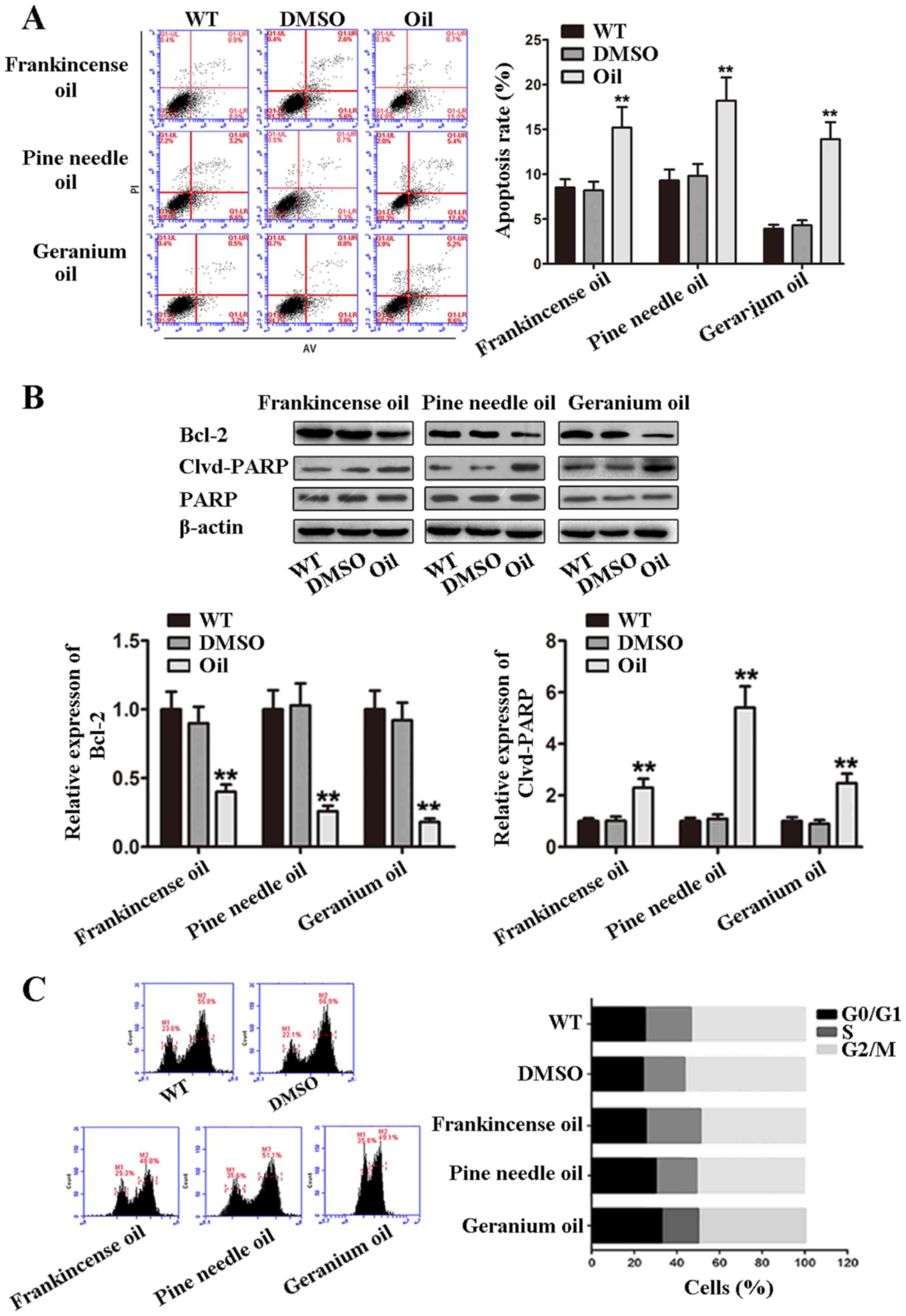
Frankincense, pine needle and geranium
essential oils regulate apoptosis and the cell cycle of MCF-7
cells
To explore the potential role of these 3 essential
oils in modulating the apoptosis and cell cycle, MCF-7 cells were
treated with oils (frankincense, 1:3,300; pine needle, 1:3,000;
geranium essential oil, 1:6,000) for 48 h, respectively. Cell
apoptosis and the cell cycle were analyzed by flow cytometry. As
shown in Fig. 3A, the number of
apoptotic cells was increased in groups treated with the oils,
compared with the WT and DMSO control groups. Cleavage of PARP was
involved in DNA repair following environmental stress (18). We also detected the expression of
cleaved PARP to evaluate the apoptotic activity of MCF-7 cells
treated with the oils. Cleaved PARP levels were increased in the
oil-treated cells (Fig. 3B). We
also determined the expression of the Bcl-2 protein, and found that
the oils induced a decrease in the expression of Bcl-2.
Furthermore, we analyzed the cell cycle of MCF-7 cells and found
that the percentage of cells arrested at the G0/G1, S and G2-M
phases were not significantly altered (Fig. 3C). Collectively, these results
indicated that frankincense, pine needle and geranium essential
oils induced apoptosis but not cell cycle arrest of MCF-7
cells.
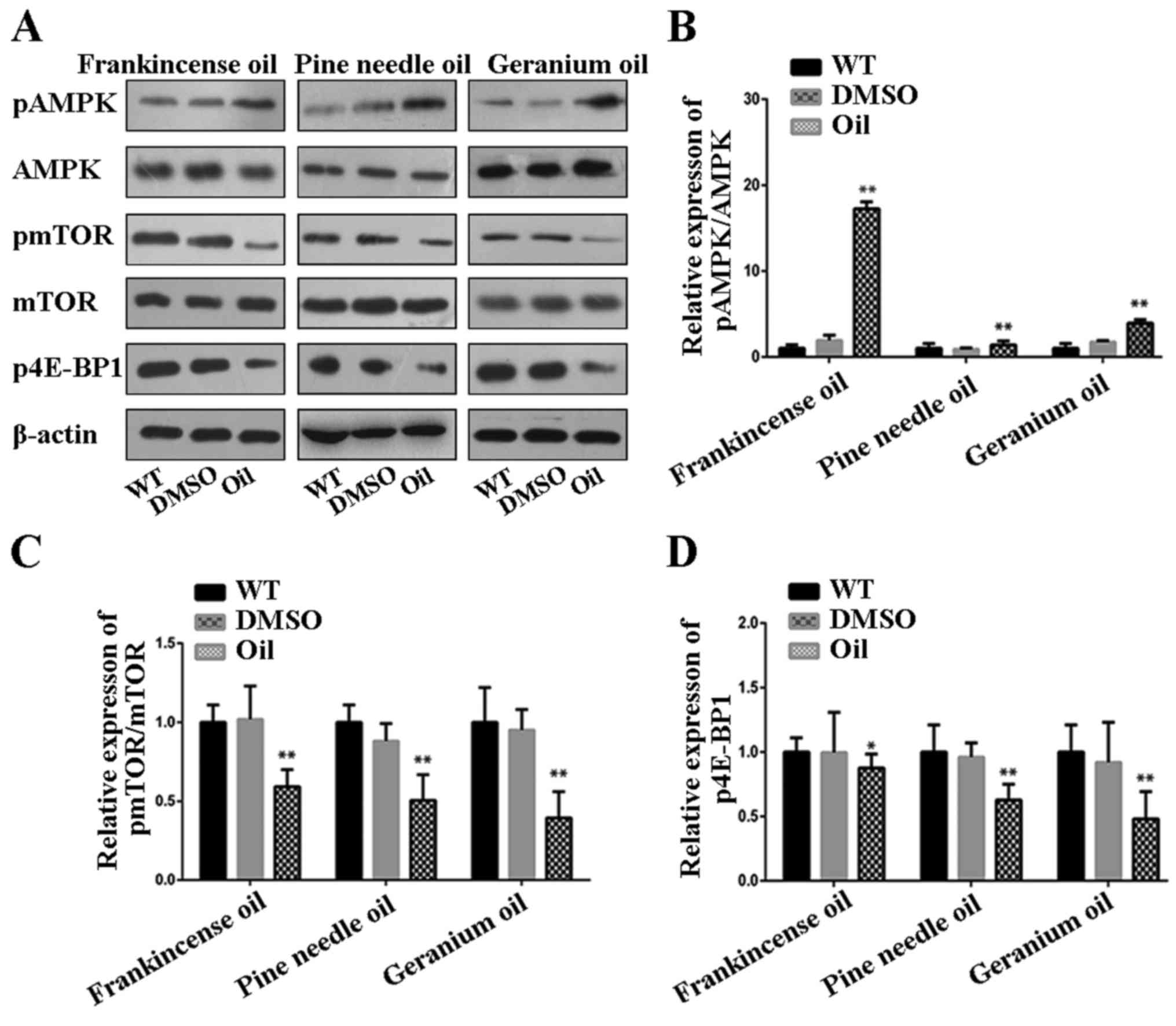
Frankincense, pine needle and geranium
essential oils mediate the activity of the AMPK/mTOR pathway
AMPK activation has been demonstrated to regulate
key proliferative signaling events such as mTOR/p70S6K (19). In addition, mTOR was demonstrated to
be a central controller of cell proliferation, growth and survival
(20). In the present study, we
assessed the activity of the AMPK signaling and its downstream
target by treatment of MCF-7 cells with frankincense, pine needle
and geranium essential oils. Western blot analysis revealed that
the levels of phosphorylated-AMPK were increased in MCF-7 cells
treated with these 3 essential oils (Fig. 4A and B). Concomitantly, the degrees
of phosphorylation of mTOR and its downstream effector 4E-BP1 were
markedly decreased in MCF-7 cells treated with these 3 essential
oils. Conversely, there was no change in the total amount of AMPK
and mTOR protein, demonstrating a true decrease in phosphorylation
status. These results revealed that the antiproliferative,
anti-invasive and induced-apoptosis effect of frankincense, pine
needle and geranium essential oils on MCF-7 cells involved
AMPK-initiated mTOR inhibition.
Frankincense essential oil modulates
tumor growth in a xenograft mouse model
Finally, we examined the tumorigenesis of
frankincense essential oil in MCF-7 cells by in vivo
experiments. Administration with frankincense subcutaneous
injection, frankincense smear, frankincense subcutaneous injection
combined with frankincense smear significantly suppressed tumor
growth in nude mice implanted with established MCF-7 tumors
(Fig. 5A). Moreover, the tumor
growth rate of the combined group was slowler than that of the
injection group and the smear group. Furthermore, TUNEL assay
revealed a significantly higher number of TUNEL-positive cells that
were detected in the experimental group, compared with the control
group. In addition, the results demonstrated that the number of
apoptotic cells in combination group was higher than that in the
other experimental control groups (Fig.
5B). Collectively, in vivo studies confirmed that
frankincense suppressed tumor growth and induced apoptosis.
Discussion
In the present study, we clearly demonstrated that
frankincense, pine needle and geranium essential oils suppressed
cell viability, proliferation and invasion in human BC cell line
MCF-7. In addition, we determined that the frankincense, pine
needle and geranium essential oils induced apoptosis, but did not
affect cell cycle progression. The frankincense essential oil was
also effective in inhibiting tumor growth and inducing tumor cell
apoptosis in human BC mouse model. We demonstrated that
frankincense, pine needle and geranium essential oils suppressed
cell progression through the AMPK/mTOR pathway.
Essential oils, which are distilled from flowers,
leaves, stems, the bark or roots of a specific plant, contained
terpenes, aldehydes, esters, alcohols and other chemical molecules.
It has been demonstrated that essential oils have an antibacterial
and anti-inflammatory effect. Moreover, research has reported that
essential oils also have a certain anticancer effect. Wu et
al demonstrated that essential oils from Angelicae
dahuricae and Inula japonica increased the sensitivity
of BC cell line MCF-7/ADR to doxorubicin (21). Essential oils distilled from the
leaves and flowers of Callistemon citrinus from the western
Himalayas gave rise to the antiproliferative effect on human lung
carcinoma cell line A549 and rat glioma C-6 cells via induction of
apoptosis (22). Thymoquinone
decreased proliferation and accelerated apoptosis in ID8-NGL (mouse
ovarian cancer cells) tumors after 10 and 30 day-treatment
(23). It has been observed
thymoquinone mediated cell cycle arrest and apoptosis in BC and
hepatocellular carcinoma (24,25).
Boswellic acids (major components of frankincense) were reported to
possess antitumor activity due to their cytostatic and
pro-apoptotic properties in many human cancer cell lines containing
meningioma (26), leukemia
(27), hepatocellular carcinoma
(28), melanoma, fibrosarcoma
(29), colon (30) and prostate cancer (31–33).
Moreover, the essential oil of frankincense inhibited proliferation
and modulated apoptosis of human cancer cell lines both in
vitro and in vivo (17,34,35).
According to a study by Jeong et al, apoptosis, oxidative
cell damage, induced by exposure to hydroxyl radical was inhibited
by the extracts from pine needle (11). In the present study, we demonstrated
that frankincense, pine needle and geranium essential oils reduced
MCF-7 cell viability in a dose-dependent manner. Moreover,
frankincense, pine needle and geranium essential oils strongly
reduced colony size and colony forming capacity of MCF-7 cells. The
treatment of frankincense, pine needle and geranium essential oils
was responsible for the altered migratory and invasive phenotype of
MCF-7 cells in vitro. Our results further indicated that the
frankincense, pine needle and geranium essential oils induced
apoptosis. However, the oils did not affect cell cycle progression.
Furthermore, we observed that the essential oil of frankincense
inhibited tumor growth and induced apoptosis in vivo. These
results clearly revealed that frankincense, pine needle and
geranium essential oils could play an important role in many
biological functions of BC cells such as proliferation, invasion as
well as apoptosis.
Further analysis of molecular mechanisms for cancer
cell progression may provide more data concerning novel molecular
targets of frankincense, pine needle and geranium essential oil
treatments for BC. AMPK is a central cellular energy-sensing system
that constructively takes part in the interaction between
metabolism and cancer progression by regulation of the mTOR pathway
(36). The activation of AMPK
directly phosphorylates and activates TSC2 by increasing its GAP
activity and inhibiting mTOR signaling (37). The serine/threonine kinase mammalian
target of rapamycin (mTOR) functions as a major regulator of
cellular growth and survival, and resides in two multiprotein
complexes, mTORC1 and mTORC2 (38).
mTORC1 regulates phosphorylation of p70 S6 kinase 1 (S6K1) and
eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4EBP1)
(20,39). It was reported that aspirin
decreased the viability and anchorage-independent growth of mutant
PIK3CA BC cells through AMP-activated protein kinase (AMPK)
activation and mTORC1 inhibition (40). Furthermore, in BC, 17-β-oestradiol
(E2) directly activated AMPK through interaction of its α-subunit
with estrogen receptors, implying its roles in cell proliferation
(41). Knockdown of AMPK inhibited
glucose metabolism and proliferation of TNBC cells (42). Based on the aforementioned, we
analyzed the correlation of the frankincense, pine needle and
geranium essential oils-mediated AMPK and mTOR signaling pathway.
We revealed that these 3 essential oils notably regulated the
activity of the AMPK/mTOR pathway in human BC cells MCF-7. These
results demonstrated that frankincense, pine needle and geranium
essential oil-modulated BC cell progression was, at least in part,
AMPK/mTOR-dependent.
In conclusion, our results demonstrated that
frankincense, pine needle and geranium essential oils have an
antitumor effect, which could be mediated by the AMPK/mTOR pathway.
The novelty of the present study was that the frankincense, pine
needle and geranium essential oils may be a promising treatment for
BC. However, the present study still had some shortcomings. To
further confirm our present findings, similar experiments using the
other BC cell lines should be performed. In addition, it is unclear
whether the oils have an effect on BC patients. Thus, further
investigation is warranted to analyze the effects and accurate
mechanisms of frankincense, pine needle and geranium essential oils
in BC.
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
PTX
|
paclitaxel
|
|
TUNEL
|
transferase-mediated deoxyuridine
triphosphate-biotin nick end labeling
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
WT
|
cells treated with nothing
|
|
PBS
|
phosphate-buffered saline
|
|
SDS
|
sodium dodecyl sulfate
|
References
|
1
|
Rahimi Z, Yari K and Rahimi Z: Matrix
metalloproteinase-9 −1562T allele and its combination with MMP-2
−735 C allele are risk factors for BC. Asian Pac J Cancer Prev.
16:1175–1179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fox P, Balleine RL, Lee C, Gao B,
Balakrishnar B, Menzies AM, Yeap SH, Ali SS, Gebski V, Provan P, et
al: Dose escalation of tamoxifen in patients with low endoxifen
level: Evidence for therapeutic drug monitoring - The TADE study.
Clin Cancer Res. 22:3164–3171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sabol M, Trnski D, Uzarevic Z, Ozretic P,
Musani V, Rafaj M, Cindric M and Levanat S: Combination of
cyclopamine and tamoxifen promotes survival and migration of mcf-7
BC cells - interaction of hedgehog-gli and estrogen receptor
signaling pathways. PLoS One. 9:e1145102014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poeckel D and Werz O: Boswellic acids:
Biological actions and molecular targets. Curr Med Chem.
13:3359–3369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ammon HP: Boswellic acids in chronic
inflammatory diseases. Planta Med. 72:1100–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Syrovets T, Büchele B, Krauss C,
Laumonnier Y and Simmet T: Acetyl-boswellic acids inhibit
lipopolysaccharide-mediated TNF-alpha induction in monocytes by
direct interaction with IkappaB kinases. J Immunol. 174:498–506.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Büchele B and Simmet T: Analysis of 12
different pentacyclic triterpenic acids from frankincense in human
plasma by high-performance liquid chromatography and photodiode
array detection. J Chromatogr B Analyt Technol Biomed Life Sci.
795:355–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siemoneit U, Pergola C, Jazzar B, Northoff
H, Skarke C, Jauch J and Werz O: On the interference of boswellic
acids with 5-lipoxygenase: Mechanistic studies in vitro and
pharmacological relevance. Eur J Pharmacol. 606:246–254. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burdine L and Kodadek T: Target
identification in chemical genetics: The (often) missing link. Chem
Biol. 11:593–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwak CS, Moon SC and Lee MS: Antioxidant,
antimutagenic, and antitumor effects of pine needles (Pinus
densiflora). Nutr Cancer. 56:162–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong JB, Seo EW and Jeong HJ: Effect of
extracts from pine needle against oxidative DNA damage and
apoptosis induced by hydroxyl radical via antioxidant activity.
Food Chem Toxicol. 47:2135–2141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lis-Balchin M and Deans SG: Antimicrobial
effects of hydrophilic extracts of Pelargonium species
(Geraniaceae). Lett Appl Microbiol. 23:205–207. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ben Slima A, Ali MB, Barkallah M, Traore
AI, Boudawara T, Allouche N and Gdoura R: Antioxidant properties of
Pelargonium graveolens L'Her essential oil on the reproductive
damage induced by deltamethrin in mice as compared to
alpha-tocopherol. Lipids Health Dis. 12:302013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boukhatem MN, Kameli A, Ferhat MA, Saidi F
and Mekarnia M: Rose geranium essential oil as a source of new and
safe anti-inflammatory drugs. Libyan J Med. 8:225202013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shim JU, Oh PS and Lim KT:
Anti-inflammatory activity of ethanol extract from Geranium
sibiricum Linne. J Ethnopharmacol. 126:90–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singla AK, Bondareva A and Jirik FR:
Combined treatment with paclitaxel and suramin prevents the
development of metastasis by inhibiting metastatic colonization of
circulating tumor cells. Clin Exp Metastasis. 31:705–714. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ni X, Suhail MM, Yang Q, Cao A, Fung KM,
Postier RG, Woolley C, Young G, Zhang J and Lin HK: Frankincense
essential oil prepared from hydrodistillation of Boswellia sacra
gum resins induces human pancreatic cancer cell death in cultures
and in a xenograft murine model. BMC Complement Altern Med.
12:2532012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satoh MS and Lindahl T: Role of
poly(ADP-ribose) formation in DNA repair. Nature. 356:356–358.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim EJ, Choi YK, Han YH, Kim HJ, Lee IK
and Lee MO: RORα suppresses proliferation of vascular smooth muscle
cells through activation of AMP-activated protein kinase. Int J
Cardiol. 175:515–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu M, Li T, Chen L, Peng S, Liao W, Bai R,
Zhao X, Yang H, Wu C, Zeng H, et al: Essential oils from Inula
japonica and Angelicae dahuricae enhance sensitivity of MCF-7/ADR
BC cells to doxorubicin via multiple mechanisms. J Ethnopharmacol.
180:18–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar D, Sukapaka M, Babu GD and Padwad Y:
Chemical composition and in vitro cytotoxicity of essential oils
from leaves and flowers of Callistemon citrinus from western
Himalayas. PLoS One. 10:e01338232015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilson AJ, Saskowski J, Barham W, Khabele
D and Yull F: Microenvironmental effects limit efficacy of
thymoquinone treatment in a mouse model of ovarian cancer. Mol
Cancer. 14:1922015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parbin S, Shilpi A, Kar S, Pradhan N,
Sengupta D, Deb M, Rath SK and Patra SK: Insights into the
molecular interactions of thymoquinone with histone deacetylase:
Evaluation of the therapeutic intervention potential against BC.
Mol Biosyst. 12:48–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ke X, Zhao Y, Lu X, Wang Z, Liu Y, Ren M,
Lu G, Zhang D, Sun Z, Xu Z, et al: TQ inhibits hepatocellular
carcinoma growth in vitro and in vivo via repression of Notch
signaling. Oncotarget. 6:32610–32621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park YS, Lee JH, Bondar J, Harwalkar JA,
Safayhi H and Golubic M: Cytotoxic action of
acetyl-11-keto-beta-boswellic acid (AKBA) on meningioma cells.
Planta Med. 68:397–401. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shao Y, Ho CT, Chin CK, Badmaev V, Ma W
and Huang MT: Inhibitory activity of boswellic acids from Boswellia
serrata against human leukemia HL-60 cells in culture. Planta Med.
64:328–331. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu JJ, Nilsson A, Oredsson S, Badmaev V
and Duan RD: Keto- and acetyl-keto-boswellic acids inhibit
proliferation and induce apoptosis in Hep G2 cells via a caspase-8
dependent pathway. Int J Mol Med. 10:501–505. 2002.PubMed/NCBI
|
|
29
|
Zhao W, Entschladen F, Liu H, Niggemann B,
Fang Q, Zaenker KS and Han R: Boswellic acid acetate induces
differentiation and apoptosis in highly metastatic melanoma and
fibrosarcoma cells. Cancer Detect Prev. 27:67–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu JJ, Nilsson A, Oredsson S, Badmaev V,
Zhao WZ and Duan RD: Boswellic acids trigger apoptosis via a
pathway dependent on caspase-8 activation but independent on
Fas/Fas ligand interaction in colon cancer HT-29 cells.
Carcinogenesis. 23:2087–2093. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pang X, Yi Z, Zhang X, Sung B, Qu W, Lian
X, Aggarwal BB and Liu M: Acetyl-11-keto-beta-boswellic acid
inhibits prostate tumor growth by suppressing vascular endothelial
growth factor receptor 2-mediated angiogenesis. Cancer Res.
69:5893–5900. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu M, Xia L, Hua H and Jing Y:
Acetyl-keto-beta-boswellic acid induces apoptosis through a death
receptor 5-mediated pathway in prostate cancer cells. Cancer Res.
68:1180–1186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Syrovets T, Gschwend JE, Büchele B,
Laumonnier Y, Zugmaier W, Genze F and Simmet T: Inhibition of
IkappaB kinase activity by acetyl-boswellic acids promotes
apoptosis in androgen-independent PC-3 prostate cancer cells in
vitro and in vivo. J Biol Chem. 280:6170–6180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frank MB, Yang Q, Osban J, Azzarello JT,
Saban MR, Saban R, Ashley RA, Welter JC, Fung KM and Lin HK:
Frankincense oil derived from Boswellia carteri induces tumor cell
specific cytotoxicity. BMC Complement Altern Med. 9:62009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suhail MM, Wu W, Cao A, Mondalek FG, Fung
KM, Shih PT, Fang YT, Woolley C, Young G and Lin HK: Boswellia
sacra essential oil induces tumor cell-specific apoptosis and
suppresses tumor aggressiveness in cultured human BC cells. BMC
Complement Altern Med. 11:1292011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin HO, Hong SE, Woo SH, Lee JH, Choe TB,
Kim EK, Noh WC, Lee JK, Hong SI, Kim JI, et al: Silencing of Twist1
sensitizes NSCLC cells to cisplatin via AMPK-activated mTOR
inhibition. Cell Death Dis. 3:e3192012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inoki K, Corradetti MN and Guan KL:
Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet.
37:19–24. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pezze P Dalle, Ruf S, Sonntag AG,
Langelaar-Makkinje M, Hall P, Heberle AM, Navas P Razquin, van
Eunen K, Tölle RC, Schwarz JJ, et al: A systems study reveals
concurrent activation of AMPK and mTOR by amino acids. Nat Commun.
7:132542016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Polak P and Hall MN: mTOR and the control
of whole body metabolism. Curr Opin Cell Biol. 21:209–218. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Henry WS, Laszewski T, Tsang T, Beca F,
Beck AH, McAllister SS and Toker A: Aspirin suppresses growth in
PI3K-mutant BC by activating AMPK and inhibiting mTORC1 signaling.
Cancer Res. 77:790–801. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lipovka Y, Chen H, Vagner J, Price TJ,
Tsao TS and Konhilas JP: Oestrogen receptors interact with the
α-catalytic subunit of AMP-activated protein kinase. Biosci Rep.
35:e002642015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu P, Ye F and Xie X, Li X, Tang H, Li S,
Huang X, Song C, Wei W and Xie X: mir-101-3p is a key regulator of
tumor metabolism in triple negative BC targeting AMPK. Oncotarget.
7:35188–35198. 2016. View Article : Google Scholar : PubMed/NCBI
|