Introduction
The efficacy of prostate cancer (PCa) detection
based on the serum level of prostate-specific antigen (PSA) coupled
with the use of ultrasound-guided random systematic prostate biopsy
has led to a marked improvement in diagnostic accuracy and
treatment. However, PCa morbidity and mortality rates have also
increased markedly in Japan and other countries.
The effectiveness of radiotherapy (RT) in particular
has improved as a result of the development and introduction of
computer software and treatment devices. RT is currently a standard
treatment for various malignant tumors, including those of the
breast, lung and prostate. RT for PCa can be classified as external
beam RT or brachytherapy. The former includes intensity-modulated
radiation therapy (IMRT), 3-dimensional conformal radiation therapy
(3D-CRT) and stereotactic radiotherapy (SRT), whereas the latter
includes iridium-192 high-dose-rate (HDR) and iodine-125
low-dose-rate (LDR) brachytherapy. Permanent iodine-125 LDR
brachytherapy is one of the important curative treatments currently
available for localized PCa. Either HDR or LDR brachytherapy can
deliver a high dose rate to the prostate parenchyma in comparison
with external irradiation treatments (1).
RT for localized PCa may directly kill tumor cells.
Clinically, however, patients receiving RT occasionally show an
abscopal effect whereby not only local, but also distant tumors
regress after localized irradiation (2). It has been reported that
treatment-associated autoantibodies were detectable in 29.2% (7 of
24), 13.8% (4 of 29), 25% (5 of 20) and 5.6% (2 of 36) of PCa
patients who had undergone neoadjuvant hormone therapy, external
beam RT, brachytherapy and radical prostatectomy, respectively
(3). It has also been reported that
patients who had received RT exhibited an increased count of
survivin-specific cytotoxic CD8+ T cells (4). In a pre-clinical study of
cancer-bearing mice, Takeshima et al found that the efficacy
of tumor reduction by RT declined after CD8+ T cell
depletion (5). Although RT may
induce some immune responses, the details are still not clear.
Irradiation has been revealed to induce many immunomodulators from
apoptotic and necrotic tumor cells, including tumor-associated
antigens, heat shock proteins (HSPs), high mobility group box 1
(HMGB1), and adenosine triphosphate (ATP) (6,7). These
immunomodulators can activate immature dendritic cells (DCs), which
in turn may enhance inflammatory responses and activate T cells,
including tumor antigen-specific T cells.
As aforementioned, permanent I-125 LDR brachytherapy
is an important curative treatment for localized PCa. We consider
that LDR may initiate continuous immune stimulation through gradual
disruption of the prostate parenchyma by the LDR seed implant. In
the present study, therefore, to clarify the immune responses of
PCa patients receiving LDR brachytherapy, we studied the dynamics
of leukocyte subsets in peripheral blood altered before and after
the treatment.
Materials and methods
Patients
The present study subjects were 36 patients with
clinically localized PCa who underwent I-125 brachytherapy. The
patients characteristics are shown in Table I. All of the patients gave
study-specific informed consent, which was approved by the
Institutional Review Board for Observation and Epidemiological
Study (KMEO B13-62). The present study approved by the Kitasato
University Medical Ethics Organization
 | Table I.Characteristics of patients who
received LDR prostate brachytherapy. |
Table I.
Characteristics of patients who
received LDR prostate brachytherapy.
| Patient | Age (years) | PSA (ng/ml) | Stage | Gleason score |
|---|
| LDR-1 | 76 | 10.7 | 2b | 4+4 |
| LDR-2 | 72 | 10.45 | 2b | 4+3 |
| LDR-3 | 73 | 11.46 | 2c | 4+3 |
| LDR-4 | 69 | 4.48 | 1c | 3+4 |
| LDR-5 | 76 | 15.08 | 2c | 4+3 |
| LDR-6 | 62 | 13.29 | 2a | 4+3 |
| LDR-7 | 52 | 4.46 | 1c | 3+3 |
| LDR-8 | 67 | 7.68 | 1c | 3+3 |
| LDR-9 | 66 | 4.74 | 2a | 3+4 |
| LDR-10 | 69 | 4.66 | 1c | 3+4 |
| LDR-11 | 76 | 5.10 | 2c | 4+3 |
| LDR-12 | 73 | 4.86 | 1c | 3+4 |
| LDR-13 | 74 | 10.54 | 1c | 3+4 |
| LDR-14 | 71 | 4.67 | 1c | 3+3 |
| LDR-15 | 67 | 5.97 | 1c | 3+4 |
| LDR-16 | 62 | 5.30 | 1c | 4+3 |
| LDR-17 | 71 | 6.56 | 1c | 3+3 |
| LDR-18 | 77 | 8.66 | 2a | 4+4 |
| LDR-19 | 80 | 12.90 | 2c | 3+4 |
| LDR-20 | 62 | 8.49 | 2a | 3+4 |
| LDR-21 | 70 | 8.23 | 2b | 3+3 |
| LDR-22 | 71 | 5.34 | 1c | 4+4 |
| LDR-23 | 69 | 9.91 | 2a | 3+4 |
| LDR-24 | 65 | 8.51 | 1c | 3+4 |
| LDR-25 | 60 | 9.97 | 2a | 4+3 |
| LDR-26 | 63 | 6.19 | 2c | 3+4 |
| LDR-27 | 64 | 6.05 | 1c | 4+3 |
| LDR-28 | 55 | 4.46 | 1c | 3+3 |
| LDR-29 | 77 | 9.72 | 1c | 3+3 |
| LDR-30 | 73 | 5.67 | 1c | 3+4 |
| LDR-31 | 77 | 6.41 | 2a | 4+3 |
| LDR-32 | 59 | 11.40 | 2a | 3+3 |
| LDR-33 | 66 | 8.97 | 2a | 3+4 |
| LDR-34 | 78 | 8.74 | 1c | 3+3 |
| LDR-35 | 76 | 7.08 | 2c | 3+3 |
| LDR-36 | 65 | 6.45 | 1c | 3+4 |
Leukocytes immunophenotyping
The immunophenotyping was monitored approximately
every ~3 months (Fig. 1).
EDTA-2Na-treated whole blood (100 µl) was incubated with the
following fluorescence-conjugated monoclonal antibodies (MAbs)
against the following: CD1c (PE/Cy7; cat. no. 331516), CD4 (PE/Cy7;
cat. no. 317414), CD8 (APC/Cy7; cat. no. 344714), CD11b (PE; cat.
no. 301306), CD14 (PerCP/Cy5.5; cat. no. 301824), CD15 (PE/Cy7;
cat. no. 323030), CD25 (FITC; cat. no. 302604), CD45RA (Pacific
Blue; cat. no. 304123), CD45RO (APC; cat. no. 304210), CD62L (FITC;
cat. no. 104406), CD66b (FITC; cat. no. 305104), CD141 (APC; cat.
no. 344106), CCR7 (PE; cat. no. 353204), HLA-DR (PerCP/Cy5.5 and
APC/Cy7) (cat. nos. 307630 and 307618) (all from BioLegend, San
Diego, CA, USA) CD3/CD16/56 (FITC/PE; Becton-Dickinson, San Jose,
CA, USA), and mouse IgG1 (Pacific Blue, FITC-PE-PE/Cy5 cocktail,
PE/Cy7, APC and APC/Cy7; cat. nos. 400151, 319201, 400125, 400119
and 400127; BioLegend). After incubation at room temperature for 15
min, red blood cells were lysed with BD FACS lysing solution
(Becton-Dickinson, San Jose, CA). FSC and SSC were set to
distinguish the lymphocyte, macrophage, and granulocyte populations
from debris with a MACSQuant flow cytometer (Miltenyi Biotec GmbH,
Bergish Gladbach, Germany). Multi-color immunofluorescence analysis
was performed using 10,000 lymphocytes for each analysis.
Statistical analysis
Statistical significance was analyzed using the
Student's t-test. Differences were considered statistically
significant at P<0.05.
Results
Comparison of circulating leukocyte
subsets before and after LDR brachytherapy
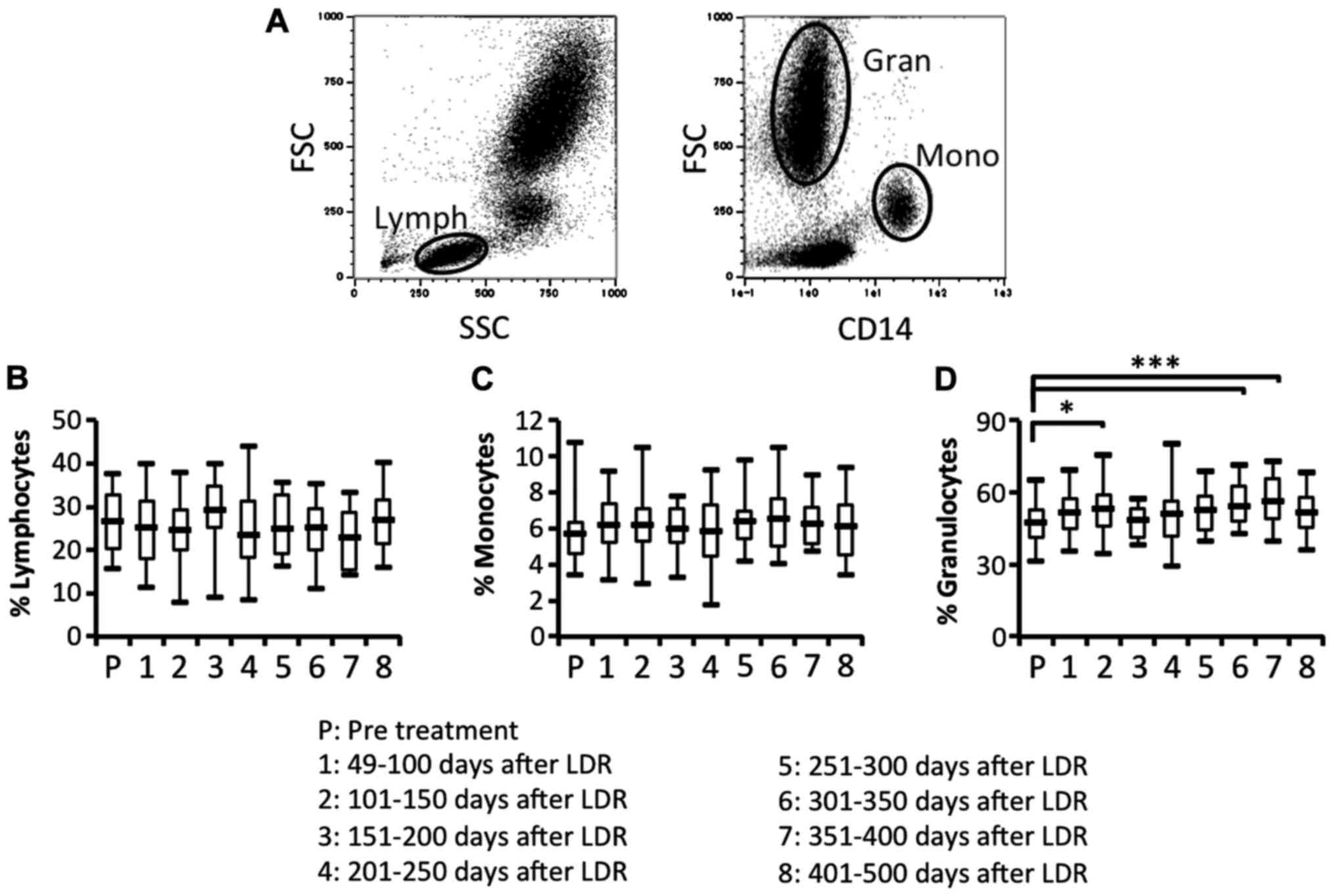
To study the efficacy of the immune responses, we
investigated whether the proportion of lymphocytes, monocytes and
granulocytes in peripheral blood was affected by the LDR
brachytherapy. We then compared the proportion of lymphocytes,
monocytes and granulocytes in peripheral blood before and after the
therapy (Fig. 2A-D). The proportion
of lymphocytes and monocytes before and after LDR brachytherapy did
not differ (Fig. 2B and C). In
contrast, the proportion of granulocytes was significantly and
bimodally increased after LDR brachytherapy (Fig. 2D). To investigate the difference in
lymphocyte subsets, we next compared the proportion of lymphocyte
subsets before and after LDR brachytherapy. The proportions of T
cells (CD3+CD19− cells gated on lymphocytes)
and natural killer (NK) cells (CD3−CD16/56+
cells gated on lymphocytes) did not significantly differ before and
after LDR brachytherapy (Fig. 3A, C and
D). In contrast, the proportion of B cells
(CD3−CD19+ cells gated on lymphocytes) was
significantly and bimodally decreased after the therapy (Fig. 3A and B). We also analyzed the
proportion of T cell subsets. The proportion of helper T cells
(CD3+CD4+ cells gated on lymphocytes) and
killer T cells (CD3+ CD8+ cells gated on
lymphocytes) before and after LDR brachytherapy did not differ
significantly (Fig. 3A, E and F).
These results revealed that granulocytes may be increased in
peripheral blood due to the induction of inflammatory responses in
the irradiated PCa, and that B cells may be depleted from
peripheral blood due to migration of antigen-recognizing B cells
from peripheral blood to the irradiated PCa.
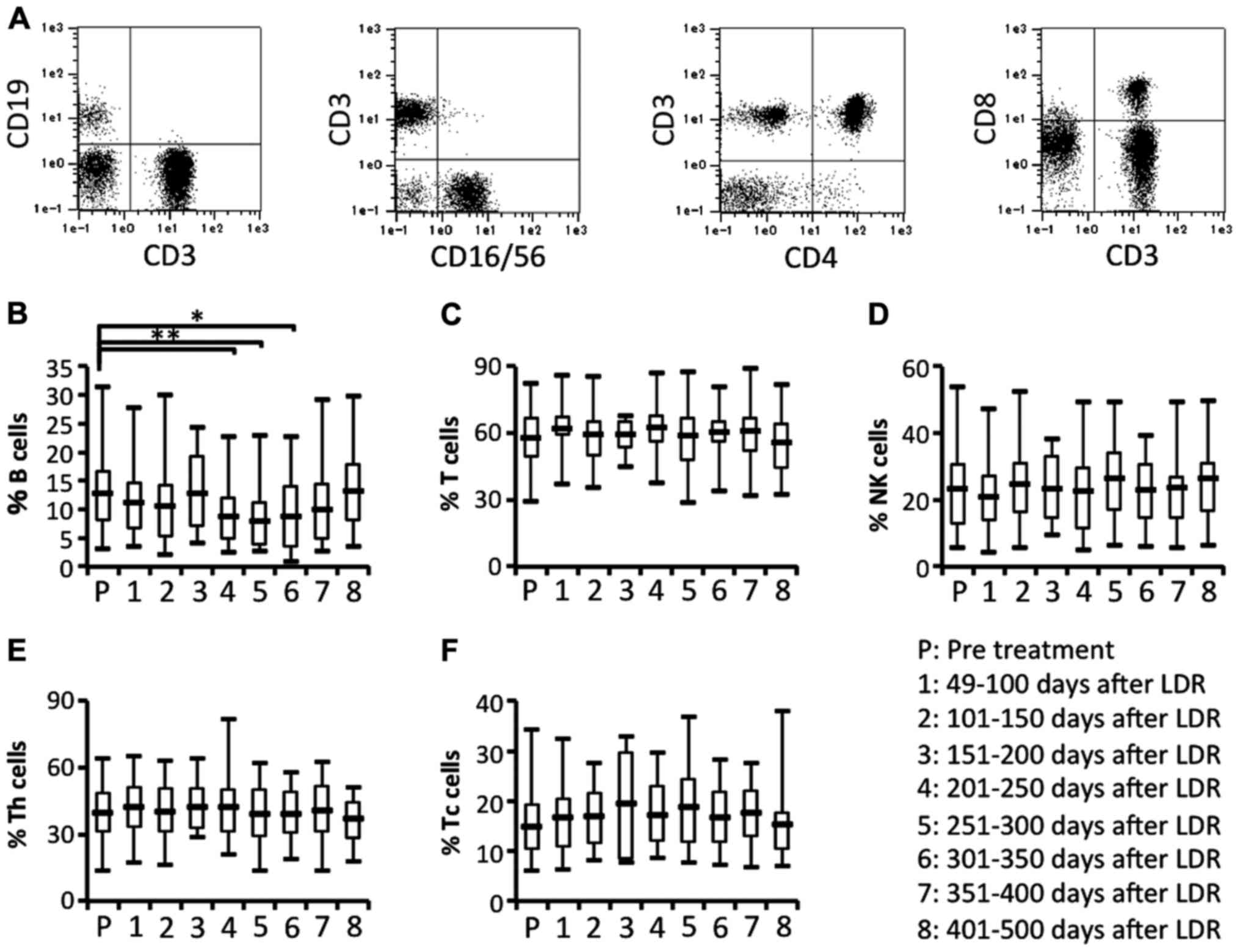 | Figure 3.Circulating lymphocyte subsets.
Circulating lymphocyte subsets in patients were characterized by
flow cytometric analysis using immunofluorescence antibody staining
for CD3, CD4, CD8, CD19 and CD16/56. (A) The representative data
for B and T cells (left panel), NK cells (2nd panel from the left),
CD4+ T cells (3rd panel from the left) and
CD8+ T cells (right panel). Data for (B) the proportion
of the B cell (CD3−CD19+ gated on
lymphocytes) subset, (C) the proportion of the T cell
(CD3+CD19− gated on lymphocytes) subset, (D)
the proportion of the NK cell (CD3−CD16/56+
gated on lymphocytes) subset, (E) the proportion of the Th cell
(CD3+CD4+ gated on lymphocytes) subset, and
(F) the proportion of the Tc cell (CD3+CD8+
gated on lymphocytes) subset; *P<0.05, **P<0.01. NK, natural
killer. |
Comparison of circulating activated T
cell subsets and monocyte-derived suppressor cells before and after
LDR brachytherapy
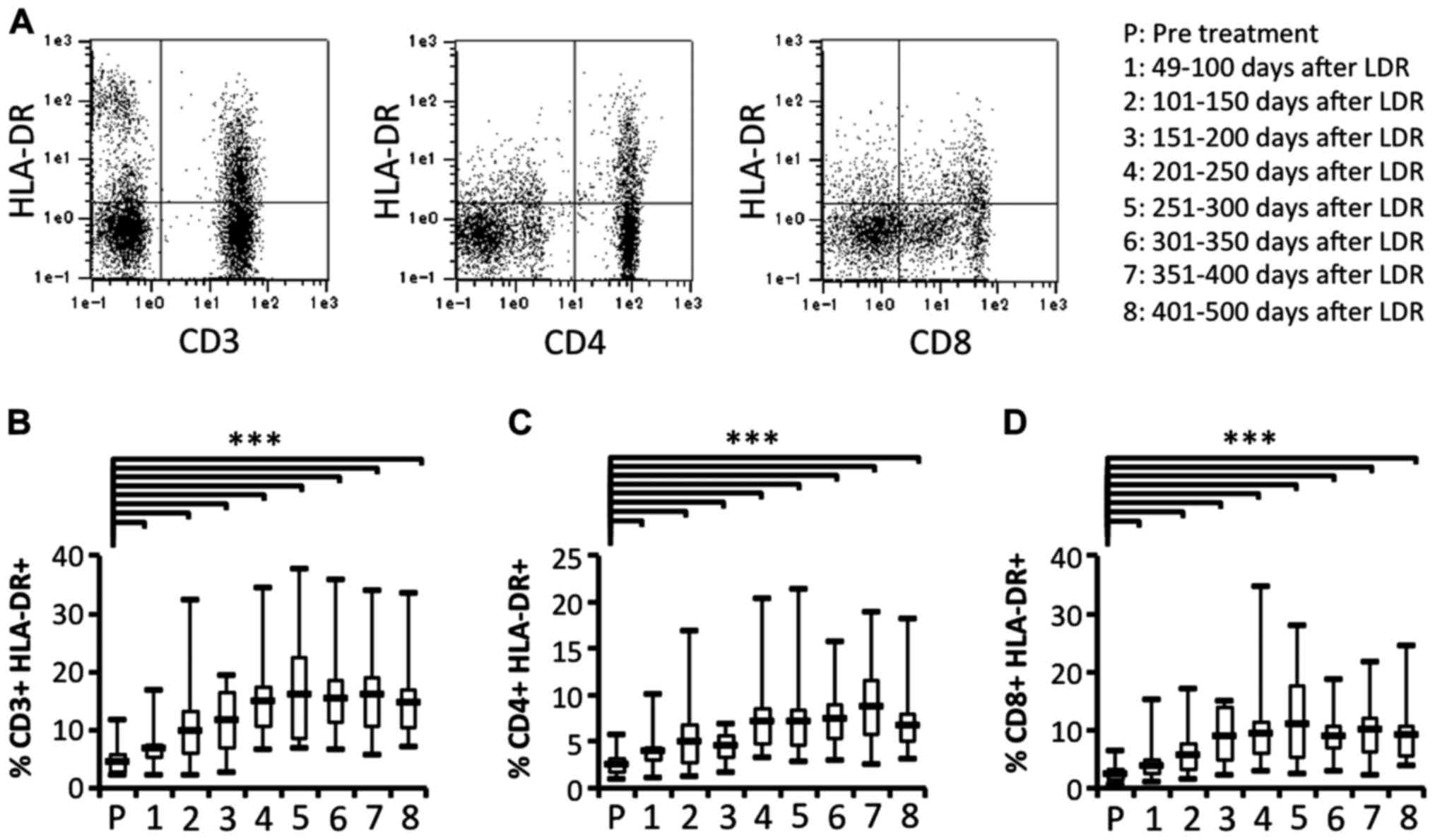
To clarify whether activated T cell subsets were
effectively induced, we next investigated the activation of T cell
subsets in patients who had received LDR brachytherapy. We also
compared the proportion of activated T cell subsets before and
after LDR brachytherapy. The proportion of activated T cell subsets
(CD3+HLA-DR+,
CD4+HLA-DR+ and CD8+
HLA-DR+) were significantly and gradually increased
after LDR brachytherapy (Fig. 4).
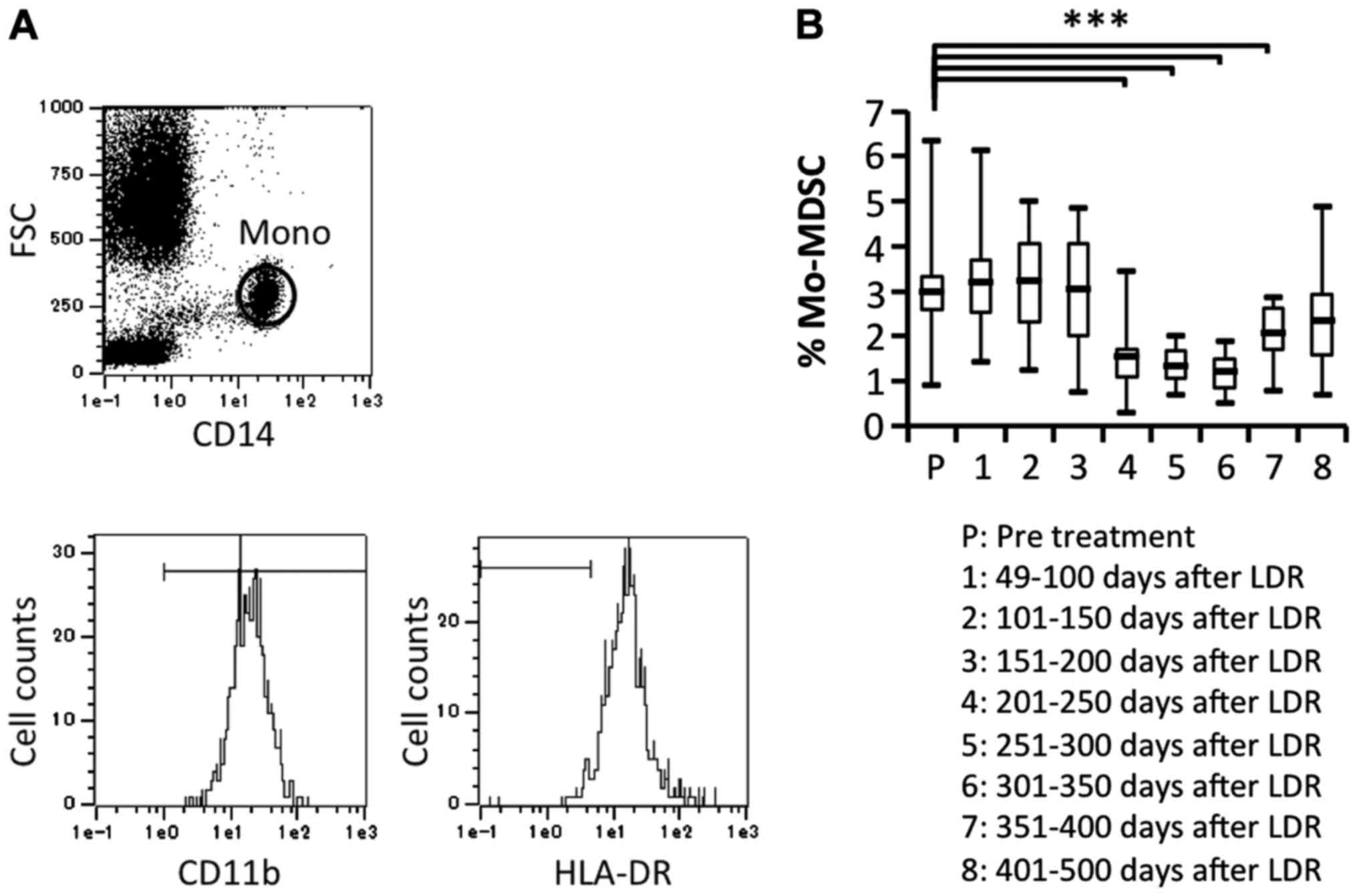
Previous studies have indicated that RT used against cancer can
induce myeloid-derived suppressor cells (MDSCs), which can suppress
immune responses by producing inhibitory cytokines. Therefore, we
investigated the proportion of MDSCs
(CD11b+CD14+HLA-DR− gated on
monocytes) before and after LDR brachytherapy and found that MDSCs
were significantly decreased after 201 days of LDR brachytherapy
(Fig. 5A and B). This suggested
that the immune responses induced by LDR brachytherapy may
gradually activate both CD4+ and CD8+ T cell
subsets, whereas reduction of MDSCs may affect T cell
activation.
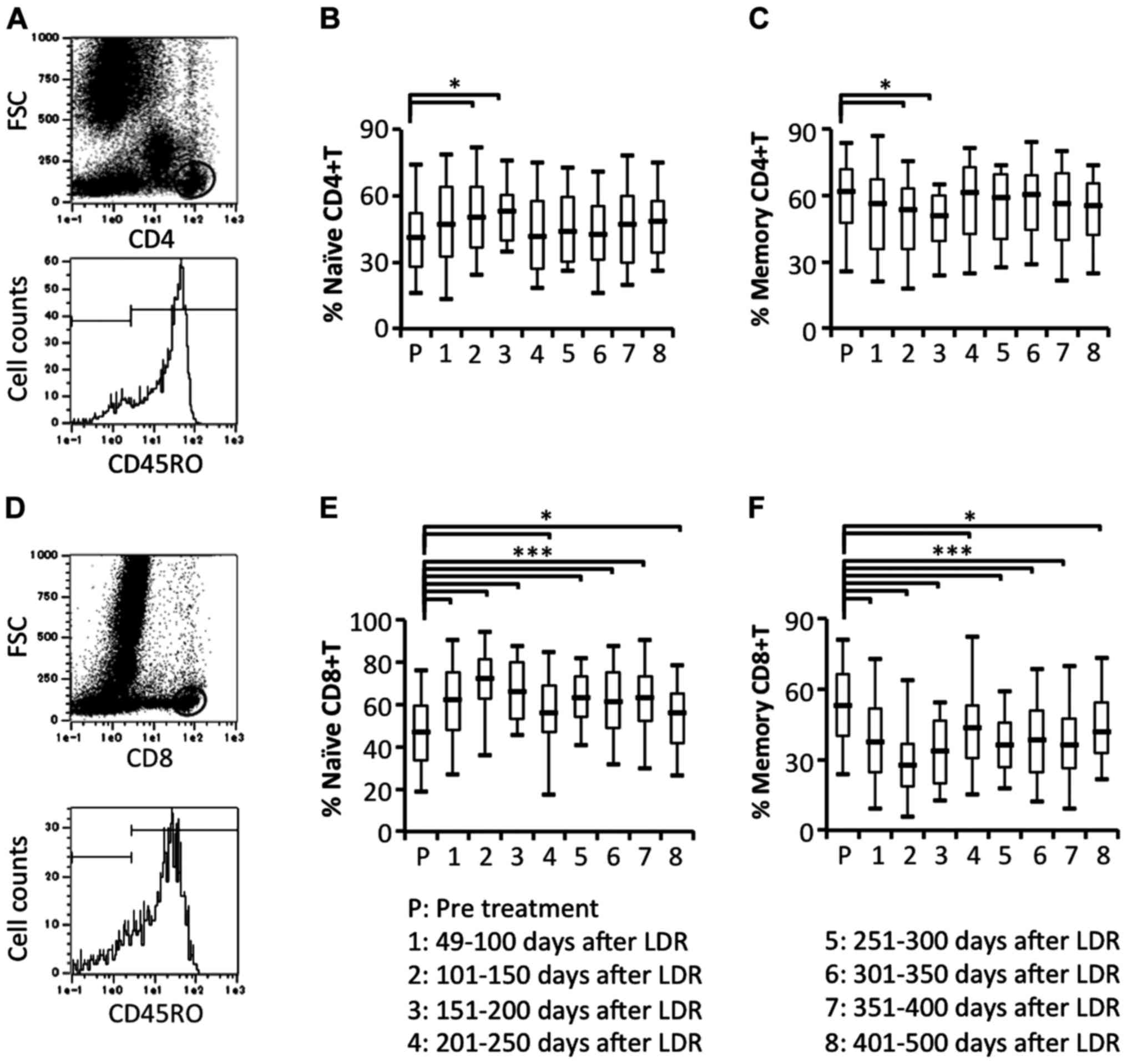
Comparison of circulating naïve and
memory T cell subsets
To study the details of naïve and memory T cell
responses in patients who had received LDR brachytherapy, we
examined the proportion of circulating naïve (CD45RO−
gated on CD4+ or CD8+ cells) and memory
(CD45RO+ gated on CD4+ or CD8+
cells) T cell subsets. Naïve CD4+ T cells were
significantly increased at an early stage after the start of LDR
brachytherapy. In contrast to naïve CD4+ T cells, memory
CD4+ T cells were significantly decreased at an early
stage after the start of LDR brachytherapy (Fig. 6A-C). In contrast, naïve
CD8+ T cells were significantly and bimodally increased
and memory CD8+ T cells were significantly and bimodally
decreased (Fig. 6D-F). These
results demonstrated that memory CD4+ and
CD8+ T cells were effectively induced to migrate from
blood vessels to the local prostate environment.
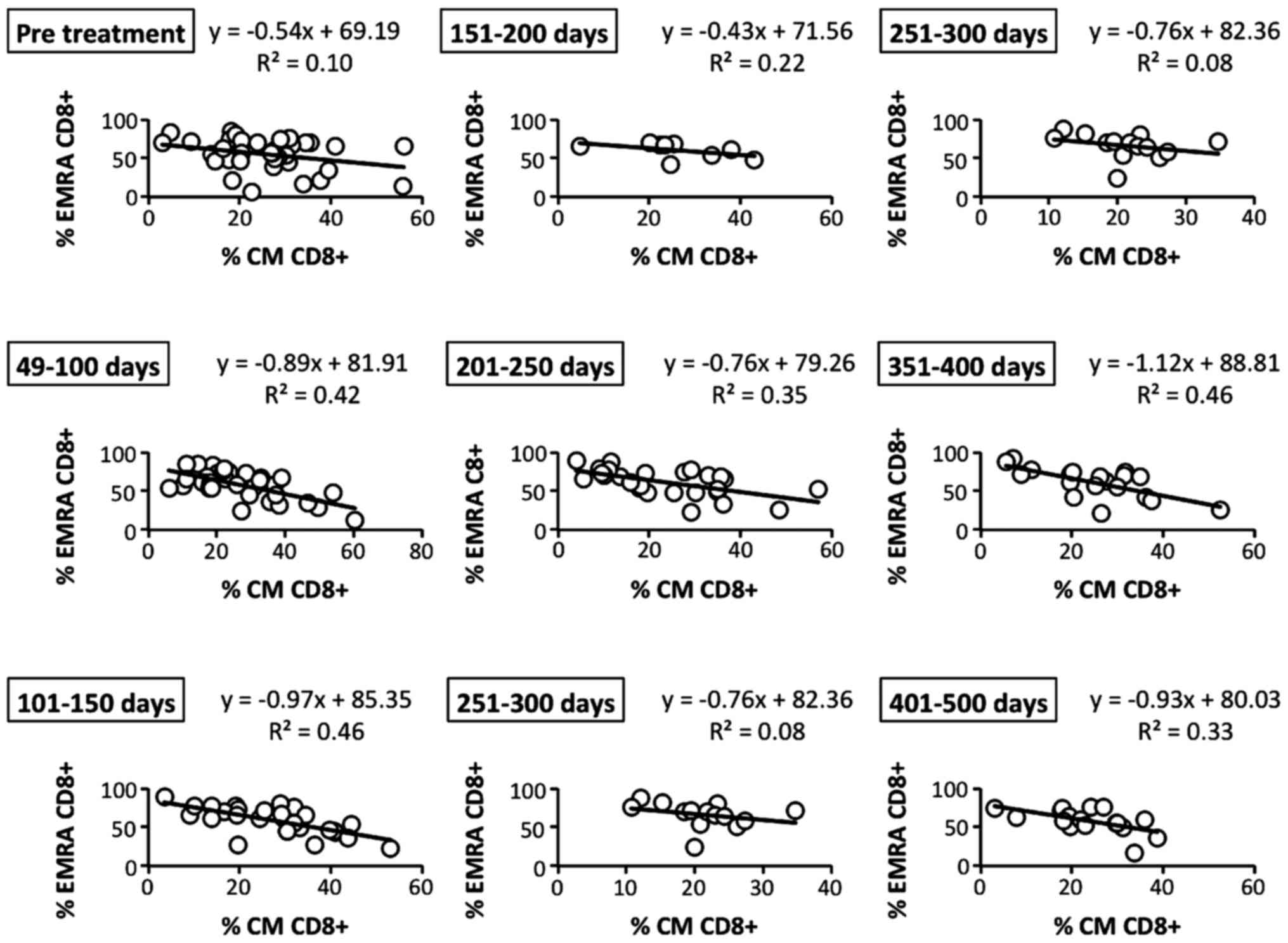
Correlations among circulating memory
CD8+ T cell subsets
To further study the details of memory
CD8+ T cell subsets in patients who had received LDR
brachytherapy, we analyzed the correlation of central memory (CM)
with effector memory (EM) CD8+ T cells before and after
LDR brachytherapy. After the therapy, the inverse correlation of CM
(CD45RO+ CD62L+ CCR7+) with EMRA
(CD45RO− CD62L− CCR7−)
CD8+ T cells became gradually and bimodally higher than
before the therapy (Fig. 7). In
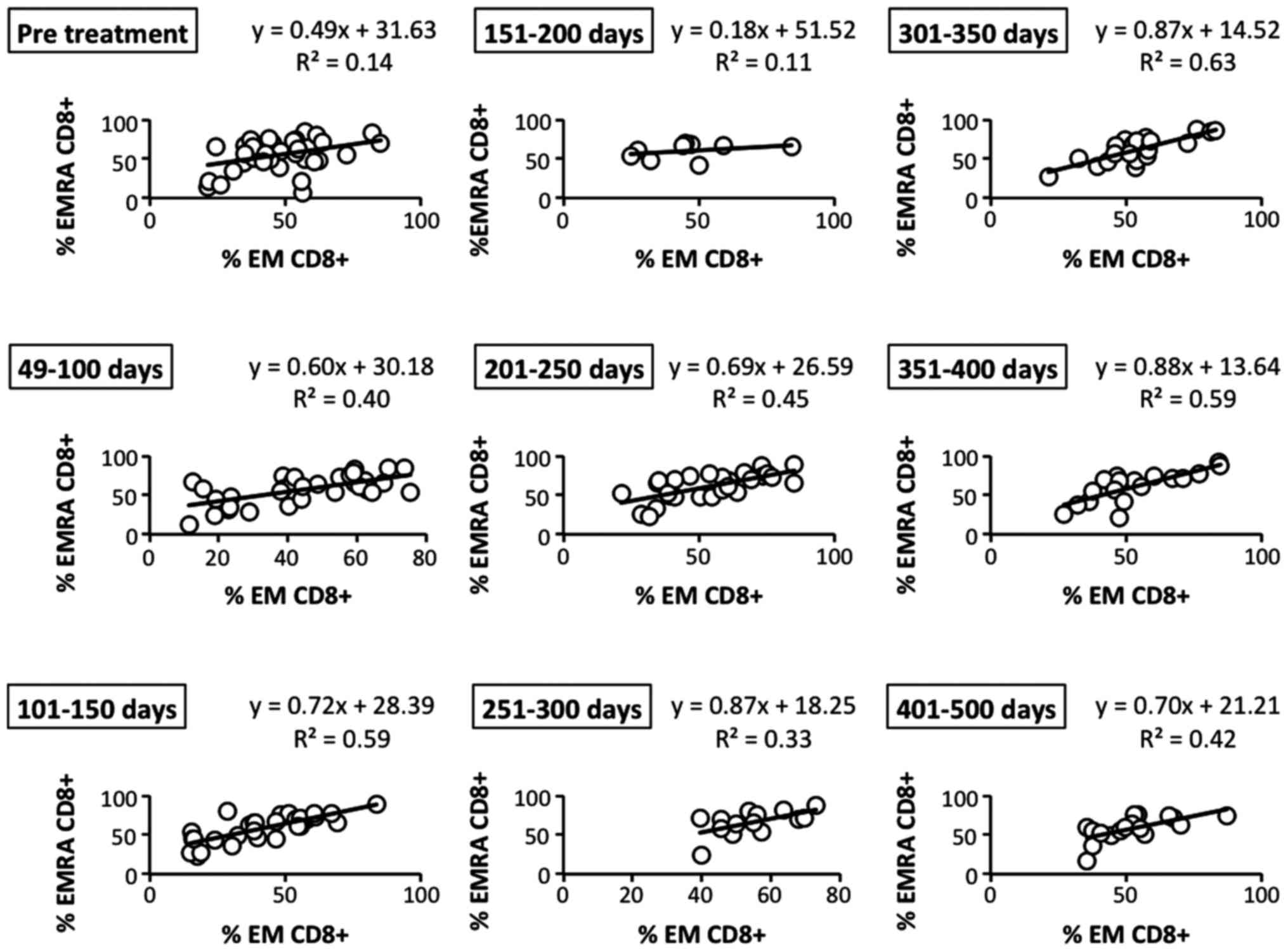
contrast, we observed that the positive correlation of EM and EMRA
(CD45RO− CD62L− CCR7−)
CD8+ T cells, another EM subset, was gradually and
bimodally increased after the therapy (Fig. 8). These results revealed that
effector memory CD8+ T cells, which play an important
role in the antitumor immune response, may be effectively induced
by LDR brachytherapy.
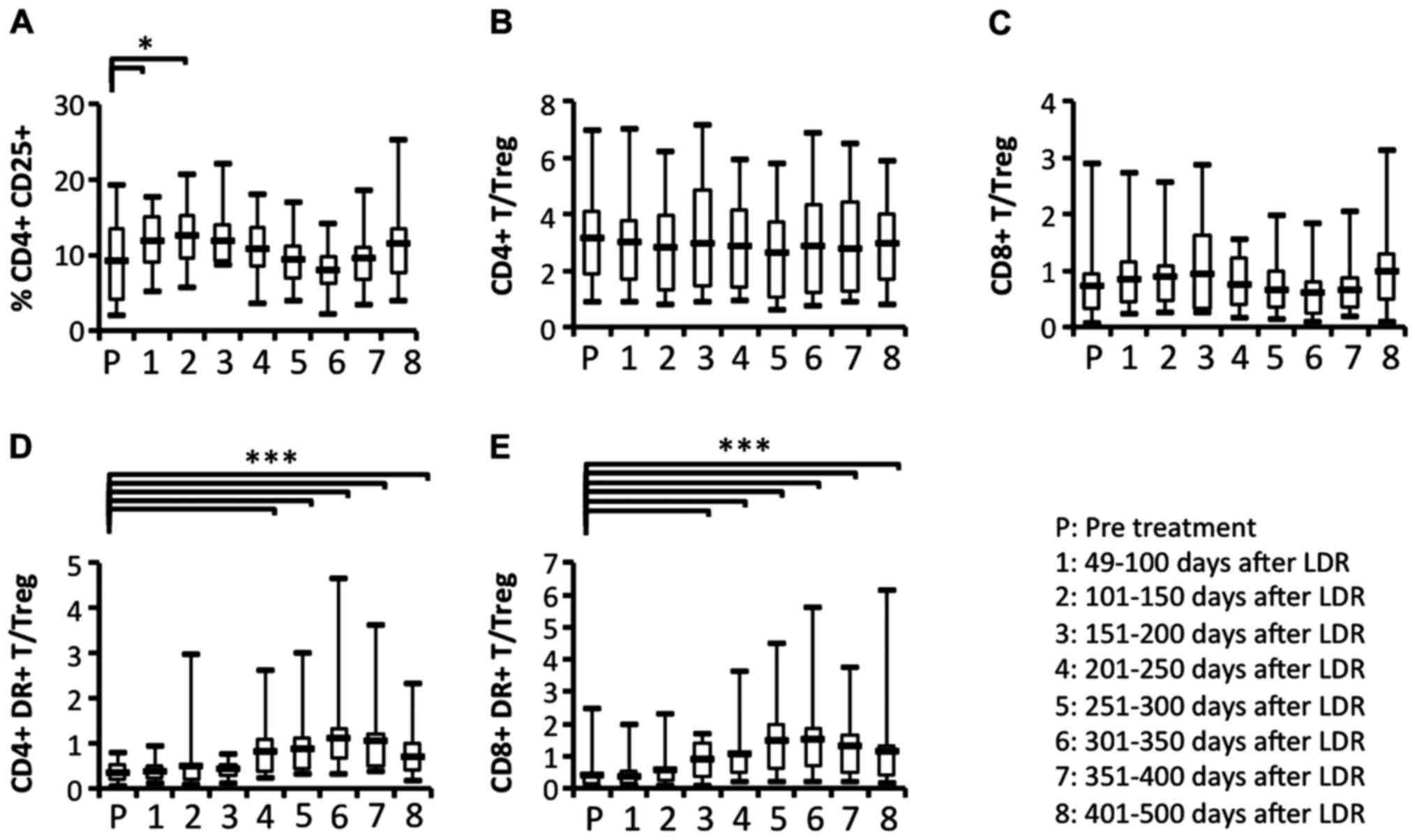
Comparison of the circulating
regulatory T cell (Treg) ratio relative to activated T cell
subsets
Our results indicated that some immune responses,
including the activation of T cell subsets, appeared to be evident
from the start of LDR brachytherapy until ~200 days later. The
proportion of the Treg subset was significantly increased after the
start of LDR brachytherapy (Fig.
9A). To determine whether Treg cells were affected by the
induction of activated T cell subsets, we analyzed the ratios of T
cell subsets or activated T cell subsets relative to Treg. The
ratios of CD4+ and CD8+ T cell subsets
relative to Tregs did not change significantly during the
observation period (Fig. 9B and C).
In contrast, we observed that the ratios of activated
CD4+ and CD8+ T cell subsets relative to
Tregs were significantly increased at ~200 days after the start of
LDR brachytherapy (Fig. 9D and E).
These results revealed that the increase in activated T cell
subsets may affect the reduction of the Treg subset induced by LDR
brachytherapy.
Discussion
Radiotherapy (RT) is a standard treatment for
prostate cancer. Clinically, although RT directly induces cancer
cell killing, an abscopal effect whereby regression of distant
tumors occurs following local irradiation is occasionally observed
(2). However, details of the
mechanisms involved and the induced immune responses in prostate
cancer (PCa) patients who received RT have not been well defined.
In the present study, we investigated in detail the dynamics of
systemic leukocyte subsets before and after RT, and found that
activated T cells subsets and effector memory CD8+ T
cells were efficiently increased in patients who received LDR
brachytherapy.
With regard to the relationship between RT and
immune responses, Stone et al revealed that immunosuppressed
thymectomized mice require more than twice the radiation dose for
control of fibrosarcoma in comparison with normal syngeneic mice
(8). Moreover, in cancer-bearing
mice, Takeshima et al reported that the efficacy of tumor
rejection induced by RT decreased after CD8+ T cell
depletion (5). Furthermore, in mice
inoculated with EL4 lymphoma cells, local irradiation successfully
controlled EL4 tumor growth and the mice survived. The mice then
rejected the tumor after re-inoculation of EL4 lymphoma cells
(9). Several clinical studies have
demonstrated an abscopal effect of RT whereby distant metastatic
tumors are rejected or decreased following localized irradiation
(2,10). Although this abscopal effect is an
infrequent phenomenon, it has recently been reported in patients
who have undergone RT coupled with immunotherapies such as
inoculation with toll-like receptor (TLR) agonists,
anti-transforming growth factor β (TGFβ), anti-CD152 (CTLA-4) and
anti-programmed death-1 (PD-1) receptor antibodies (11,12).
CTLA-4 and PD-1 have received notable attention as important immune
checkpoint molecules. As aforementioned, the antitumor efficacy by
RT is considered to be closely associated with the induction of
immune responses. However, the immune responses in patients who
have received RT are not well defined. In particular, the
alterations in the immune responses of patients receiving LDR
brachytherapy have never been previously reported.
In the present study, we found that activated T cell
subsets were significantly and gradually increased after LDR
brachytherapy. Although the precise mechanism responsible for this
T cell-subset activation was not clear, an inverse reduction of
MDSCs and regulatory T cells (Tregs) may be involved. Kachikwu
et al reported that radiation treatment increased the
population of Tregs in mice (13).
We also observed that Tregs were significantly increased after 150
days of LDR brachytherapy, as compared with the situation before
RT. Although our findings were partly consistent with theirs, we
also observed a tendency for Treg reduction after 200 days of LDR
brachytherapy. Wu et al indicated that splenic MDSCs were
increased in prostate tumor-bearing mice 48 h after radiation
(14). Although we did not observe
an increase of MDSCs after LDR brachytherapy, we recognized a
significant reduction in the ratio of MDSCs at 200 days after the
start of LDR brachytherapy. Certain factors may influence the
populations of Tregs and MDSCs. Xu et al has reported that
blockade of macrophage colony-stimulating factor (CSF1) signaling
improves the efficacy of RT for PCa (15). Therefore there is a need to
investigate cytokines and chemokines in the plasma of patients
before and after LDR brachytherapy.
We also observed that naïve T cells in peripheral
blood were bimodally and significantly increased after LDR
brachytherapy, whereas memory T cells were decreased. These results
suggest that memory T cells, particularly memory CD8+ T
cells, may infiltrate from the peripheral blood to the irradiated
PCa, leading to a relative increase of naïve T cells in peripheral
blood. However, Tabi et al previously reported that the
proportion of apoptotic and Fas+ naïve
(CD45RA+) T cells was increased in patients who received
external beam radiotherapy (EBRT) (16). Our findings were inconsistent with
their results. LDR brachytherapy may have limited influence on the
immune system due to internal localized irradiation. In the present
study, we found that some leukocyte subsets in patients who
received LDR brachytherapy were dynamically and sequentially
changed. In the future, to demonstrate the role of activated T
cells and regulatory cells in remission and relapse rates, we will
endeavor to compare overall survival rates, relapse rates, and T
cell activation in PCa patients receiving LDR brachytherapy.
Although we observed some dynamic immune responses in LDR patients,
we did not investigate details of the immune response in patients
who received other radiotherapies, including HDR. In the next step,
a comparison of the immune responses of patients who have received
other treatments is warranted.
In conclusion, we have shown for the first time that
the proportion of activated T cell subsets in peripheral blood was
gradually and significantly increased after LDR brachytherapy. In
contrast, the proportion of Tregs and Mo-MDSCs was significantly
decreased after 200 days of LDR brachytherapy. The increase of
activated T cell subsets resulting from LDR brachytherapy may help
to maintain remission and reduce relapse rates. The decrease of
Tregs and Mo-MDSCs may be related to the increase of activated T
cell subsets.
Acknowledgements
The present study was supported by the JSPS KAKENHI
grant nos. C25462500 and 16K11028, and grants from the Kitasato
University School of Allied Health Sciences (Grant-in-aid for
Research Project 2012-2014).
References
|
1
|
Ishiyama H, Satoh T, Kawakami S, Tsumura
H, Komori S, Tabata K, Sekiguchi A, Takahashi R, Soda I, Takenaka
K, et al: A prospective quasi-randomized comparison of
intraoperatively built custom-linked seeds versus loose seeds for
prostate brachytherapy. Int J Radiat Oncol Biol Phys. 90:134–139.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nesslinger NJ, Sahota RA, Stone B, Johnson
K, Chima N, King C, Rasmussen D, Bishop D, Rennie PS, Gleave M, et
al: Standard treatments induce antigen-specific immune responses in
prostate cancer. Clin Cancer Res. 13:1493–1502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schaue D, Comin-Anduix B, Ribas A, Zhang
L, Goodglick L, Sayre JW, Debucquoy A, Haustermans K and McBride
WH: T-cell responses to survivin in cancer patients undergoing
radiation therapy. Clin Cancer Res. 14:4883–4890. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeshima T, Chamoto K, Wakita D, Ohkuri
T, Togashi Y, Shirato H, Kitamura H and Nishimura T: Local
radiation therapy inhibits tumor growth through the generation of
tumor-specific CTL: Its potentiation by combination with Th1 cell
therapy. Cancer Res. 70:2697–2706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma Y, Kepp O, Ghiringhelli F, Apetoh L,
Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM, et al:
Chemotherapy and radiotherapy: Cryptic anticancer vaccines. Semin
Immunol. 22:113–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gameiro SR, Jammeh ML, Wattenberg MM,
Tsang KY, Ferrone S and Hodge JW: Radiation-induced immunogenic
modulation of tumor enhances antigen processing and calreticulin
exposure, resulting in enhanced T-cell killing. Oncotarget.
5:403–416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stone HB, Peters LJ and Milas L: Effect of
host immune capability on radiocurability and subsequent
transplantability of a murine fibrosarcoma. J Natl Cancer Inst.
63:1229–1235. 1979.PubMed/NCBI
|
|
9
|
Yoshimoto Y, Suzuki Y, Mimura K, Ando K,
Oike T, Sato H, Okonogi N, Maruyama T, Izawa S, Noda SE, et al:
Radiotherapy-induced anti-tumor immunity contributes to the
therapeutic efficacy of irradiation and can be augmented by CTLA-4
blockade in a mouse model. PLoS One. 9:e925722014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ludgate CM: Optimizing cancer treatments
to induce an acute immune response: Radiation abscopal effects,
PAMPs, and DAMPs. Clin Cancer Res. 18:4522–4525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marabelle A, Filatenkov A, Sagiv-Barfi I
and Kohrt H: Radiotherapy and toll-like receptor agonists. Semin
Radiat Oncol. 25:34–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernández-García EM, Vera-Badillo FE,
Perez-Valderrama B, Matos-Pita AS and Duran I: Immunotherapy in
prostate cancer: Review of the current evidence. Clin Transl Oncol.
17:339–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kachikwu EL, Iwamoto KS, Liao YP, DeMarco
JJ, Agazaryan N, Economou JS, McBride WH and Schaue D: Radiation
enhances regulatory T cell representation. Int J Radiat Oncol Biol
Phys. 81:1128–1135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu CT, Chen MF, Chen WC and Hsieh CC: The
role of IL-6 in the radiation response of prostate cancer. Radiat
Oncol. 8:159–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu J, Escamilla J, Mok S, David J,
Priceman S, West B, Bollag G, McBride W and Wu L: CSF1R signaling
blockade stanches tumor-infiltrating myeloid cells and improves the
efficacy of radiotherapy in prostate cancer. Cancer Res.
73:2782–2794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tabi Z, Spary LK, Coleman S, Clayton A,
Mason MD and Staffurth J: Resistance of CD45RA- T cells to
apoptosis and functional impairment, and activation of
tumor-antigen specific T cells during radiation therapy of prostate
cancer. J Immunol. 185:1330–1339. 2010. View Article : Google Scholar : PubMed/NCBI
|