Introduction
Osteoclasts, derived from the mononuclear phagocyte
system, are the main cells capable of resorbing bone (1). Physiologically, bone homeostasis is
maintained by the balance between osteoblastic bone formation and
osteoclastic bone resorption (2).
However, excess mass bone resorption and an imbalance in weight are
observed in various osteolytic diseases, including arthritis,
periodontitis, loosened tooth implants and malignancy-induced bone
metastasis (3,4). Regardless of disease type, osteoclasts
play important roles in the body. Once osteoclasts are recruited to
bone sites, the bone architecture or function becomes abnormal
(5).
In contrast to distant bone metastasis caused by
breast or prostate cancer, local bone invasion is typically
detected in oral cancer, particularly OSCC. Bone invasion by OSCC
exhibits specific characteristics, which depend on crosstalk
between osteoblasts, osteoclasts and tumour cells (6). Our research group focused on these
complex interactions. By using an indirect cell co-culture model,
we found that CM from both an osteoblast cell line and primary
cultured osteoblasts stimulated the invasive properties of OSCC
cells, while CM of OSCC cells induced the generation of
osteoclastic factors in osteoblasts (7). To determine which component in the CM
caused gene expression changes during cell co-culture, transforming
growth factor-β (TGF-β) was utilized to treat OSCC cells, and
partial epithelial-mesenchymal transition (EMT) was confirmed in
OSCC cells. CM of OSCC cells pre-treated with TGF-β promoted the
survival of mature osteoclasts (8).
In addition, we demonstrated that specific inhibition of monocyte
chemoattractant protein-1 (MCP-1) in OSCC cells by transfection
with by 7 amino acids truncated (7ND), reduced osteoclast
differentiation in vitro, and the bone invasion area within
the calvariae of nude mice (9).
These studies revealed molecules with the potential to inhibit bone
invasion for future biotherapy.
In the present study, we used a calvaria bone
invasion mouse model of OSCC, to examine how osteoclast precursors
are recruited by tumour cells. First, we isolated BMCs from nude
mice and started primary osteoclast culture, which generated only a
few osteoclasts. Therefore, we used PBMCs and specific blood
centrifuge tubes to obtain large numbers of monocytes in
vitro. By MACS and the cytokines CSF and RANKL, human
osteoclasts were differentiated from CD14+ monocytes of
PBMCs. Bone resorption function was further explored. Finally,
Transwell inserts were utilized for indirect cell co-culture
between human OSCC cells and CD14+ monocytes. Expression
of specific osteoclast markers were detected by real-time PCR and
western blotting. The results suggested that CM of tumour cells can
induce the expression of osteoclast markers, and differentiation of
monocytes to mature osteoclasts to resorb adjacent bone.
Materials and methods
Reagent
Dulbeccos modified Eagles medium (DMEM), α-MEM,
fetal bovine serum (FBS), trypsin-EDTA, antibiotics and
phosphate-buffered saline (PBS) were purchased from Thermo Fisher
Scientific (Waltham, MA, USA). The primary antibodies were mouse
anti-human monoclonal matrix metalloproteinase-9 (MMP-9) and
anti-cathepsin K (CTSK; Santa Cruz Biotechnology, Santa Cruz, CA,
USA), rabbit anti-human polyclonal nuclear factor of activated T
cell 1 (NFATc1; Cell Signaling Technology, Danvers, MA, USA), and
mouse anti-human monoclonal α-tubulin (Abcam, Cambridge, UK). The
horseradish peroxidase (HRP)-conjugated secondary antibody was
supplied by Bio-Rad Laboratories (Hercules, CA, USA). Recombinant
murine and human cytokines CSF and RANKL were obtained from
PeproTech (Rocky Hill, NJ, USA). The TRAP staining kit was from
Sigma-Aldrich (St. Louis, MO, USA). The OSCC cell line SCC25 was
obtained from the American Type Tissue Collection (ATCC; Manassas,
VA, USA), and was maintained in DMEM with 10% FBS and antibiotics
(100 U/ml of penicillin G and 100 mg/ml of streptomycin) at 37°C in
an incubator (5% CO2/20% O2).
Establishment of animal model of bone
invasion using SCC25 cells
BALB/c nude mice were purchased from the animal
resources center, housed in an animal facility and cared for by
animal house staff. All protocols were reviewed and approved by the
university ethics committee (2016–334QX). At 6–7 weeks of age, the
mice were utilized to develop an animal model of bone invasion by
OSCC (9). Under sterile condition,
SCC25 (5×106 cells/100 µl) were injected subcutaneously
overlaying the calvaria. Body weight and tumour volume were
recorded each week. All animals were sacrificed at week 4, and BMCs
were obtained immediately for subsequent cell culture
experiments.
Micro-computed tomography (μCT)
imaging
All calvariae were surgically removed from SCC25
tumour-bearing nude mice, fixed in 70% ethanol and scanned using a
μCT instrument (Scanco Medica AG, Brüttisellen, Switzerland).
μCT-analyzer software was used to analyze the structure of the
calvaria using a global segmentation method. Two-dimensional images
were used for three-dimensional reconstruction. The resorpted area
of each calvaria was determined for analysis and quantification as
previously reported (9).
Histological and immunohistochemical
analysis
Tumour specimens from nude mice were embedded in
paraffin using a tissue processor. Serial 5-µm paraffin sections
were cut using a rotary microtome (Leica Microsystems, Wetzlar,
Germany) and stained with hematoxylin and eosin (H&E). For
tumour-bearing calvaria, all calvariae were decalcified in 10% EDTA
(pH 7.4) for 2 weeks and then processed for paraffin embedding.
Serial 5-µm sections were stained with both H&E and TRAP. TRAP
staining was performed using a standard protocol. Giant
TRAP-positive cells along the tumour-bone interface were considered
osteoclasts.
Primary osteoclast culture from bone
marrow mononuclear cells of nude mice
Nude model mice were sacrificed and dissected to
obtain humerus and tibia (10). All
cell culture experiments were conducted in α-MEM supplemented with
10% FBS and 1% penicillin-streptomycin, in a 5% CO2
atmosphere at 37°C. BMCs were flushed from the bone with medium and
then filtered through a 600-µm cell strainer. Cells were seeded in
150×25 mm cell culture dishes at a density of 1.5×104
cells/cm2 with 30 ng/ml murine CSF. After 2 days,
adherent cells were separated from non-adherent cells and plated in
24-well plates at a density of 1×105 cells/well.
Osteoclast-like cells were generated by adding murine CSF (30
ng/ml) and RANKL (35 ng/ml). After continuous culture for 10 days,
cells were fixed with 10% formaldehyde solution and TRAP-stained
according to the manufacturers instructions. Comparing with single
cells, these round and giant cells, strongly TRAP stained and three
or more cell fusion, were considered multinucleated osteoclasts.
Four fields were randomly selected and non-overlapping images were
taken for each of triplicate culture wells. In each image the total
numbers of TRAP-positive multinucleated osteoclasts were counted by
two independent assessors.
MACS of CD14+
monocytes
Human PBMCs were isolated from the blood of healthy
volunteers using BD Vacutainer cell preparation tubes (BD
Biosciences, Franklin Lakes, NJ, USA) containing sodium citrate
(9). Informed consent was obtained
from each patient. After centrifugation at 1,500 × g for 30 min,
the cell layer on top of the Ficoll-Paque was collected,
resuspended in 10 ml of α-MEM and centrifuged (1,250 rpm, 10 min).
CD14+ monocytes were purified by incubation with MACS
CD14+ MicroBeads (Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany) for 15 min at 4°C. Cells were then washed with
CD14+ isolation buffer [0.5% fetal calf serum (FCS), 2
mM EDTA, pH 8.0] and passed through a MACS cell separator.
CD14+ monocytes were collected and utilized for
subsequent experiments.
Osteoclast differentiation assay
CD14+ monocytes were plated in 24-well
plates (1×105 cells/well) containing 600 µl medium
(α-MEM, pH 7.4, containing 10% FBS and 1% penicillin/streptomycin)
in each well. To induce osteoclast differentiation, groups were
arranged as follows: Group 1, CD14+ monocytes only;
Group 2, CD14+ monocytes with CSF (25 ng/ml) and RANKL
(40 ng/ml). Total medium was changed every 3 days and osteoclasts
appeared in 1 week. Osteoclasts were subsequently fixed in 10%
formaldehyde solution. TRAP staining was used to characterize
osteoclasts. Comparing with single cells, these round and giant
cells, strongly TRAP stained and three or more cell fusion, were
considered multinucleated osteoclasts. Four fields were randomly
selected and non-overlapping images were taken for each of
triplicate culture wells. In each image the total numbers of
TRAP-positive multinucleated osteoclasts were counted by two
independent assessors.
Immunofluorescence
To further confirm staining of the F-actin ring,
immunofluorescence (IF) was utilized. Briefly, osteoclasts were
fixed in 10% formaldehyde solution, permeabilized with 0.5% Triton
X-100 and stained in the dark. Rhodamine-conjugated phalloidin
(Life Technologies, Carlsbad, CA, USA) was used to label F-actin.
DAPI staining (Life Technologies) was used to visualize the nuclei.
Cells were visualized using a fluorescence microscope. Four fields
were randomly selected and counted for the numbers of F-actin rings
by two independent assessors.
Bone resorption assay
To confirm bone resorption activity,
CD14+ monocytes were plated on dentin slices of 96-well
plates. After 21 days of culture with cytokines of CSF and RANKL,
mature osteoclasts were fixed in acetone, citrate and formaldehyde
solution and stained with TRAP. Bone resorption assays were
performed on dentine slices as previously described (12). Dentine slices were sputter-coated
with gold and observed with a scanning electron microscope. All
dentin slices were kindly provided by Dr Nigel A. Morrison from
Griffith University (Southport, Gold Coast, Australia).
Indirect cell co-culture between SCC25
cells and CD14+ monocytes
Transwell inserts (0.4-µm pore; Corning, Inc.,
Corning, NY, USA) were used for indirect cell co-culture. SCC25
cells (5×103 cells/well) were seeded in the upper
chamber, and human CD14+ monocytes (5×104
cells/well) were placed in the bottom of 24-well plates. The
chambers were incubated for 3 and 6 days, respectively. Cytokines
of CSF and RANKL were added to the bottom of 24-well plates, while
the negative control group contained no cytokines. The medium was
changed and new cytokine was added every 2 days; both RNA and
protein were extracted from monocytes at each of the tested
time-points.
Real-time PCR
Total RNA was isolated from monocytes using a
PureLink RNA Mini kit (Invitrogen, Carlsbad, CA, USA), and reverse
transcribed to cDNA using an iScript cDNA Synthesis kit (Bio-Rad
Laboratories) according to the manufacturers instructions.
Quantitative gene analysis was performed for NFATc1, MMP-9 and CTSK
using the Express SYBR GreenER qPCR Supermix Universal kit
(Invitrogen) and LightCycler 480 Real-time PCR system (Roche). Data
were normalized to the internal control GAPDH to obtain ∆Cq. The
fold change in genes of interest relative to untreated samples was
determined using the 2−∆∆Cq method (11). The primer sequences have been
previously reported (12,13).
Western blotting
Total protein was extracted from monocytes by using
lysis buffer (Thermo Fisher Scientific). Protein concentration was
determined with a BCA Protein Assay kit (Pierce, Rockford, IL,
USA). Next, 40 µg of protein was subjected to SDS-PAGE using 10%
poly-acrylamide gels. Proteins were transferred to polyvinylidene
fluoride membranes, and blocked with 5% non-fat dry milk in
Tris-buffered saline (TBS) for 1 h at room temperature. The
membranes were then incubated with primary antibodies for NFATc1
(1:500), MMP-9 (1:200), CTSK (1:200), and α-tubulin (1:3,000)
overnight at 4°C, washed twice, and incubated with horseradish
peroxidase-conjugated (HRP) secondary antibodies for 1 h at room
temperature. Protein bands were detected with SuperSignal West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific) and
visualized using VersaDoc MP Imaging Systems (Bio-Rad
Laboratories).
Differentiation of osteoclasts from
indirect cell co-culture
After co-culture for 3 and 6 days, CD14+
monocytes were fixed in 10% formaldehyde solution. TRAP and F-actin
staining were performed to characterize the osteoclasts.
TRAP-positive cells with three or more nuclei were considered as
multinucleated osteoclasts. Rhodamine-conjugated phalloidin was
used to stain F-actin, while DAPI was used to stain the nuclei.
Four fields were randomly selected and counted for osteoclast and
F-actin numbers by two independent assessors.
Statistical analysis
Results were presented as the mean ± standard error
(SE) of at least 3 independent experiments. Data analysis was
performed using SPSS Software (version 20.0; SPSS, Inc., Chicago,
IL, USA). Students t-test was used to compare two groups. One-way
analysis of variance was applied to compare two or more groups,
followed by Student-Newman-Keuls test. A P<0.05 was regarded as
statistically significant.
Results
Typical bone resorption in calvarial
bone invasion model of OSCC
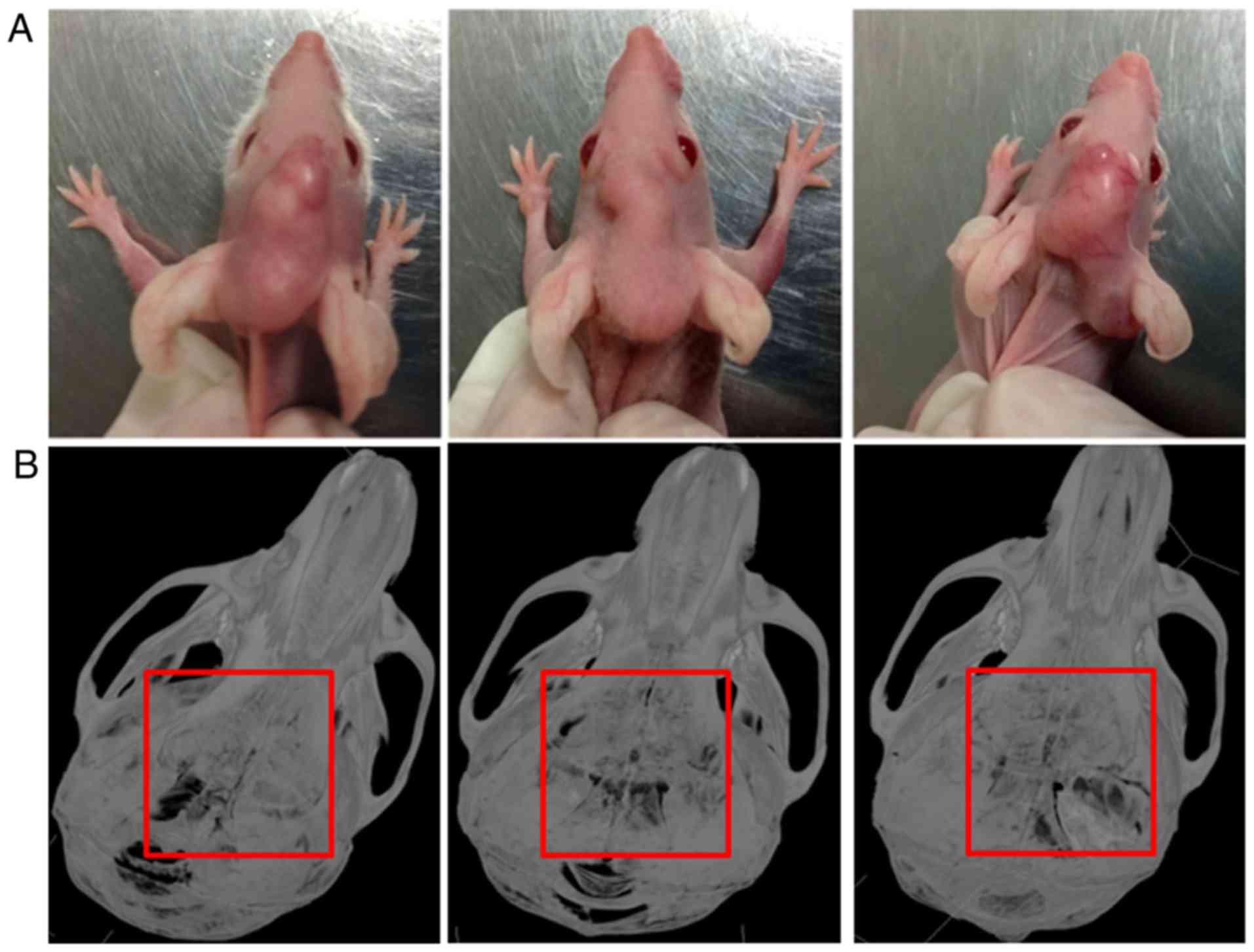
By injecting SCC25 cells into the center of
calvariae in nude mice, a local bone invasion animal model of OSCC
was established (Fig. 1A). All 6
nude mice showed typical bone resorption areas (Fig. 1B). No mice died during the
experiment.
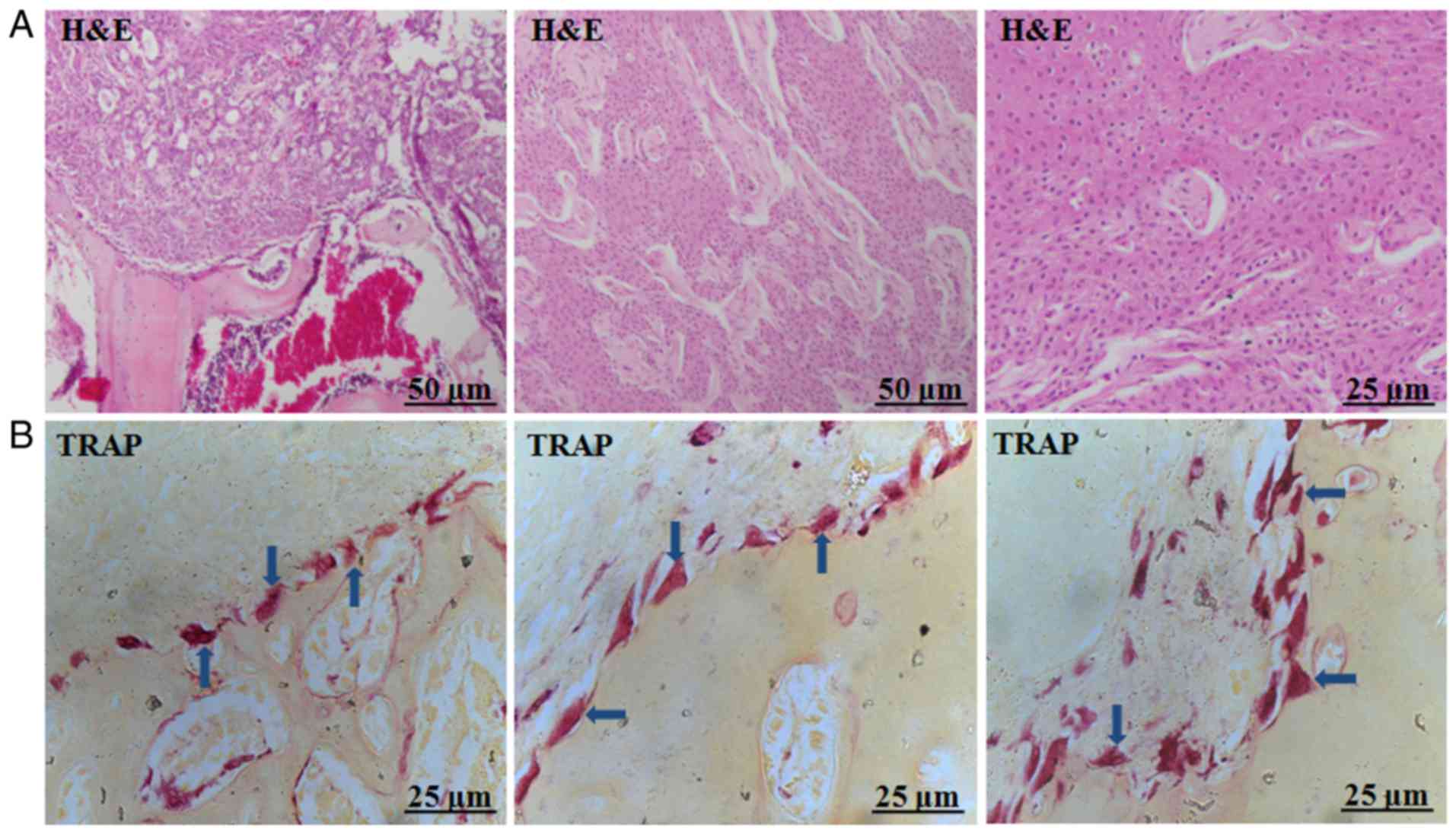
Various types of giant osteoclasts in
the tumour-bone interface
Histological analysis of tumour samples from the
animal model suggested that the formation of squamous cell
carcinoma in vivo, which invaded adjacent bone tissue
(Fig. 2A). TRAP staining revealed
various types of giant osteoclasts in the tumour-bone interface,
displaying different phenotypes including round, triangle, or
slabstone shapes (Fig. 2B).
Few osteoclasts were generated from
BMCs of nude mice
A primary osteoclast culture from BMCs was
established in vitro. BMCs were obtained from nude mice as
previously reported (10). However,
after continuous culture for 10 days, few osteoclasts were
generated in each of the 6 nude mice. TRAP staining observed
several round and giant osteoclast precursors (Fig. 3A). Normal control and tumour-bearing
nude mice showed similar results (Fig.
3B).
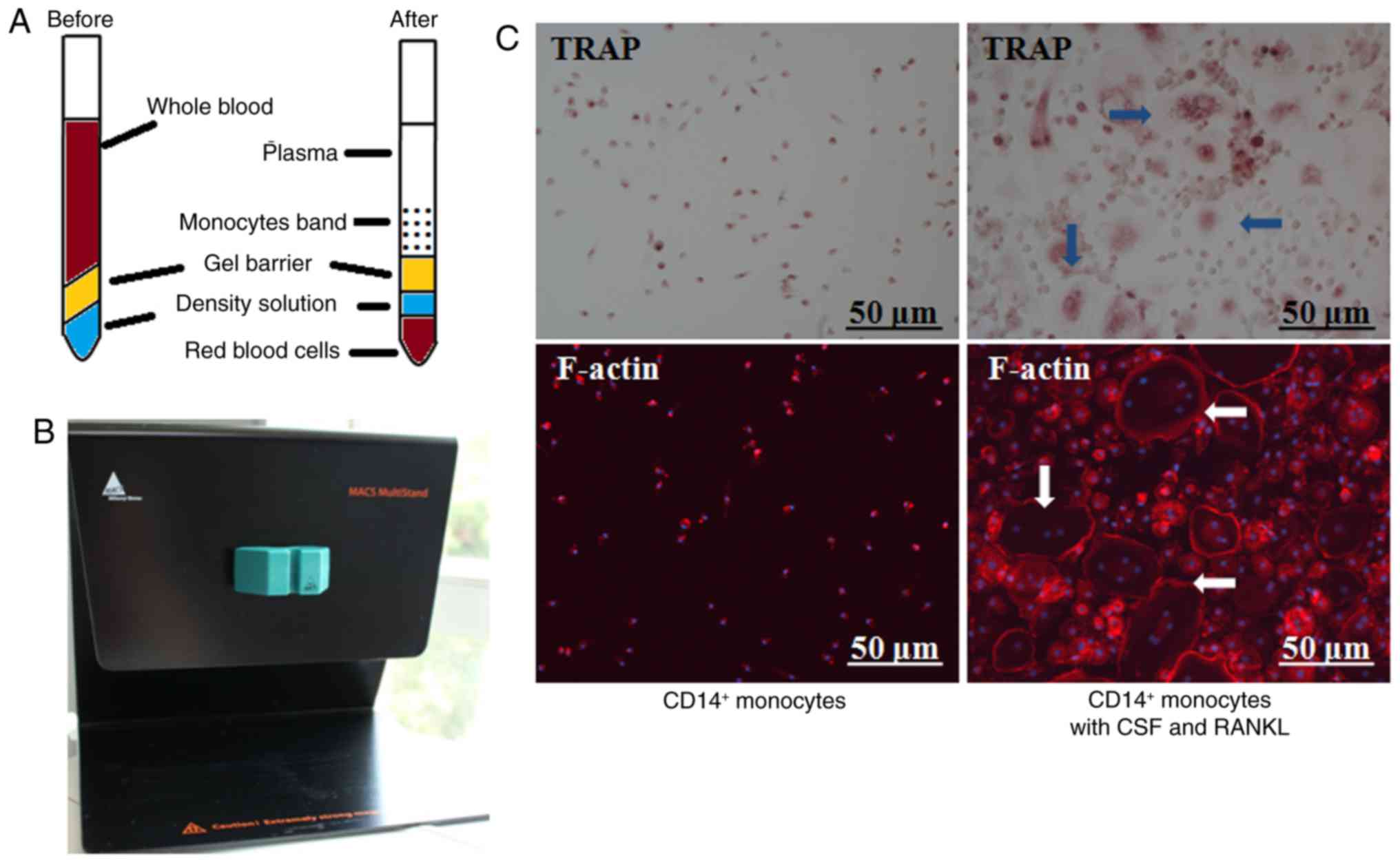
Special centrifuge blood tubes for
monocyte collection and primary human osteoclast culture by
MACS
Special centrifuge blood tubes were utilized to
enrich human PBMCs (Fig. 4A), which
were further labeled with a CD14+ magnetic antibody and
flowed through a MACS selector (Fig.
4B). In the presence of CSF and RANKL, osteoclasts
differentiated after continuous culture for 6 days. TRAP staining
indicated typical round and giant osteoclasts and IF confirmed the
formation of F-actin ring (Fig.
4C).
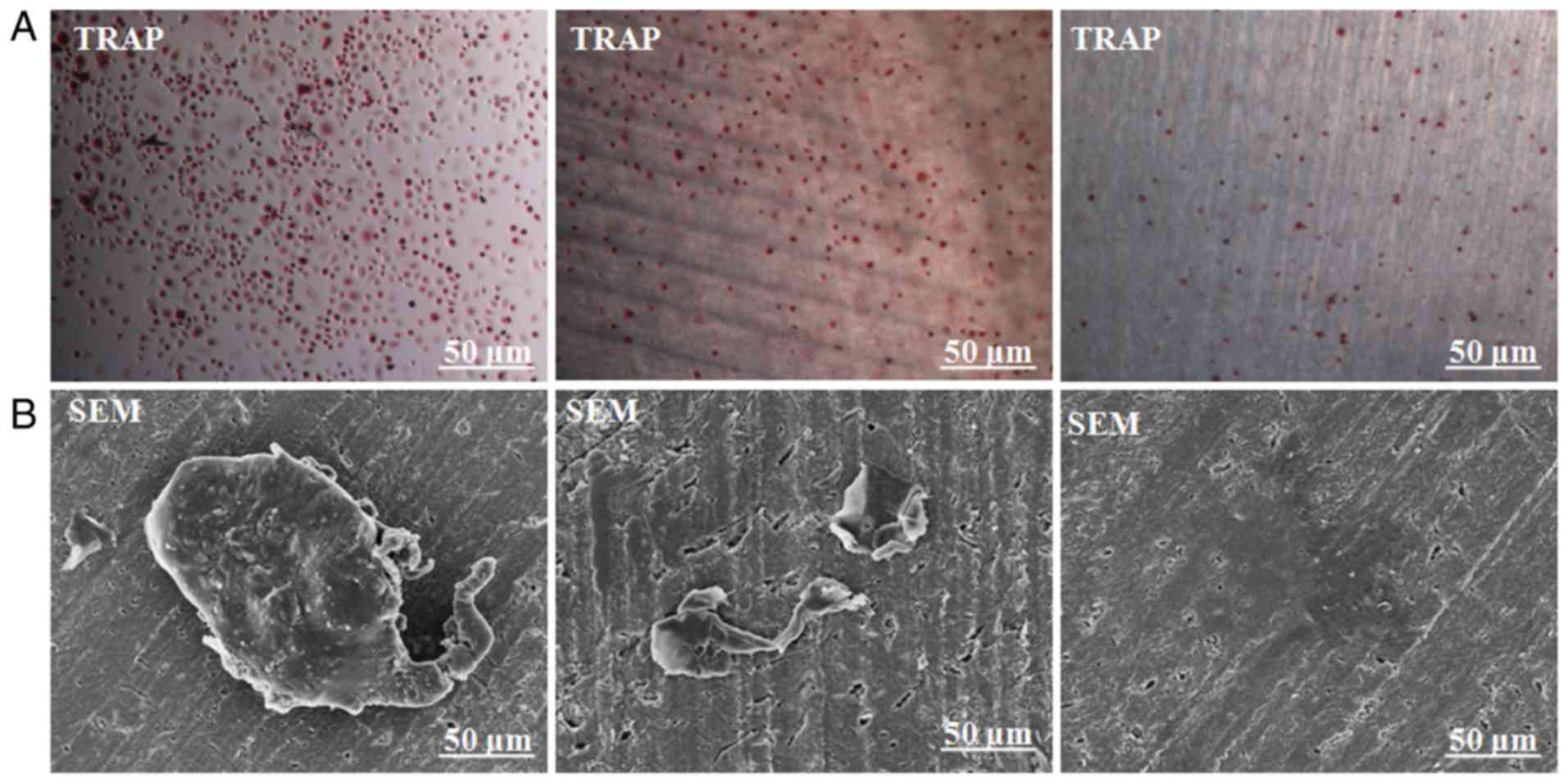
Scanning electron microscopy (SEM)
confirmed bone resorption pits of dentin slides degraded by
osteoclasts
To further confirm whether the obtained osteoclasts
could resorb bone, CD14+ monocytes were implanted on
dentin slides and cultured for 21 days. TRAP staining of both cells
with or without dentin slides suggested normal TRAP staining
(Fig. 5A). SEM further confirmed
the typical osteoclast morphology (Fig.
5B). Negative control slides showed CD14+ monocytes
only (Fig. 5B). Different
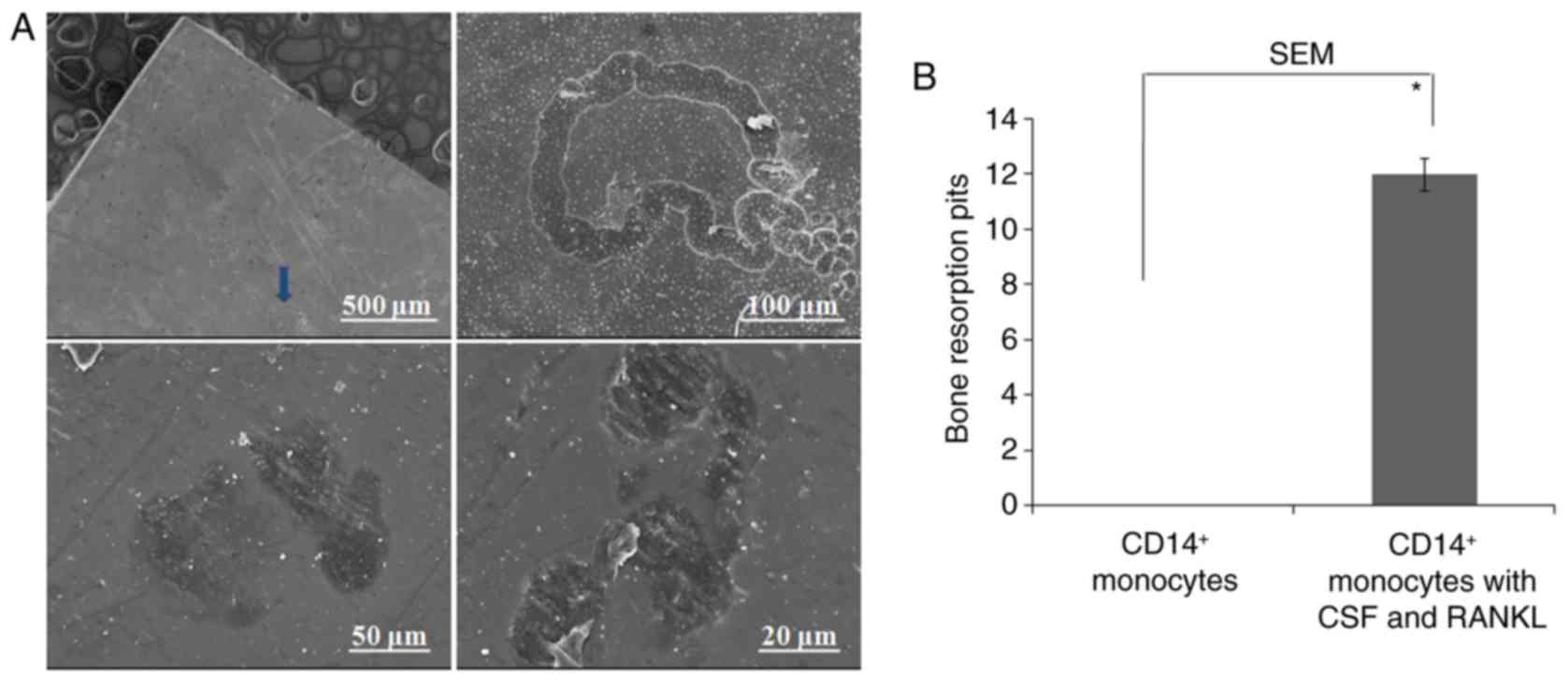
magnifications were utilized to observe resorption pits, which were
in the forms of either single, small resorption tracks or discrete
areas of lacunar excavations (Fig.
6A). No resorption pits were observed in the control group
containing only CD14+ monocytes (Fig. 6B).
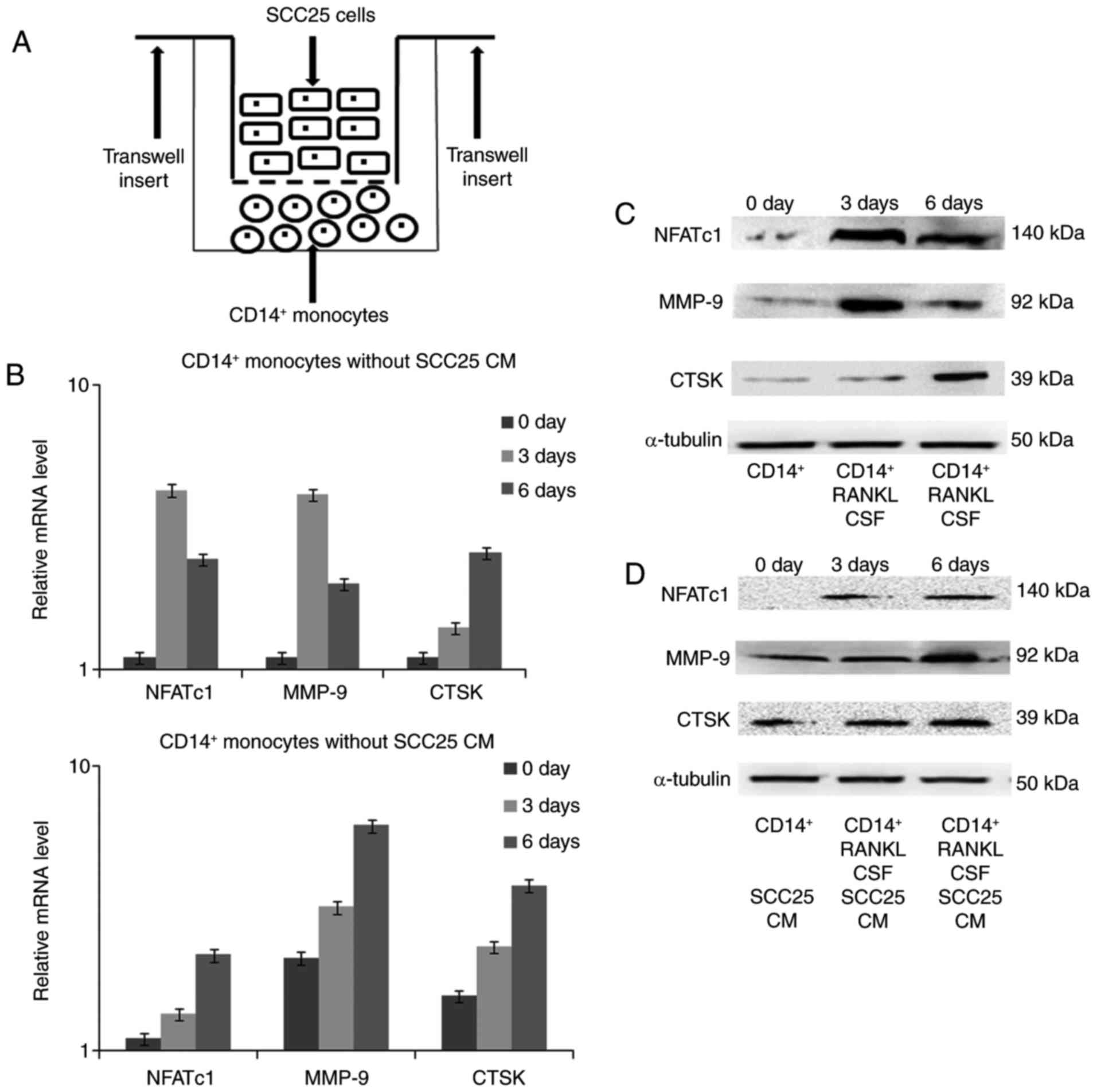
Expression changes in osteoclast
markers detected by real-time PCR and western blotting
The indirect co-culture model was established
between SCC25 cells and CD14+ monocytes (Fig. 7A). After co-culture for 3 and 6
days, RNA was extracted and real-time PCR was performed to detect
gene expression changes. In monocytes cultured without CM of SCC25
cells, the transcriptional factor NFATc1 was expressed from day 3
until day 6, and production of the proteinases MMP-9 and CTSK
increased on day 3 and day 6 (Fig.
7B), respectively. Similar expression trends for NFATc1 were
found in monocytes after stimulation with CM of SCC25. For MMP-9
and CTSK, CM of SCC25 stimulated expression from the start of
co-culture, which reached a maximum on day 6. Changes in the
protein levels of these targeted markers were further confirmed by
western blotting (Fig. 7C and
D).
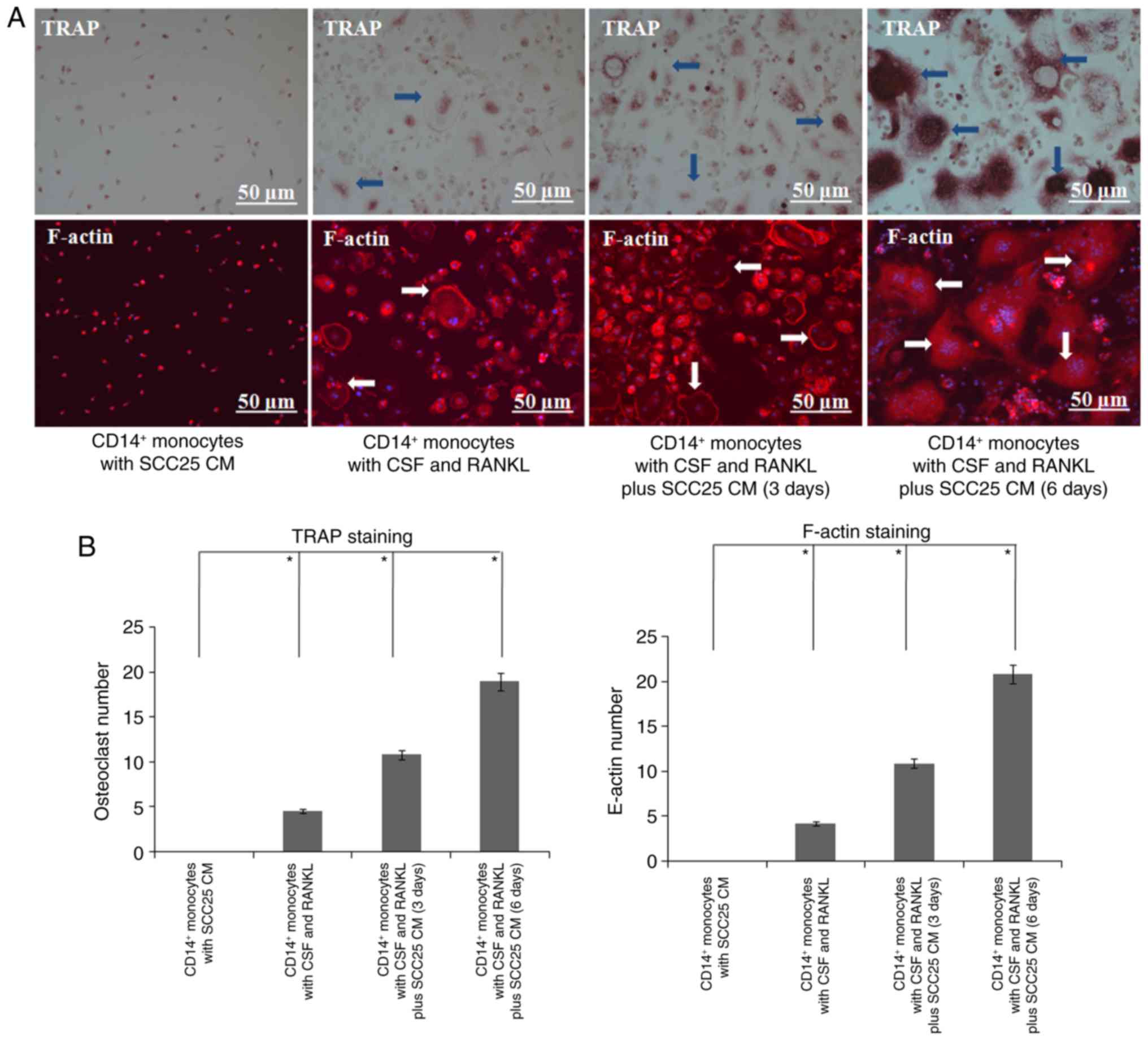
CM of SCC25 cells induced more
differentiation of human osteoclasts
To confirm the expression level changes of
osteoclast markers in human monocytes, an osteoclast
differentiation assay was further performed. Compared with the
control group, more osteoclasts were observed on day 3 after
stimulation with CM of SCC25 cells (Fig. 8). A great number of osteoclasts were
observed on day 6, and several differentiated osteoclasts were
linked together. Similar results were obtained in IF, and larger
numbers of F-actin rings were observed in both groups on days 3 and
6 (Fig. 8).
Discussion
The cultivation of osteoclasts in vitro is a
pre-requisite for studying osteoclastogenesis (14). Current methods for isolating
osteoclasts include using convenient immortal macrophage cell lines
and primary mononuclear cells isolated from bone marrow (13). Raw 264.7 and THP1 cells are
macrophage cell lines frequently used in osteoclast culture. Both
are tumour-derived cancer cell lines that can differentiate into
mature osteoclasts upon induction with RANKL (15). However, cell lines often fail to
mimic their primary counterparts (16), and not all observed changes are
relevant to osteoclast development in vivo. Primary
osteoclast culture from BMCs relies on the multiple differentiation
ability of these cells. Cells from the bone marrow are
heterogeneous and contain monocytes, blood cells, mesenchymal stem
cells and other multipotent progenitor cells (17). Therefore, it is difficult to
distinguish the exact cell types that differentiate into
osteoclasts. In the present study, we isolated BMCs from the bone
marrow of nude mice and attempted to differentiate them into
osteoclasts. However, we did not obtain a sufficient number of
osteoclasts for co-culture with tumour cells. The culture required
a long time and the cell number was limited, possibly because of
deficient proliferative and differentiative ability of BMCs from
nude mice. Thus, we used human PBMCs in the subsequent
experiments.
Numerous studies have reported that osteoclasts can
be generated from the PBMC populations, and these cells are
commonly isolated by apheresis and density gradient centrifugation
(18). However, this method is
time-consuming and can result in a mixed red blood cell lysis
solution (19). Currently, MACS is
widely used to purify specific cell populations from PBMCs
(20). In the present study, we
used a specific centrifuge tube to obtain increased numbers of
monocytes. As shown in Fig. 7, this
centrifuge tube could enrich the monocytes as a single layer after
centrifuging the blood, and was mainly composed of leukocytes,
which could be easily removed into the collection tube. After
washing and centrifugation, the collected monocytes were used for
magnetic labeling with MACS. This procedure was previously shown to
be more specific and less time-consuming than other methods
(19). Other factors affecting the
osteoclast culture using MACS method include the freshness of
peripheral blood, the centrifuging speed and temperature, the
quality and quantity of collected leukocytes, and the handling
skill of cell culture. Any of these factors can affect the
abilities of cell proliferation and cell fusion, which may lead to
the failure of osteoclast differentiation. In the present study
differentiated osteoclasts were obtained from CD14+
monocytes after 6 days of culture. More importantly, a bone
resorption assay was applied and dentin slices were observed after
20 days of continuous culture. Typical bone resorption pits in
dentin slices were observed at different magnifications.
Furthermore, a single giant osteoclast was observed and captured by
SEM, which revealed the typical structure of mature osteoclasts.
The ability to resorb the mineralized matrix is a crucial hallmark
of these cells (21). Bone
resorption occurs in intricate and dynamic patterns, which
facilitates the formation of complex bone shapes (1).
We next evaluated how tumour cells recruit
osteoclast precursors to adjacent bone tissue. For this, we
utilized Transwell inserts and established an indirect cell
co-culture. The co-culture system is a useful tool for stimulating
cell-cell communication, which occurs in the tumour
microenvironment (22). The
Transwell membrane allows secreted soluble factors to pass through,
but prevents direct cell contact (22). A previous study showed that 10% CM
of SCC25 cells induces the formation of osteoclasts within 1 week
of culture (9). However, expression
of marker genes was not examined. Osteoclast markers, including
TRAP, CTSK, c-FOS and NFATc1, are crucial for osteoclast
differentiation (13,23). The results of the present study
showed that the master transcriptional regulator NFATc1 was present
with the cytokines CSF and RANKL, and was increased on days 3 and
6. MMP-9 and CTSK were increased from days 3 to 6. MMP-9 and CTSK,
as the main proteinases, are typically observed during the late
stage of osteoclast differentiation (24,25).
After stimulating with CM of SCC25 cells, NFATc1 still appeared on
day 3 but was not observed in the initial culture. In contrast to
expression patterns observed without SCC25 CM, MMP-9 and CTSK
appeared and reached a maximum on day 6. Additionally, CM of SCC25
stimulated MMP-9 and CTSK expression, indicating anabolic effects.
The interactions between OSCC cells and osteoclast precursors
promote the production of cytokines that stimulate osteoclastic
cell function (22). The direct
cell co-culture between monocytes and tumour cells will be the
research plan of our future work. Based on published studies
(26–28), the direct cell co-culture has been
frequently utilized in studies seeking to understand the mechanisms
of cancer progression and the preclinical trials of anticancer
drugs (7). Since monocytes are
derived from leukocytes of human peripheral blood, and the direct
cell co-culture involves physical contact, whether these monocytes
would identify and eliminate tumour cells as innate immune response
is hard to know. Therefore, both the cell types and cell density
are extremely important for establishing the direct co-culture
model.
In summary, we observed different phenotypes of
osteoclasts by establishing a bone invasion animal model and
utilizing various primary cell cultures. Our results demonstrate
that CM of OSCC cells can be used to induce the differentiation of
osteoclasts from monocytes in the blood, with expression changes of
osteoclast markers. This information can be used in studies of bone
invasion by OSCC and may guide the development of biotherapies.
Acknowledgements
The present study was supported by the Medical
Scientific Research Foundation of Guangdong Province (B2014164) and
the National Natural Science Foundation of China (81500839).
References
|
1
|
Chambers TJ: The birth of the osteoclast.
Ann NY Acad Sci. 1192:19–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dou C, Cao Z, Yang B, Ding N, Hou T, Luo
F, Kang F, Li J, Yang X, Jiang H, et al: Changing expression
profiles of lncRNAs, mRNAs, circRNAs and miRNAs during
osteoclastogenesis. Sci Rep. 6:214992016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM
and Xie C: Osteoblast-osteoclast interactions. Connect Tissue Res.
8:1–9. 2017. View Article : Google Scholar
|
|
4
|
Strålberg F, Kassem A, Kasprzykowski F,
Abrahamson M, Grubb A, Lindholm C and Lerner UH: Inhibition of
lipopolysaccharide-induced osteoclast formation and bone resorption
in vitro and in vivo by cysteine proteinase inhibitors. J Leukoc
Biol. 101:1233–1243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin TJ and Sims NA: RANKL/OPG; Critical
role in bone physiology. Rev Endocr Metab Disord. 16:131–139. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quan J, Johnson NW, Zhou G, Parsons PG,
Boyle GM and Gao J: Potential molecular targets for inhibiting bone
invasion by oral squamous cell carcinoma: A review of mechanisms.
Cancer Metastasis Rev. 31:209–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quan J, Zhou C, Johnson NW, Francis G,
Dahlstrom JE and Gao J: Molecular pathways involved in crosstalk
between cancer cells, osteoblasts and osteoclasts in the invasion
of bone by oral squamous cell carcinoma. Pathology. 44:221–227.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quan J, Elhousiny M, Johnson NW and Gao J:
Transforming growth factor-β1 treatment of oral cancer induces
epithelial-mesenchymal transition and promotes bone invasion via
enhanced activity of osteoclasts. Clin Exp Metastasis. 30:659–670.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quan J, Morrison NA, Johnson NW and Gao J:
MCP-1 as a potential target to inhibit the bone invasion by oral
squamous cell carcinoma. J Cell Biochem. 115:1787–1798. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan UA, Hashimi SM, Bakr MM, Forwood MR
and Morrison NA: Foreign body giant cells and osteoclasts are TRAP
positive, have podosome-belts and both require OC-STAMP for cell
fusion. J Cell Biochem. 114:1772–1778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim MS, Day CJ, Selinger CI, Magno CL,
Stephens SR and Morrison NA: MCP-1-induced human osteoclast-like
cells are tartrate-resistant acid phosphatase, NFATc1, and
calcitonin receptor-positive but require receptor activator of
NFkappaB ligand for bone resorption. J Biol Chem. 281:1274–1285.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morrison NA, Day CJ and Nicholson GC:
Dominant negative MCP-1 blocks human osteoclast differentiation. J
Cell Biochem. 115:303–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong Q, Zhang L, Zhan S, Ge W and Tang P:
Investigation of proteome changes in osteoclastogenesis in low
serum culture system using quantitative proteomics. Proteome Sci.
14:82016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pivetta E, Wassermann B, Bulian P, Steffan
A, Colombatti A, Polesel J and Spessotto P: Functional
osteoclastogenesis: The baseline variability in blood donor
precursors is not associated with age and gender. Oncotarget.
6:31889–31900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Penolazzi L, Lolli A, Sardelli L,
Angelozzi M, Lambertini E, Trombelli L, Ciarpella F, Vecchiatini R
and Piva R: Establishment of a 3D-dynamic osteoblasts-osteoclasts
co-culture model to simulate the jawbone microenvironment in vitro.
Life Sci. 152:82–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szpalski C, Barbaro M, Sagebin F and
Warren SM: Bone tissue engineering: current strategies and
techniques - part II: Cell types. Tissue Eng Part B Rev.
18:258–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M and Huang B: The
multi-differentiation potential of peripheral blood mononuclear
cells. Stem Cell Res Ther. 3:482012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayer A, Lee S, Lendlein A, Jung F and
Hiebl B: Efficacy of CD14+ blood monocytes/macrophages
isolation: Positive versus negative MACS protocol. Clin Hemorheol
Microcirc. 48:57–63. 2011.PubMed/NCBI
|
|
20
|
Sprangers S, Schoenmaker T, Cao Y, Everts
V and de Vries TJ: Integrin αMβ2 is differently expressed by
subsets of human osteoclast precursors and mediates adhesion of
classical monocytes to bone. Exp Cell Res. 350:161–168. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castillo LM, Guerrero CA and Acosta O:
Expression of typical osteoclast markers by PBMCs after PEG-induced
fusion as a model for studying osteoclast differentiation. J Mol
Histol. 48:169–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teixeira LN, de Castro Raucci LM, Alonso
GC, Coletta RD, Rosa AL and de Oliveira PT: Osteopontin expression
in co-cultures of human squamous cell carcinoma-derived cells and
osteoblastic cells and its effects on the neoplastic cell phenotype
and osteoclastic activation. Tumour Biol. 37:12371–12385. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hemingway F, Cheng X, Knowles HJ, Estrada
FM, Gordon S and Athanasou NA: In vitro generation of mature human
osteoclasts. Calcif Tissue Int. 89:389–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khan UA, Hashimi SM, Khan S, Quan J, Bakr
MM, Forwood MR and Morrison NM: Differential expression of
chemokines, chemokine receptors and proteinases by foreign body
giant cells (FBGCs) and osteoclasts. J Cell Biochem. 115:1290–1298.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khan UA, Hashimi SM, Bakr MM, Forwood MR
and Morrison NA: CCL2 and CCR2 are essential for the formation of
osteoclasts and foreign body giant cells. J Cell Biochem.
117:382–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shabo I, Midtbö K, Andersson H, Åkerlund
E, Olsson H, Wegman P, Gunnarsson C and Lindström A: Macrophage
traits in cancer cells are induced by macrophage-cancer cell fusion
and cannot be explained by cellular interaction. BMC Cancer.
15:9222015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mercatali L, Spadazzi C, Miserocchi G,
Liverani C, De Vita A, Bongiovanni A, Recine F, Amadori D and
Ibrahim T: The effect of everolimus in an in vitro model of triple
negative breast cancer and osteoclasts. Int J Mol Sci. 1:17,
18272016.
|
|
28
|
Low HB, Png CW, Li C, Wang Y, Wong SB and
Zhang Y: Monocyte-derived factors including PLA2G7 induced by
macrophage-nasopharyngeal carcinoma cell interaction promote tumor
cell invasiveness. Oncotarget. 7:55473–55490. 2016. View Article : Google Scholar : PubMed/NCBI
|