Introduction
Chronic myelogenous leukemia (CML) is a cancer of
the blood cells and bone marrow (1,2). This
myeloproliferative disorder is predominantly diagnosed in adults
and is characterized by uncontrolled cell growth (3). The exact cause of CML remains elusive.
CML is associated with the Philadelphia chromosome, which is a
reciprocal chromosome translocation and fusion of the BCR gene on
chromosome 22 and the ABL gene on chromosome 9 (4–6). The
resulting BCR-ABL fusion protein possesses constitutive kinase
activity and promotes the unregulated proliferation of
hematopoietic stem cells (5,7).
The properties of differentiation and self-renewal
in hematopoietic stem cells are modulated by various
lineage-specific transcription factors (8,9).
Consensus DNA sequences for the family of GATA transcription
factors can be found in the promoters of many hematopoietic
linkage-related genes, including β-globin (10,11).
GATA binding protein 1 (GATA-1) and nuclear factor, erythroid
derived 2 (NF-E2) are specifically involved in the transcriptional
regulation of terminal differentiated erythroid cells (12,13).
In contrast, GATA binding protein 2 (GATA-2) and myeloproliferative
leukemia virus oncogene (c-MPL) are required for the expansion of
multipotential hematopoietic progenitors and the formation of mast
cells but not for the terminal differentiation of erythroid cells
and macrophages (14,15). c-MYC has been found to be involved
in growth, differentiation and apoptosis (16,17).
During erythroid and myelomonocytic differentiation, c-MYC plays a
critical role. The overexpression of c-MYC leads to the partial
inhibition of erythroid differentiation in K562 cells (18–21).
In addition, c-MYC suppresses the differentiation induced by
imatinib in chronic myeloid leukemia cells (22–24).
BCR-ABL kinase inhibitors, such as imatinib and dasatinib, have
been used to successfully treat CML (22,23).
ITR-284 [N-(2-dimethylaminoethyl)-4,8-dihydrobenzo
(1,2-b;4,5-b') dithio-phene-2-carboxamide phosphoric acid salt], a
carboxamide derivative, exhibits potent anticancer effects on
various human cancer cell lines (25–27).
Our previous study indicated that ITR-284 had anti-proliferative
effects on HL60 and WEHI-3 leukemia cells, but it had very low
toxicity toward normal cells (26,27).
ITR-284 also showed growth inhibitory effects on human
hepatocellular cancer cell lines (HepG2, Hep3B, SK-HEP-1 and J5)
and colorectal cancer cell lines (HT-29, COLO-205, HCT-116 and
SW-620) (26). In this study, we
investigated the efficacy of ITR-284 in treating myeloid leukemia
and compared its induction of human chronic myelogenous leukemia
K562 cell differentiation with that of all-trans retinoic
acid (ATRA).
Materials and methods
Chemicals and reagents
Fetal bovine serum (FBS), L-glutamine,
penicillin-streptomycin, and RPMI-1640 medium were obtained from
Thermo Fisher Scientific Inc. (Grand Island, NY, USA).
All-trans retinoic acid (ATRA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
May-Grünwald stain and nitro blue tetrazolium were purchased from
Sigma (Sigma, St. Louis, MO, USA). Primary antibodies against
FOXM1, GLI 1, c-MYC, BCL-2, caspase-3 and β-actin were purchased
from GeneTex (Hsinchu, Taiwan). ITR-284 was synthesized by Dr
Yen-Fang Wen at China Medical University.
Cell culture
Human chronic myelogenous leukemia cell line K562
was obtained from the American Type Culture Collection and grown in
RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells were passaged every
two days and maintained in a humidified environment with 95% air
and 5% CO2 at 37°C for the following experiments
(5,28,29).
Cell viability assay
Cell viability was determined by MTT assay. Briefly,
K562 cells were seeded at 1×104 cells per well in
96-well plates (Costar, Corning Inc., Corning, NY, USA), allowed to
attach overnight, and then exposed to various concentrations of
ITR-284 (0, 2, 4, 6, 8 and 10 nM) or ATRA (0, 0.1, 0.5, 1, 5 and 10
µM) for 24 h. The culture media was removed, and 100 µl of 0.5
mg/ml MTT solution was added to each well. After 4 h of incubation
at 37°C, the supernatant was removed, and 100 µl of DMSO was added
to each well. The absorbance at 595 nm was measured by using an
enzyme-linked immunosorbent assay reader, and the control
absorbance was normalized to 100%. Six replicate wells were
included in each group, and at least three independent experiments
were done (23,25,30).
Trypan blue exclusion assay for cell
death
Cells were treated with ITR-284 and cell death was
evaluated by trypan blue exclusion assay as previously described
(31). Briefly, K562, HL60, U937
and WEHI-3 cells were seeded in a 24-well plate (2.5×105
cells/per well) and were incubated with ITR-284 as indicated. After
24 h, cells were stained with 0.25% trypan blue solution and the
amount of dead cells were determined by Countess Automated Cell
Counter (Invitrogen/Life Technologies) (31).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining
Apoptotic DNA fragmentation was detected using In
Situ Cell Death Detection kit, Fluorescein (Roche, Mannheim,
Germany) according to the manufacturer's protocol. Briefly, K562
cells (2×105 cells/per well) were seeded into 12-well
plates and incubated with 0, 5, 10 and 15 µM of ITR-284 for 48 h.
Cells were harvested and assayed by In Situ Cell Death
Detection kit, Fluorescein (31).
May-Grünwald Giemsa staining
May-Grünwald Giemsa (MGG) staining was used to
analyze the morphological features of megakaryocytes.
Approximately, 5×104 cells were seeded in each well of a
24-well plate for 24 h and treated with ITR-284 and ATRA at the
indicated concentrations for another 24 h. Cells were centrifuged
onto a microscope slide at 800 × g for 5 min and fixed with 10%
formaldehyde. After air drying, the slides were stained with
May-Grünwald solution (Sigma) for 5 min; the solution was removed,
and the cells were then stained with Giemsa solution for another 20
min. The stained cells were examined, and images were captured with
an inverted microscope (Nikon Eclipse TE2000U) (9).
Cell differentiation
Nitro blue tetrazolium (NBT) reduction assay is used
to determine the differentiation of blood leukocytes by measuring
the production of alkaline phosphatase. Briefly, 1×105
cells were seeded in each well of a 24-well plate for 24 h and
treated with ITR-284 and ATRA at the indicated concentrations for
another 24 h. Cells were centrifuged into a 1.5-ml microscope tube
at 6000 × g for 5 min and washed with 1 ml of PBS. After
centrifugation, the cell pellets were mixed with NBT reagent for 5
min at 37°C and then stained with NBT for 10 min at room
temperature. The proportion of blue-stained cells was calculated
using a total of 200 cells randomly selected for each sample in
triplicate by light microscopy. The mean ± SD of a representative
experiment is shown (8,32).
RNA extract
Approximately 1×105 cells from each
treatment were harvested. Total RNA was isolated using the TRIzol
reagent (Invitrogen, USA) according to the manufacturer's
instructions. Briefly, cell pellets were treated with 1 ml of
TRIzol at room temperature for 5 min; next, 0.2 ml of chloroform
was added to the tubes and mixed well. After centrifugation at
12,000 rpm for 15 min, the upper aqueous layers were transferred to
new tubes and mixed with 0.5 ml of isopropanol for 10 min. The
tubes were centrifuged at 12,000 rpm for 15 min. The pellets were
dried and dissolved in DEPC-treated H2O. Absorbance was
measured at 260 and 280 nm. A ratio of A260:A280 between 1.8 and
2.0 was used to verify the purity of the samples (29,33,34).
Quantitation of gene expression by
real-time RT-PCR
Quantitative RT-PCR was performed according to the
Takara One Step SYBR Ex Taq™ qRT-PCR kit. Briefly, one-step RT-PCR
was performed in a 20-µl reaction volume containing 100 ng of total
RNA, 8 pmol of each forward and reverse primer, Prime Script 1 Step
Enzyme Mix, 2X one-step SYBR RT-PCR buffer and RNase-free
dH2O. Quantitative RT-PCR was performed on an ABI 7900HT
system (Applied Biosystems). Quantitative RT-PCR conditions were as
follows: stage 1 was cDNA synthesis at 42°C for 5 min and then
denaturation at 95°C for 10 min. Stage 2 was RT-PCR amplification
for 40 cycles, denaturation at 94°C for 30 sec and annealing and
elongation at 60°C for 15 sec. The relative expression level of
target genes was determined by normalizing the RNA concentration to
that of the β-actin internal control. The relative expression
levels of mRNA represent the mean ± SD of duplicates. The primer
sequences used for quantitative RT-PCR were as follows: i) actin,
forward: 5′-CCAACCGCGAGAAGATGA3′ and reverse:
5′-TCCATCACGATGCCAGTG-3′; ii) GATA-1, forward:
5′CTGAGGGCTTGGATGCAG-3′ and reverse: 5′-TGGGTACACCTGAAAGACTGG-3′;
iii) GATA-2, forward: 5′-TGGCGCACAACTACATGG-3′ and reverse:
5′-GCGAGTCGAGGTGATTGAAG-3′; iv) NF-E2, forward:
5′-GCTGTCCACTTCAGAGCTAGG-3′ and reverse:
5′-GCTCACTTGAGACATTCAGA-3′; v) c-mpl, forward:
5′-CCTCTTCATGGTCACCTCCT-3′ and reverse: 5′-AGGAGACATCTTGGCTGCTG-3′;
vi) c-myc, forward: 5′-CGGTGCAGCCGTATTTCTAC-3′ and reverse:
5′-CAGCAGCTCGAATTTCTTCC-3′; and vii) BCR-ABL, forward:
5′-CACAGCATTCCGCTGACCATCA-3′ and reverse:
5′-GCTTCACACCATTCCCCATTGT-3′ (29,33,34).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). One-way analysis of variance (ANOVA) with a
one-tailed Student's t-test was used for multiple comparisons among
groups. A value of p<0.05 was considered statistically
significant (5,33,35).
Results
ITR-284 and ATRA inhibit cell
viability of K562 cells
The trypan blue exclusion assays were performed to
investigate the cytotoxic effects of ITR-284 on several leukemic
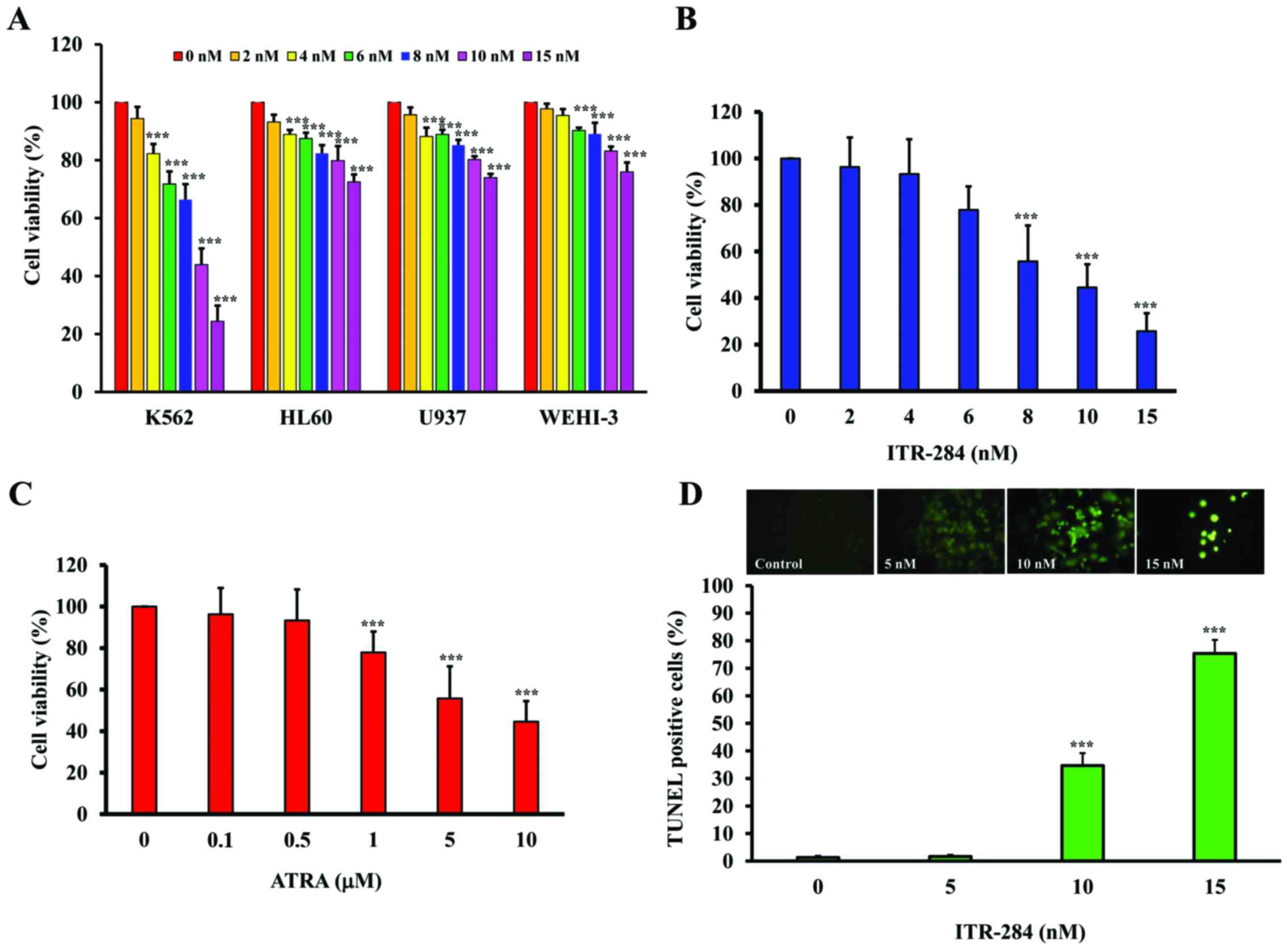
cell lines including K562, HL60, U937 and WEHI-3 (Fig. 1A). K562 cells were more sensitive
than other cells. We investigated the cytotoxic effects of ITR-284
and ATRA on the proliferation of K562 cells by the MTT assays.
ITR-284 (Fig. 1B) and ATRA
(Fig. 1C) inhibited the viability
of K562 cells in a concentration-dependent manner. The
IC50 values of ITR-284 and ATRA were 9 nM and 8.5 µM,
respectively. When K562 cells were treated with concentrations
which were higher than IC50, we were able to observe
apoptotic cells using TUNEL assays (Fig. 1D).
Morphological changes in ITR-284- and
ATRA-treated K562 cells
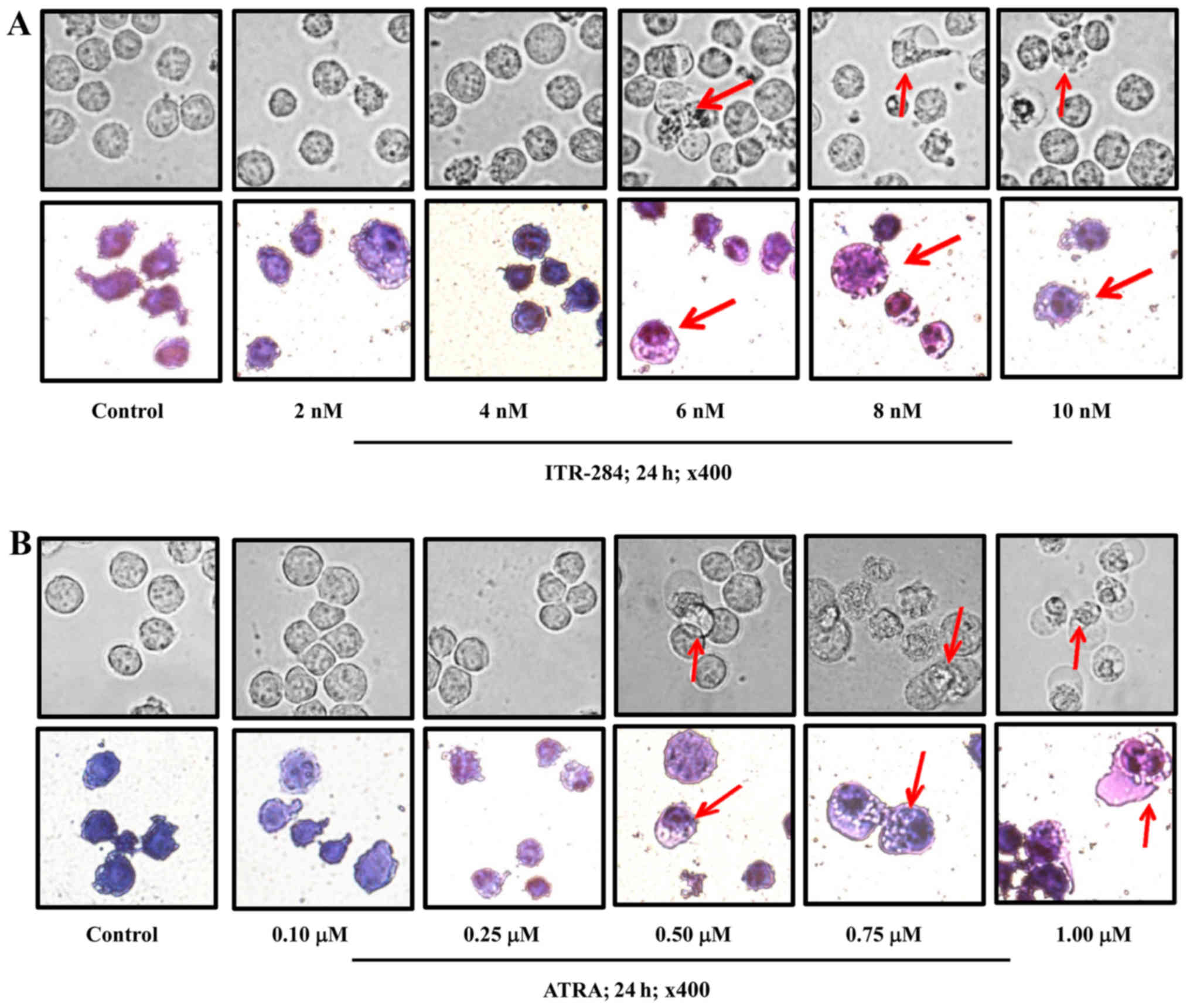
The May-Grünwald-Giemsa staining method was used to
observe the morphological changes and differentiation of blood
cells via microscopy. ITR-284-treated cells had morphological
changes at concentrations >6 nM. Cells were differentiated and
showed the nuclei characteristics of white blood cells, not those
of red blood cells (Fig. 2A).
Likewise, ATRA-284 treated cells had morphological changes at
concentrations >0.5 µM. More nuclei were observed in
ATRA-284-treated cells than in ITR-284-treated cells (Fig. 2B). Furthermore, to examine whether
ITR-284 and ATRA promote cell differentiation, we used the nitro
blue tetrazolium (NBT) assay to detect the production of alkaline
phosphatase. NBT was used as a substrate for alkaline phosphatase,
which converted the yellow NBT dye to purple-blue formazan in
cells. After treatment with either ITR-284 or ATRA for 24 h, the
K562 cells had differentiated significantly (Fig. 3).
ITR-284 and ATRA regulated GATA-1 and
NF-E2 mRNA expression
To determine the relationship of GATA transcription
factors and EF-E2 in ITR-284- and ATRA-induced cell
differentiation, we examined the mRNA expression levels of
GATA-1 and NF-E2 by quantitative RT-PCR.
GATA-1 (Fig. 4A) and
NF-E2 (Fig. 5A) mRNA levels
increased in the ITR-284-treated K562 cells, while GATA-1
(Fig. 4B) and NF-E2
(Fig. 5B) mRNA levels decreased in
the ATRA-treated K562 cells compared to those in the untreated
cells.
ITR-284 and ATRA regulated GATA-2 and
c-MPL mRNA expression
Myeloproliferative leukemia virus oncogene (c-MPL,
TPO receptor) plays a crucial role in the differentiation of
megakaryocytes and platelets (36–38).
To determine whether ITR-284 and ATRA are involved in megakaryocyte
differentiation, we treated K562 cells with either ITR-284 or ATRA
for 24 h and examined the mRNA expression levels of GATA-2
and c-MPL by quantitative RT-PCR. GATA-2 mRNA levels
increased in the ITR-284-treated K562 cells (Fig. 6A), while GATA-2 mRNA levels
decreased in the ATRA-treated K562 cells compared to those in the
untreated cells (Fig. 6B). The mRNA
expression levels of c-MPL increased in the ITR-284-treated
K562 cells (Fig. 7A), while the
c-MPL mRNA levels did not change in the ATRA-treated K562
cells (Fig. 7B).
ITR-284 inhibits BCR-ABL mRNA
expression in K562 cells
To examine whether ITR-284 and ATRA regulate BCR-ABL
mRNA expression, we treated K562 cells with either ITR-284 or ATRA
for 24 h and measured BCR-ABL mRNA expression by
quantitative RT-PCR. The BCR-ABL mRNA levels decreased in
the ITR-284-treated K562 cells (Fig.
8A), while there was no significant change in the ATRA-treated
K562 cells compared to untreated cells (Fig. 8B). Our results suggest that ITR-284
inhibits BCR-ABL oncogene expression.
ITR-284 regulates cell
differentiation- and apoptosis-related protein expression in K562
cells
Cell differentiation- and apoptosis-related protein
expression levels were analyzed. We treated K562 cells with ITR-284
for 24 h and measured the protein levels of forkhead box (Fox) M1
(FOXM1), GLI 1, c-MYC, BCL-2 and caspase-3 by western blotting As
shown in Fig. 9, the protein levels
of FOXM1, GLI 1, c-MYC and BCL-2 decreased in the ITR-284-treated
K562 cells.
Discussion
Human chronic myelogenous leukemia (CML) is
characterized by the presence of the Philadelphia chromosome,
BCR-ABL gene fusion with the constitutively active tyrosine kinase,
and increased myeloid cells in the bone marrow and the peripheral
blood (3). K562 cells contain a
Philadelphia chromosome with the chimeric BCR-ABL gene
transcript. The BCR-ABL fusion protein acts a constitutively active
tyrosine kinase in malignant transformation. In recent years,
target therapeutic agents have been successfully used to improve
the efficacy and adverse effects observed for traditional
chemotherapeutic agents (39,40).
Many studies have demonstrated that the novel compounds exhibit
significant effects in leukemia treatment by inducing cell
differentiation and apoptosis (39). The K562 cell line is a good model to
investigate hematological cell differentiation and cell death. K562
cells can be differentiated to erythroid cells, macrophages and
megakaryocytes by phorbol 12-myristate 13-acetate (PMA) (41,42),
arsenic trioxide (As2O3) (43,44)
and ATRA (45,46). In this study, we evaluated the
anti-leukemia effects of ITR-284 on cell differentiation and
investigated its mechanism of action in K562 cells. We found that
ITR-284 and ATAR simultaneously induced anti-proliferation
(Fig. 1), morphological changes
(Fig. 2) and megakaryocytic
differentiation (Fig. 3) in K562
cells according to the results of the MTT assay,
May-Grünwald-Giemsa staining and nitro blue tetrazolium (NBT)
assay.
Many lineage-specific transcription factors are
involved in hematopoietic stem cell differentiation (47–49).
The GATA transcription factor is one of the regulators of
hematopoietic stem cell differentiation. GATA-1 and GATA2 bind to
the consensus DNA sequence (A/T)GATA(A/G) and regulate
lineage-restricted gene expression during hematopoietic stem and
progenitor cell differentiation (47,49).
GATA-1 is required for the differentiation of erythroid cells,
granulocytic cells, mast cells, megakaryocytes, and eosinophils,
whereas GATA2 is indispensable for the maintenance of hematopoietic
stem and progenitor cells (49).
Our results showed that GATA-1 (Fig. 4A) and GATA-2 (Fig. 6A) mRNA levels increased in the
ITR-284-treated K562 cells, but GATA-2 (Fig. 6B) mRNA levels decreased in the
ATRA-treated K562 cells compared to untreated cells. No report has
demonstrated the exact roles of GATA-1 and GATA2 in K562 cell
differentiation. Ryningen et al demonstrated that ATRA
decreased the levels of GATA-2 in acute promyelocytic leukemia
(APL) (50). The transcription
factor nuclear factor-erythroid 2 (NF-E2) is another specific
transcription factor that is functionally linked to the
megakaryocytic lineage (51). It
has been reported that NF-E2 was significantly reduced in malignant
megakaryocytes from essential thrombocythemia (ET) patients and in
K562 cells (51). The NF-E2
mRNA levels were increased in the ITR-284-treated K562 cells
(Fig. 5A), suggesting that NF-E2
plays a crucial role in the ITR-284-induced differentiation of K562
cells.
Myeloproliferative leukemia virus oncogene (c-MPL,
hematopoietic cytokine receptor) mainly controls the megakaryocytic
lineage of cell differentiation. When K562 cells were treated with
either ITR-284 or ATRA, c-MPL mRNA was increased (Fig. 7A). Targeting the BCR-ABL kinase is
an attractive approach for CML treatment. Elevated BCR-ABL kinase
activity causes cell proliferation, while the reduction of BCR-ABL
kinase activity induces hematopoietic stem cell to undergo
differentiation, which leads to apoptosis (52,53).
Our results showed that the BCR-ABL mRNA levels decreased in the
ITR-284-treated K562 cells (Fig.
8A). Therefore, BCR-ABL may be a target of ITR-284.
Forkhead box (Fox) M1 (FOXM1) and c-MYC have been
characterized as human proto-oncogenes and play an important role
in human malignancies (54,55). The FOXM1 protein, a transcriptional
regulator, is involved in leukemia cell survival, proliferation and
chemotherapy resistance (56–58).
The c-MYC protein encodes a transcription factor that regulates the
expression of various genes in cell proliferation, cell death and
differentiation (59,60). In this study, our results
demonstrate the downregulation of the cell survival proteins FOXM1,
GLI 1, c-MYC and Bcl-2. In contrast, the cleaved caspase-3 protein
levels were increased in the ITR-284-treated K562 cells. These
findings suggest that ITR-284 can inhibit K562 cell proliferation
by inducing cell differentiation and apoptotic cell death.
In conclusion, our data showed that ITR-284 inhibits
growth and cell differentiation in leukemia cells. The possible
mechanisms of action of ITR-284 in K562 cells are summarized in
Fig. 10. ITR-284 induces cell
differentiation by upregulating GATA-1, NF-E2 and
GATA-2, as well as c-MPL, and downregulating
BCR-ABL mRNA expression in K562 cells. Taken together, our
findings provide important new insight into the possible molecular
mechanism of the anti-chronic myelogenous leukemia activity of
ITR-284.
Acknowledgements
This study was supported by a grant to Dr Jai-Sing
Yang from China Medical University Hospital, Taichung, Taiwan
(DMR-106-122). This study was supported by research grants (no.
CMU100-TC-08 and no. CMU106-S-26) to Dr Shih-Chang Tsai from China
Medical University, Taichung, Taiwan.
References
|
1
|
Xu LP, Wu DP, Han MZ, Huang H, Liu QF, Liu
DH, Sun ZM, Xia LH, Chen J, Wang HX, et al: A review of
hematopoietic cell transplantation in China: Data and trends during
2008–2016. Bone Marrow Transplant. Apr 24–2017.(Epub ahead of
print). View Article : Google Scholar
|
|
2
|
National Cancer Institute (US), . Chronic
myelogenous leukemia treatment: (PDQ(R)): Health professional
versionPDQ Cancer Information Summaries. Bethesda, MD: 2002
|
|
3
|
Vrontaki E, Melagraki G, Voskou S,
Phylactides MS, Mavromoustakos T, Kleanthous M and Afantitis A:
Development of a predictive pharmacophore model and a 3D-QSAR study
for an in silico screening of new potent Bcr-Abl kinase inhibitors.
Mini Rev Med Chem. 17:188–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Webb KC, Harasimowicz M, Janeczek M,
Speiser J, Swan J and Tung R: Development of asymmetric facial
depigmentation in a patient treated with dasatinib with new-onset
hypovitaminosis D: Case report and review of the literature. Case
Rep Dermatol Med. 2017:93590862017.PubMed/NCBI
|
|
5
|
Antoszewska-Smith J, Pawlowska E and
Blasiak J: Reactive oxygen species in BCR-ABL1-expressing cells -
relevance to chronic myeloid leukemia. Acta Biochim Pol. 64:1–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saponaro C, Maffia M, Di Renzo N and
Coluccia AM: Is going for cure in CML targeting aberrant glycogen
synthase kinase 3β? Curr Drug Targets. 18:396–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haguet H, Douxfils J, Mullier F, Chatelain
C, Graux C and Dogné JM: Risk of arterial and venous occlusive
events in chronic myeloid leukemia patients treated with new
generation BCR-ABL tyrosine kinase inhibitors: A systematic review
and meta-analysis. Expert Opin Drug Saf. 16:5–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang WW, Yang JS, Lin CF, Ho WJ and Lee
MR: Pycnogenol induces differentiation and apoptosis in human
promyeloid leukemia HL-60 cells. Leuk Res. 29:685–692. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saleh M, Shamsasanjan K, Movassaghpour AA,
Akbarzadehlaleh P and Molaeipour Z: Inhibitory effect of
mesenchymal stem cell co-culture on erythroid differentiation of
K562 cells compared to the control croup. Cell J. 19:127–136.
2017.PubMed/NCBI
|
|
10
|
Zhu Y, Wang D, Wang F, Li T, Dong L, Liu
H, Ma Y, Jiang F, Yin H, Yan W, et al: A comprehensive analysis of
GATA-1-regulated miRNAs reveals miR-23a to be a positive modulator
of erythropoiesis. Nucleic Acids Res. 41:4129–4143. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishimura S, Takahashi S, Kuroha T, Suwabe
N, Nagasawa T, Trainor C and Yamamoto M: A GATA box in the GATA-1
gene hematopoietic enhancer is a critical element in the network of
GATA factors and sites that regulate this gene. Mol Cell Biol.
20:713–723. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raich N and Romeo PH: Erythroid regulatory
elements. Stem Cells. 11:95–104. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gourdon G, Morlé F, Roche J, Tourneur N,
Joulain V and Godet J: Identification of GATA-1 and NF-E2 binding
sites in the flanking regions of the human alpha-globin genes. Acta
Haematol. 87:136–144. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ayala RM, Martínez-López J, Albízua E,
Diez A and Gilsanz F: Clinical significance of Gata-1, Gata-2,
EKLF, and c-MPL expression in acute myeloid leukemia. Am J Hematol.
84:79–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jordan CT and Van Zant G: Recent progress
in identifying genes regulating hematopoietic stem cell function
and fate. Curr Opin Cell Biol. 10:716–720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Honeycutt KA and Roop DR: c-Myc and
epidermal stem cell fate determination. J Dermatol. 31:368–375.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Nigris F, Sica V, Herrmann J,
Condorelli G, Chade AR, Tajana G, Lerman A, Lerman LO and Napoli C:
c-Myc oncoprotein: Cell cycle-related events and new therapeutic
challenges in cancer and cardiovascular diseases. Cell Cycle.
2:325–328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan XN, Chen JJ, Wang LX, Xiao RZ, Liu LL,
Fang ZG, Liu Q, Long ZJ and Lin DJ: Inhibition of c-Myc overcomes
cytotoxic drug resistance in acute myeloid leukemia cells by
promoting differentiation. PLoS One. 9:e1053812014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Yang Y, Meng W, Li Y, Jia Y and Liu
T: Study on the differentiation of K562 cell-line induced by
Tanshinone II A. Hua Xi Yi Ke Da Xue Xue Bao. 33:80–83. 2002.(In
Chinese). PubMed/NCBI
|
|
20
|
Yang Y and Yang M: Effects of C-myc
antisense transcripts on differentiation of k562 cells. Int J
Oncol. 6:419–424. 1995.PubMed/NCBI
|
|
21
|
Sasaki D, Kosunago S, Mikami T, Matsumoto
T and Suzuki M: Growth-inhibition by hemin in K562 human leukemic
cells is related to hemoglobin-producing activity. Biol Pharm Bull.
17:586–590. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu N, Li P, Zang S, Liu Q, Ma D, Sun X
and Ji C: Novel agent nitidine chloride induces erythroid
differentiation and apoptosis in CML cells through c-Myc-miRNAs
axis. PLoS One. 10:e01168802015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gómez-Casares MT, García-Alegria E,
López-Jorge CE, Ferrándiz N, Blanco R, Alvarez S, Vaqué JP,
Bretones G, Caraballo JM, Sánchez-Bailón P, et al: MYC antagonizes
the differentiation induced by imatinib in chronic myeloid leukemia
cells through downregulation of p27(KIP1). Oncogene. 32:2239–2246.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawano T, Horiguchi-Yamada J, Saito S,
Iwase S, Furukawa Y, Kano Y and Yamada H: Ectopic cyclin D1
expression blocks STI571-induced erythroid differentiation of K562
cells. Leuk Res. 28:623–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM,
Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC: Resveratrol-induced
autophagy and apoptosis in cisplatin-resistant human oral cancer
CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol.
50:873–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao YR, Lu CC, Lai KC, Yang JS, Kuo SC,
Wen YF, Fushiya S and Wu TS: The novel carboxamide analog ITR-284
induces caspase-dependent apoptotic cell death in human
hepatocellular and colorectal cancer cells. Mol Med Rep.
7:1539–1544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen YF, Yang JS, Kuo SC, Hwang CS, Chung
JG, Wu HC, Huang WW, Jhan JH, Lin CM and Chen HJ: Investigation of
anti-leukemia molecular mechanism of ITR-284, a carboxamide analog,
in leukemia cells and its effects in WEHI-3 leukemia mice. Biochem
Pharmacol. 79:389–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang SM, Yang JS, Tsai SC, Chen MH, Hsu
MH, Lin HY, Chou LC, Chinag JH, Lee KH, Huang LJ, et al: The novel
synthesized
2-(3-(methylamino)phenyl)-6-(pyrrolidin-1-yl)quinolin-4-one (Smh-3)
compound induces G2/M phase arrest and mitochondrial-dependent
apoptotic cell death through inhibition of CDK1 and AKT activity in
HL-60 human leukemia cells. Int J Oncol. 38:1357–1364. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen HY, Lu HF, Yang JS, Kuo SC, Lo C,
Yang MD, Chiu TH, Chueh FS, Ho HC, Ko YC, et al: The novel
quinolone CHM-1 induces DNA damage and inhibits DNA repair gene
expressions in a human osterogenic sarcoma cell line. Anticancer
Res. 30:4187–4192. 2010.PubMed/NCBI
|
|
30
|
Liu CY, Yang JS, Huang SM, Chiang JH, Chen
MH, Huang LJ, Ha HY, Fushiya S and Kuo SC: Smh-3 induces G(2)/M
arrest and apoptosis through calcium-mediated endoplasmic reticulum
stress and mitochondrial signaling in human hepatocellular
carcinoma Hep3B cells. Oncol Rep. 29:751–762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin C, Tsai SC, Tseng MT, Peng SF, Kuo SC,
Lin MW, Hsu YM, Lee MR, Amagaya S, Huang WW, et al: AKT
serine/threonine protein kinase modulates baicalin-triggered
autophagy in human bladder cancer T24 cells. Int J Oncol.
42:993–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song QF, Qu YC, Zheng HB, Zhang GH, Lin HG
and Yang JL: Differentiation of erythroleukemia K562 cells induced
by piperine. Ai Zheng. 27:571–574. 2008.(In Chinese). PubMed/NCBI
|
|
33
|
Gu C, Yang Y, Sompallae R, Xu H, Tompkins
VS, Holman C, Hose D, Goldschmidt H, Tricot G, Zhan F, et al: FOXM1
is a therapeutic target for high-risk multiple myeloma. Leukemia.
30:873–882. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liao CL, Lai KC, Huang AC, Yang JS, Lin
JJ, Wu SH, Wood W Gibson, Lin JG and Chung JG: Gallic acid inhibits
migration and invasion in human osteosarcoma U-2 OS cells through
suppressing the matrix metalloproteinase-2/-9, protein kinase B
(PKB) and PKC signaling pathways. Food Chem Toxicol. 50:1734–1740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin CF, Yang JS, Lin C, Tsai FJ, Lu CC and
Lee MR: CCY-1a-E2 induces G2/M phase arrest and apoptotic cell
death in HL-60 leukemia cells through cyclin-dependent kinase 1
signaling and the mitochondria-dependent caspase pathway. Oncol
Rep. 36:1633–1639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deutsch VR and Tomer A: Megakaryocyte
development and platelet production. Br J Haematol. 134:453–466.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hofmann WK, Ottmann OG and Hoelzer D:
Megakaryocytic growth factors: Is there a new approach for
management of thrombocytopenia in patients with malignancies?
Leukemia. 13:14–18. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Katayama N, Itoh R, Kato T, Sugawara T,
Mahmud N, Ohishi K, Masuya M, Aoki M, Minami N, Miyazaki H, et al:
Role for C-MPL and its ligand thrombopoietin in early
hematopoiesis. Leuk Lymphoma. 28:51–56. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chi HT, Ly BT, Kano Y, Tojo A, Watanabe T
and Sato Y: ETV6-NTRK3 as a therapeutic target of small molecule
inhibitor PKC412. Biochem Biophys Res Commun. 429:87–92. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang YC, Shieh HR and Chen YJ:
Midostaurin (PKC412) modulates differentiation and maturation of
human myeloid dendritic cells. Toxicol In Vitro. 24:1705–1710.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anisimov AG, Volkova TO, Chekmasova AA and
Nemova NN: Phorbol-12-myristate-13-acetate prevents the inhibitory
effect of A23187 on erythroid differentiation of K562 cells induced
by dimethylsulfoxide. Izv Akad Nauk Ser Biol. 142–148. 2002.(In
Russian). PubMed/NCBI
|
|
42
|
Lee CH, Yun HJ, Kang HS and Kim HD:
ERK/MAPK pathway is required for changes of cyclin D1 and B1 during
phorbol 12-myristate 13-acetate-induced differentiation of K562
cells. IUBMB Life. 48:585–591. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yuan FF, Zhang XH, Mi RH, Fan RH, Yin QS
and Wei XD: Effects of As2O3 in combination with TPA on K562 cells.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 22:943–949. 2014.(In Chinese).
PubMed/NCBI
|
|
44
|
Xu W, Wei W, Yu Q, Wu C, Ye C, Wu Y and
Yan H: Arsenic trioxide and bortezomib interact synergistically to
induce apoptosis in chronic myelogenous leukemia cells resistant to
imatinib mesylate through Bcr/Abl-dependent mechanisms. Mol Med
Rep. 10:1519–1524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xiang L, Dong W, Wang R, Wei J, Qiu G, Cen
J, Chen Z, Zheng X, Hu S, Xie X, et al: All-trans retinoic acid
enhances the effect of 5-aza-2′-deoxycytidine on p16INK4a
demethylation, and the two drugs synergistically activate retinoic
acid receptor β gene expression in the human erythroleukemia K562
cell line. Oncol Lett. 8:117–122. 2014.PubMed/NCBI
|
|
46
|
Tang XY, Wang CH and Xiao GF: The effect
of ATRA-induced leukemic cell differentiation on Brd7 gene
expression in leukemia cell lines. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 18:593–596. 2010.(In Chinese). PubMed/NCBI
|
|
47
|
Sive JI and Göttgens B: Transcriptional
network control of normal and leukaemic haematopoiesis. Exp Cell
Res. 329:255–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ebina W and Rossi DJ: Transcription
factor-mediated reprogramming toward hematopoietic stem cells. EMBO
J. 34:694–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shimizu R and Yamamoto M: GATA-related
hematologic disorders. Exp Hematol. 44:696–705. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ryningen A, Stapnes C, Paulsen K, Lassalle
P, Gjertsen BT and Bruserud O: In vivo biological effects of ATRA
in the treatment of AML. Expert Opin Investig Drugs. 17:1623–1633.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Catani L, Vianelli N, Amabile M, Pattacini
L, Valdrè L, Fagioli ME, Poli M, Gugliotta L, Moi P, Marini MG, et
al: Nuclear factor-erythroid 2 (NF-E2) expression in normal and
malignant megakaryocytopoiesis. Leukemia. 16:1773–1781. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fierro FA, Taubenberger A, Puech PH,
Ehninger G, Bornhauser M, Muller DJ and Illmer T: BCR/ABL
expression of myeloid progenitors increases beta1-integrin mediated
adhesion to stromal cells. J Mol Biol. 377:1082–1093. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen GQ, Wang LS, Wu YL and Yu Y:
Leukemia, an effective model for chemical biology and target
therapy. Acta Pharmacol Sin. 28:1316–1324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Z, Zhu P, Zhou Y, Sheng Y, Hong Y,
Xiang D, Qian Z, Mosenson J and Wu WS: A novel slug-containing
negative-feedback loop regulates SCF/c-Kit-mediated hematopoietic
stem cell self-renewal. Leukemia. 31:403–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Green MR, Aya-Bonilla C, Gandhi MK, Lea
RA, Wellwood J, Wood P, Marlton P and Griffiths LR: Integrative
genomic profiling reveals conserved genetic mechanisms for
tumorigenesis in common entities of non-Hodgkin's lymphoma. Genes
Chromosomes Cancer. 50:313–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mencalha AL, Binato R, Ferreira GM, Du
Rocher B and Abdelhay E: Forkhead box M1 (FoxM1) gene is a new
STAT3 transcriptional factor target and is essential for
proliferation, survival and DNA repair of K562 cell line. PLoS One.
7:e481602012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Carrett-Dias M, Almeida LK, Pereira JL,
Almeida DV, Filgueira DM, Marins LF, Votto AP and Trindade GS: Cell
differentiation and the multiple drug resistance phenotype in human
erythroleukemic cells. Leuk Res. 42:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nakamura S, Hirano I, Okinaka K, Takemura
T, Yokota D, Ono T, Shigeno K, Shibata K, Fujisawa S and Ohnishi K:
The FOXM1 transcriptional factor promotes the proliferation of
leukemia cells through modulation of cell cycle progression in
acute myeloid leukemia. Carcinogenesis. 31:2012–2021. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shin JM, Jeong YJ, Cho HJ, Magae J, Bae YS
and Chang YC: Suppression of c-Myc induces apoptosis via an
AMPK/mTOR-dependent pathway by 4-O-methyl-ascochlorin in leukemia
cells. Apoptosis. 21:657–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shi P, Huang Z and Chen G: Rhein induces
apoptosis and cell cycle arrest in human hepatocellular carcinoma
BEL-7402 cells. Am J Chin Med. 36:805–813. 2008. View Article : Google Scholar : PubMed/NCBI
|