Introduction
Bladder cancer remains one of the most commonly
diagnosed urological malignancies worldwide (1). Approximately one-fourth of patients
present with muscle-invasive bladder cancer (MIBC) at diagnosis.
The outcomes for MIBC are poor and progress in regards to the
treatment of bladder cancer has been stagnant for decades (2). Recently, The Cancer Genome Atlas
(TCGA) project revealed the whole genomic characteristics of
bladder cancer based on next generation sequencing (3). This provided new insight into the
understanding of the molecular features of bladder cancer.
YWHAZ, a gene that encodes 14-3-3ζ, has been
demonstrated to be overexpressed in some MIBC patients (4). 14-3-3 proteins are a family of
conserved regulatory molecules that bind to phosphorylate serine or
threonine residue on their target molecules. Through 14-3-3
protein's binding ability, it regulates critical proteins in a
variety of cell processes. To date, seven different isoforms (β, γ,
ε, σ, η, τ and ζ) have been identified (5). The role of 14-3-3ζ in cancer remains
controversial. The overexpression of 14-3-3ζ is generally
associated with malignant biological behavior and worse survival
outcomes in many other tumor types, such as prostate, lung and
breast cancer (6–8). However, in other cancer types such as
squamous cell carcinoma of the tongue, 14-3-3ζ silencing also
retards tumor growth (9).
In our previous study, we revealed that the
amplification of YWHAZ, along with TP53 or
CDKN2A loss predicted better survival (10). This finding demonstrated that YWHAZ
may play an antitumor effect in bladder cancer development.
Therefore, in the present study, we aimed to investigate the
function of YWHAZ by downregulation of its expression both
in vitro and in vivo, and its related pathway was
also explored in silico through gene set enrichment analysis
(GSEA).
Materials and methods
Data mining and analysis in the TCGA
database
An in silico reproduction using the TCGA
database was performed in the present study, as previously
described (11–13). The TCGA bladder cancer
(provisional), pan lung cancer (provisional), invasive breast
cancer (provisional) and prostate adenocarcinoma (provisional) was
respectively chosen on the cBioPortal online platform (www.cbioportal.org). Cases with YWHAZ alteration were
queried. The ‘Survival’ function was used to plot Kaplan-Meier
curves for overall survival and disease-free survival.
Cell culture
Two MIBC cell lines, T24 and 5637, were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). The cells were cultured in RPMI-1640 medium (Life
Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and
0.1 mg/ml streptomycin. The cells were maintained in an atmosphere
of 5% CO2 at 37°C.
RNA extraction and real-time
quantitative PCR (qPCR)
Total cellular RNA was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Reverse transcription of RNA was
carried out using a M-MLV Reverse Transcription kit (Promega,
Madison, WI, USA) according to the manufacturer's instructions. The
relative expression level of YWHAZ was determined by the real-time
quantitative PCR using SYBR Premix Ex Taq II (Takara, Tokyo,
Japan). The primer was synthesized by Shanghai Genechem Co., Ltd.
(Shanghai, China) for YWHAZ (forward primer, AGCCATTGCTGAACTTGATACA
and reverse primer, AATTTTCCCCTCCTTCTCCTG); and reference gene
(GAPDH) (forward primer, TGACTTCAACAGCGACACCCA and reverse primer,
CACCCTGTTGCTGTAGCCAAA). The 2−ΔΔCt method was used to
calculate the relative expression ratio of YWHAZ.
Lentiviral RNA interference
Lentivirus encoding YWHAZ shRNA was generated by
Shanghai Genechem Co., Ltd. Lentivirus expressing scramble shRNA
were used as negative control (NC). Cells were plated in 12-well
plates (1×105 cells/well), transduced with 5 MOI
lentiviral particles [using 8 µg/ml hexadimethrine bromide
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)] and incubated at
37°C with 5% CO2. Suppression of YWHAZ in stable cells
was confirmed by qPCR.
Cell proliferation assay
Cell viability in each group was measured by using
MTT assay. The cells were seeded into 96-well plates at a density
of 2×104 cells/well, cultured overnight and treated as
mentioned above. Next, 10 µl of 5 mg/ml MTT was added to each well.
After 4 h of further incubation, dimethyl sulfoxide (DMSO) was
added to solve the formazan, then the optical density at 490 nm of
each well was measured with a microplate reader. Cell proliferation
ability was analyzed daily for 5 consecutive days.
Colony-formation assay
A total of 10 ml complete medium containing 200
cells were added to each well of a 6-well plate. The plates were
incubated at 37°C in 5% CO2 for 2 weeks. Next, the cells
were washed with phosphate-buffered saline (PBS) solution and fixed
by 4% paraformaldehyde, and then were stained with Giemsa for 10
min. Colonies containing at least 50 cells were counted under a
microscope (XDS-100; Carl Zeiss, Göttingen, Germany).
Cell migration and invasion
assays
Migration and invasive abilities were assessed using
a cell invasion assay kit (Chemicon International, Inc., Temecula,
CA, USA). Cells (5×104 cells/well) were cultured in the
upper chamber of the 8.0-µm pore-size cell culture inserts that
were either coated or uncoated with Matrigel for migration and
invasive assays, respectively. The insets were then placed in a
24-well plate filled with medium with 5% FBS. The cells that
penetrated to the underside of the surfaces of the inserts were
fixed and stained with the Diff-Quick method (Thermo Fisher
Scientific, Inc.) and were counted under the microscope (XDS-100;
Carl Zeiss). The mean cell number of three high power fields at
×100 magnification for each condition was calculated. Assays were
repeated at least three times.
Wound healing assay
When the cells reached 95% confluence in 6-well
plates after transfection, a 200-µl pipette tip was used to draw a
wound with the same width. After washing and removing the cell
debris, the cells were cultured using a serum-free medium under
standard conditions. The same area of the gap was taken at ×100
magnifications using a microscope equipped with a digital camera
(Olympus Corp., Centre Valley, PA, USA) at 0, 4, 8 and 24 h after
scratching, and the scratch width was quantified using ImageJ
software (http://rsbweb.nih.gov/ij/). The
migration rate was calculated as the difference between the initial
width and the width at the different time-points.
Cell cycle assay
Cells were plated in 25-cm2 flasks and
incubated overnight. The cells were then collected and fixed in
pre-cold 70% ethanol for 1.5 h at 4°C. After fixation, the cells
were washed in PBS again and centrifuged for 5 min at 1,000 rpm.
The PBS was discarded and propidium iodide (PI) was added to a
final concentration of 50 µg/ml in dark at 4°C for 30 min. Flow
cytometric analysis was performed on the FACSCalibur flow cytometer
(Becton-Dickinson; BD Biosciences, San Jose, CA, USA). The cell
cycle was analyzed using CellQuest software (BD Biosciences).
Cell apoptosis assay
The cells were plated in 25-cm2 flasks
and were incubated overnight. The cells were then collected and
adjusted with staining buffer at a density of 106
cells/ml. After incubation with 5 µl of Annexin V-FITC and 10 µl of
PI in dark for 15 min, the cells were analyzed by flow cytometry
(Becton-Dickinson). Apoptotic cells were recognized as having a
high Annexin V fluorescence signal with a low PI signal. The
percentages of apoptotic cells were calculated by data from FACS
analysis.
Bladder cancer xenograft model
Twelve male BALB/c nude mice at 6 weeks of age,
weighing 18–22 g, were obtain from SLAC Laboratory Animal Co., Ltd.
(Shanghai, China) and were bred in a special pathogen-free (SPF)
grade laboratory in Fudan University. The mice were housed
5–10/cage, in a 12:12-h light:dark cycle environment, with ad
libitum access to food and water. Mice were randomly divided
into 2 groups (T24; treatment vs. control). A total of
107 cancer cells resuspended in 100 ml of PBS were
injected subcutaneously at the left axilla of each mouse. Tumors
became perceptible at ~5 mm in diameter on day 7. Thereafter,
intratumoral injection of 50 µl of the lentivirus at 108
U/ml or control (PBS) with the same volume was administered. All
mice were sacrificed on day 35 and the tumors were extracted. The
tumor size was calculated with the following formula: Volume =
length × width2/2. All experimental protocols were
approved by the Institutional Review Board of the Department of
Laboratory Animal Science of Fudan University (Shanghai,
China).
Immunohistological analysis and
assessment of Ki-67
Firstly, 2-µm unstained xenograft tumor sections
from formalin-fixed paraffin embedded (FFPE) tissue were
deparaffinized in xylene and rehydrated through graded alcohols.
After antigen retrieval, the sections were stained with primary
antibody, Ki-67 (dilution 1:200; 28-9; Abcam), followed by antibody
localization using the Dako EnVision+ System-HRP labelled polymer
(Dako; Agilent Technologies, Inc., Santa Clara, CA, USA). Staining
was visualized by a 5-min incubation with diaminobenzidine.
The staining patterns were assessed by two
independent pathologists using standard light microscopy (XDS-100;
Carl Zeiss). Positive staining was considered when >10% of tumor
cells showed reactivity. Stain intensity and the percentage of
tumor cells stained were categorized as <25%, 25–50%, >50–75%
and >75% according to the number of positive tumor cells
stained.
GSEA of TCGA data
To investigate the YWHAZ overexpression and
the cancer-related pathway, GSEA was performed using TCGA bladder
cancer (provisional) level 3 RNASeq V2 datasets. This analytical
technique is designed to test a priori defined gene sets for
association with phenotypes (14).
KEGG gene sets (v6.0) and phenotype label files were created and
loaded into GSEA software (v2.0.13; Broad Institute, Cambridge, MA,
USA). Cases with YWHAZ mRNA expression were queried on the
cBioPortal online platform. The phenotype label was YWHAZ
overexpression vs. YWHAZ normal expression based on the
cBioPortal platform. The number of permutations was set to 1,000. A
ranked-list metric was generated by calculating the signal-to-noise
ratio, which is based on the difference of means scaled according
to the standard deviation.
Statistical analysis
Statistical analysis was performed using IBM SPSS
version 20.0 (IBM SPSS, Armonk, NY, USA). Data are shown as means ±
SD. The difference between two groups was analyzed using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
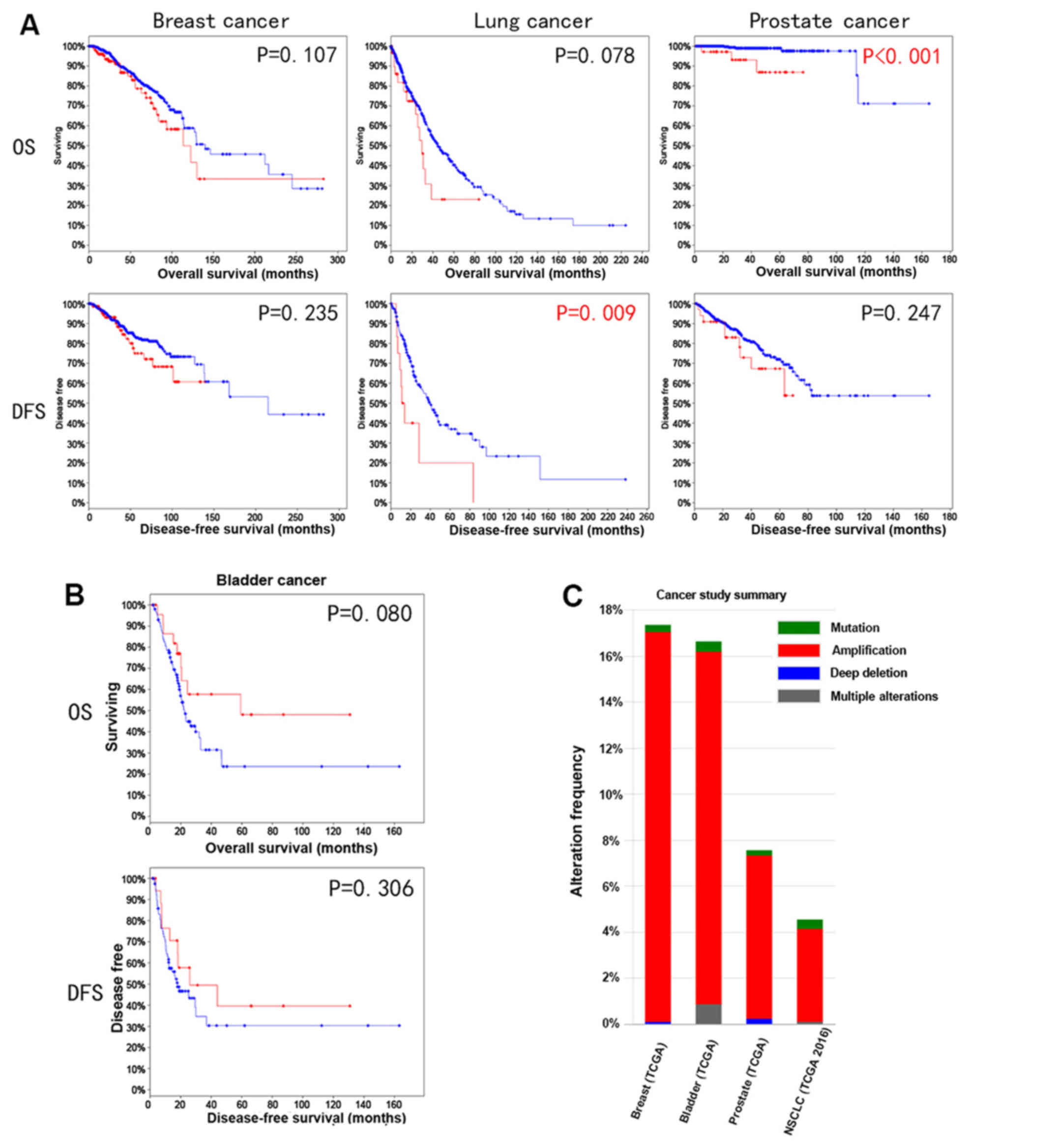
YWHAZ amplification indicates a better
survival trend in bladder cancer
Since YWHAZ amplification is commonly
observed in many types of cancer, such as breast, lung and prostate
cancer, we therefore investigated its correlation with survival
outcomes among these types of cancer. Amplification was determined
using algorithm GISTIC value as ≥2 according to the TCGA
definition. Amplification was found in 35/442 (7%) patients with
prostate cancer, 47/1,144 (4%) patients with lung cancer, 180/1,080
(17%) patients with breast cancer and 69/408 (17%) patients with
bladder cancer (Fig. 1C). A worse
survival trend among breast, lung and prostate cancer both in
overall survival and disease-free survival was noted (Fig. 1A). Of note, lung cancer patients
with YWHAZ amplification had significantly worse
disease-free survival compared with those without YWHAZ
amplification (P=0.009). Similarly, the overall survival defects in
prostate cancer patients with YWHAZ amplification also
reached statistical significance (P<0.001). In bladder cancer,
there was a better survival trend for patients with YWHAZ
amplification. Specifically, the significance for overall survival
was marginal (P=0.08) (Fig.
1B).
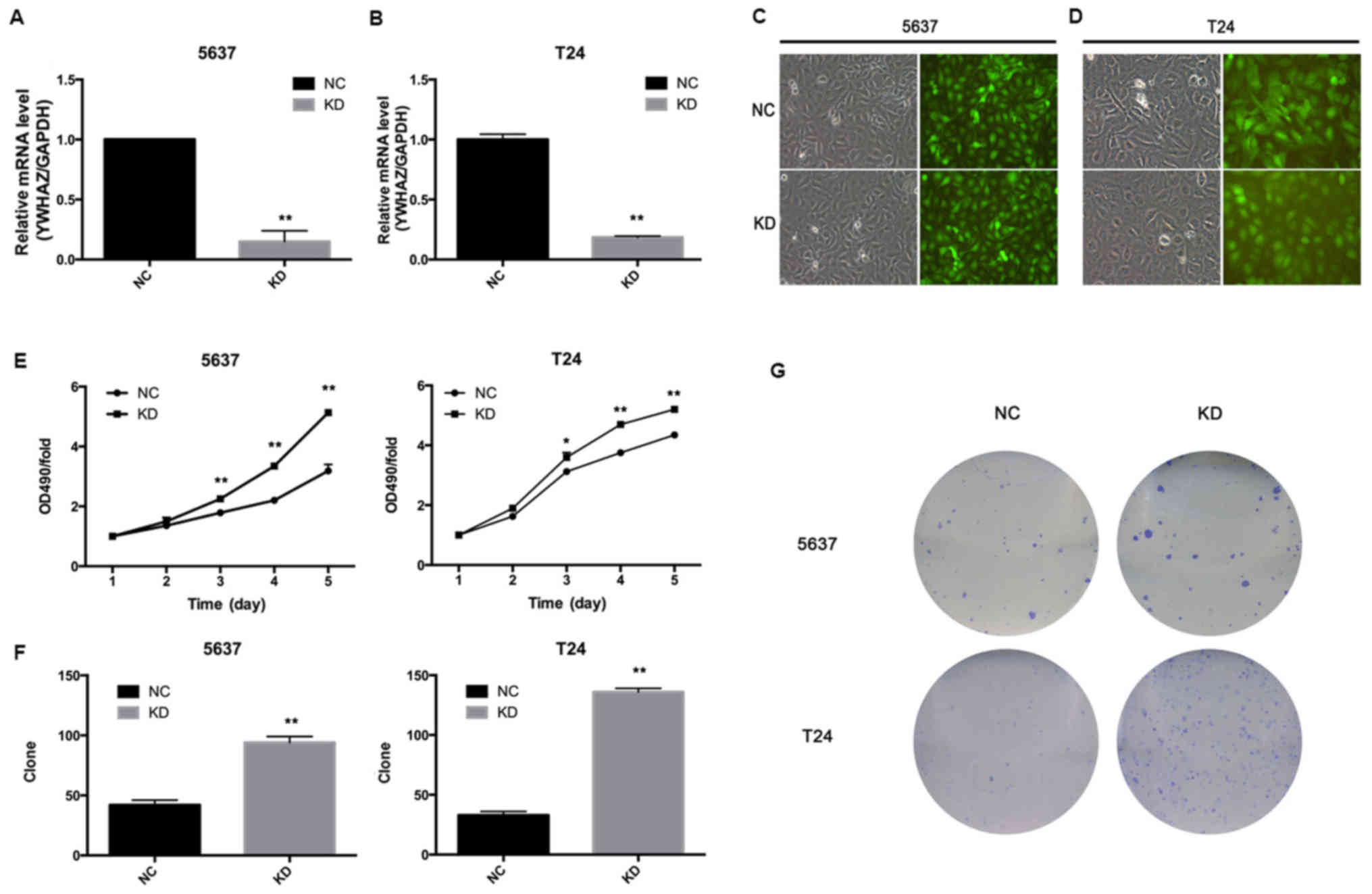
Downregulation of YWHAZ enhances
bladder cancer cell proliferation and colony number
Next, we investigated the effect of YWHAZ on
bladder cancer cell growth, and we infected T24 and 5637 cells with
Lenti-YWHAZ (knockdown, KD group) or Lenti-NC (negative
control, NC group). Transfection of 5637 and T24 cells with
Lenti-YWHAZ significantly inhibited YWHAZ gene
expression (P<0.05) (Fig. 2A and
B). Fluorescence was observed using fluorescence microscope 96
h after infection with the indicated lentivirus (Fig. 2C and D). MTT assay demonstrated that
the proliferation ability of the T24 and 5637 cells infected with
Lenti-YWHAZ was markedly enhanced compared with the the
Lenti-NC-infected group 3 days after infection (Fig. 2E). Colony-formation assay showed
that the colony numbers of the T24 (136±3 vs. 33±3; P<0.01) and
5637 (94±5 vs. 42±4; P<0.01) cells infected with
Lenti-YWHAZ were significantly higher compared with these
values in the Lenti-NC-infected group (Fig. 2F and G). Therefore, it is suggested
that knockdown of YWHAZ enhances bladder cancer cell
proliferation.
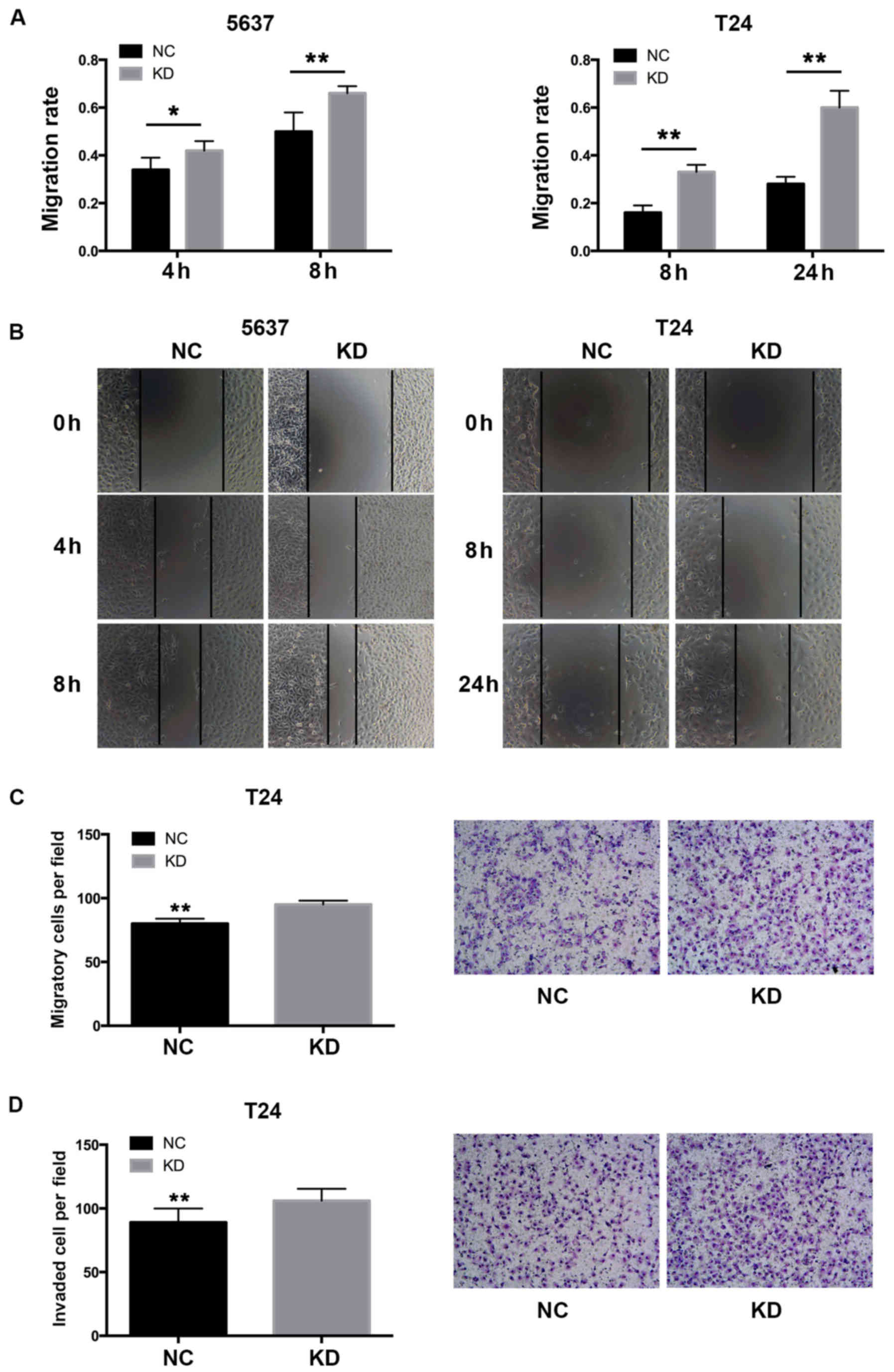
Downregulation of YWHAZ enhances the
migration and invasiveness of bladder cancer cells
To investigate whether the overexpression of
YWHAZ has any influence on the migration ability of bladder
cancer cells, wound healing assays were performed. Knockdown of
YWHAZ significantly enhanced the migration rate of the
bladder cancer cells in both the T24 (0.33±0.03 vs. 0.16±0.03 at 8
h, P<0.01; 0.6±0.07 vs. 0.28±0.03 at 24 h, P<0.01) and 5637
(0.42±0.04 vs. 0.34±0.05 at 4 h, P=0.03; 0.66±0.03 vs. 0.5±0.08 at
8 h, P<0.01) cells (Fig. 3). Of
note, T24 cells with downregulated YWHAZ demonstrated almost
doubled migration capacity compared to the respective controls. We
further performed Transwell assays to investigate the migration and
invasive ability of the T24 bladder cancer cells. The result showed
that the Lenti-YWHAZ group had significantly elevated
migrated (95±3.02 vs. 80±3.94; P<0.01) and invasive cells
(88±7.02 vs. 106±5.76; P<0.01) compared with the Lenti-NC
infected group (Fig. 3). These
results demonstrated that YWHAZ downregulation significantly
contributed to the migration and invasiveness of bladder cancer
cells.
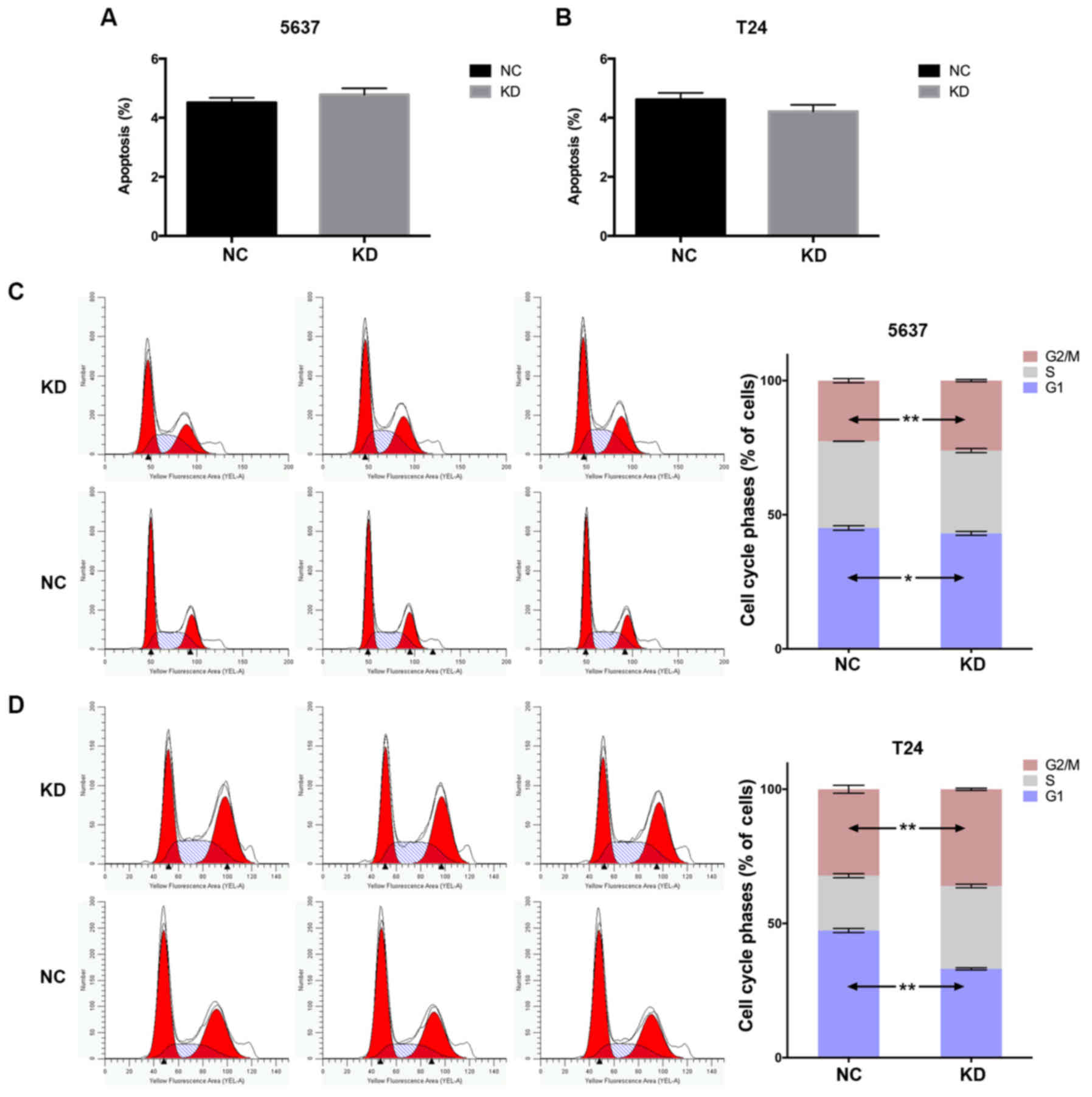
YWHAZ knockdown promotes cell cycle
progression
To address the mechanisms underlying YWHAZ
downregulation of proliferation and migration of bladder cancer
cells, we further investigated the influence of YWHAZ
knockdown on apoptosis. The percentage of apoptotic cells were
comparable with those of the Lenti-NC infected group (P>0.05)
(Fig. 4A and B). Furthermore, the
cell cycle profile was analyzed through flow cytometry. The
distribution of cell cycle phase was significantly different
between two groups in both the T24 and 5637 cell lines. The
percentage of cells in G1 phase infected with Lenti-YWHAZ
were significantly lower than those of cells infected with
Lenti-NC. An obvious decrease in the G1 phase (45.08±0.84 vs.
43.06±0.73%; P=0.03) and a significant increase in G2/M (26.07±0.41
vs. 22.58±0.78%; P<0.01) cell populations were observed compared
with the control group in the 5637 cell line. Similarly, a
significant decrease in the G1 phase (45.08±0.84 vs. 43.06±0.73%;
P<0.01), while a significant increase in S phase (30.85±0.68 vs.
20.45±0.75%; P<0.001) and G2/M (36.04±0.41 vs. 32.19±1.48%;
P=0.001) cell populations were observed compared with the control
group in the T24 cell line (Fig. 4C and
D). These results demonstrated that YWHAZ knockdown
significantly promoted G1/S transition.
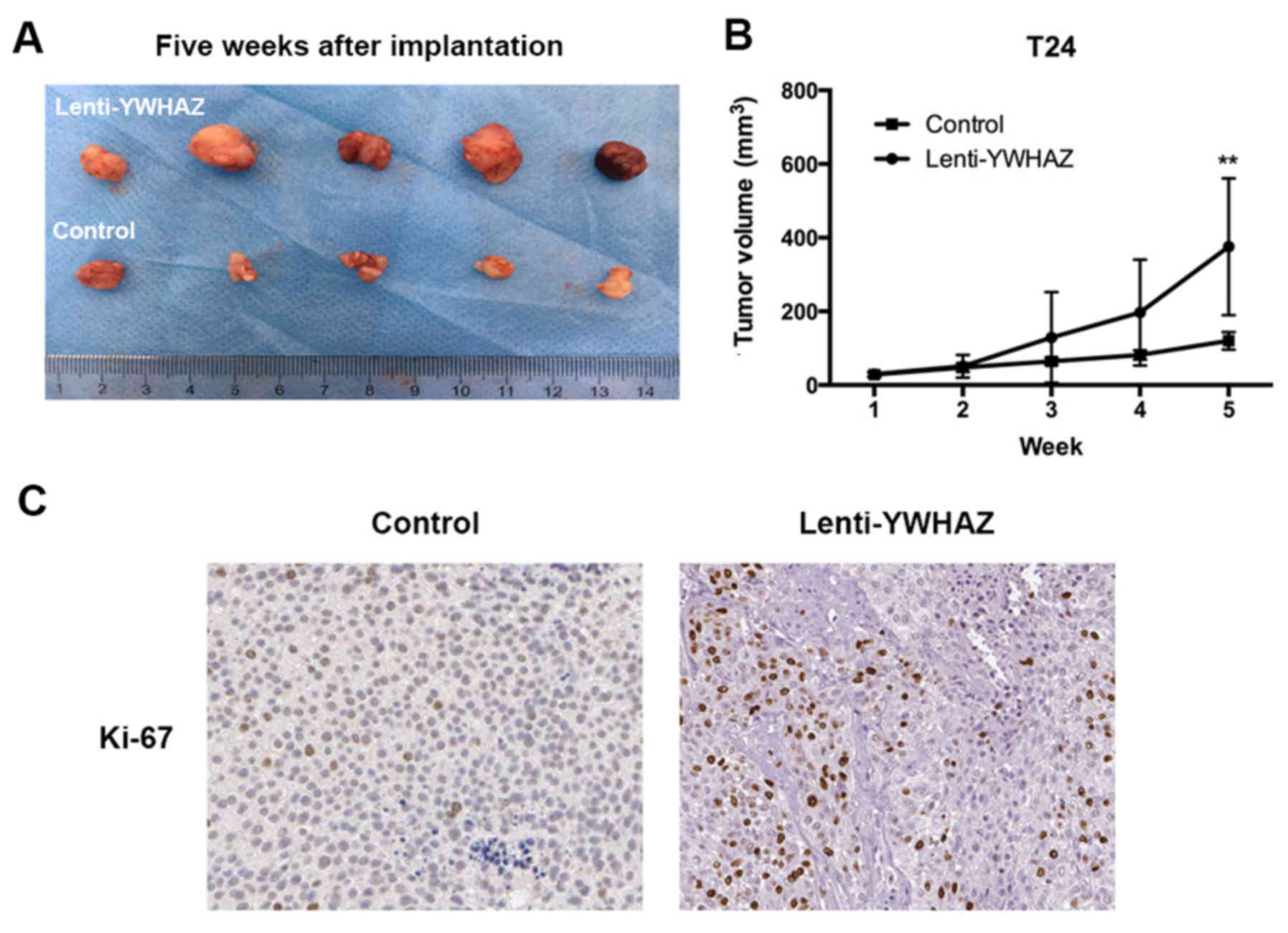
YWHAZ downregulation promotes tumor
growth in vivo
To investigate the effect of YWHAZ
downregulation on antitumor activities in vivo, we
established a T24 xenograft tumor model in nude mice. Assessment of
tumor volume indicated that the YWHAZ downregulation group
had enhanced tumor growth compared to the control group and the
group treated with PBS (Fig. 5A and
B). The xenografts were further evaluated by immunohistology in
relation to the proliferation marker Ki-67. The results
demonstrated that the percentage of Ki-67-positive cells was higher
in the YWHAZ downregulation group than that noted in the
control group (Fig. 5C and Table I).
 | Table I.Comparison of immunoexpression of
tumor marker Ki-67 in the YWHAZ-knockdown and control group
in the xenograft model. |
Table I.
Comparison of immunoexpression of
tumor marker Ki-67 in the YWHAZ-knockdown and control group
in the xenograft model.
| Ki-67 expression
levels (%) | Lenti-YWHAZ
group (no. of tumors) | Control group (no.
of tumors) | P-value |
|---|
| <25 | 0 | 0 | 0.02 |
| 25–50 | 1 | 5 |
|
| >50–75 | 3 | 1 |
|
| >75 | 2 | 0 |
|
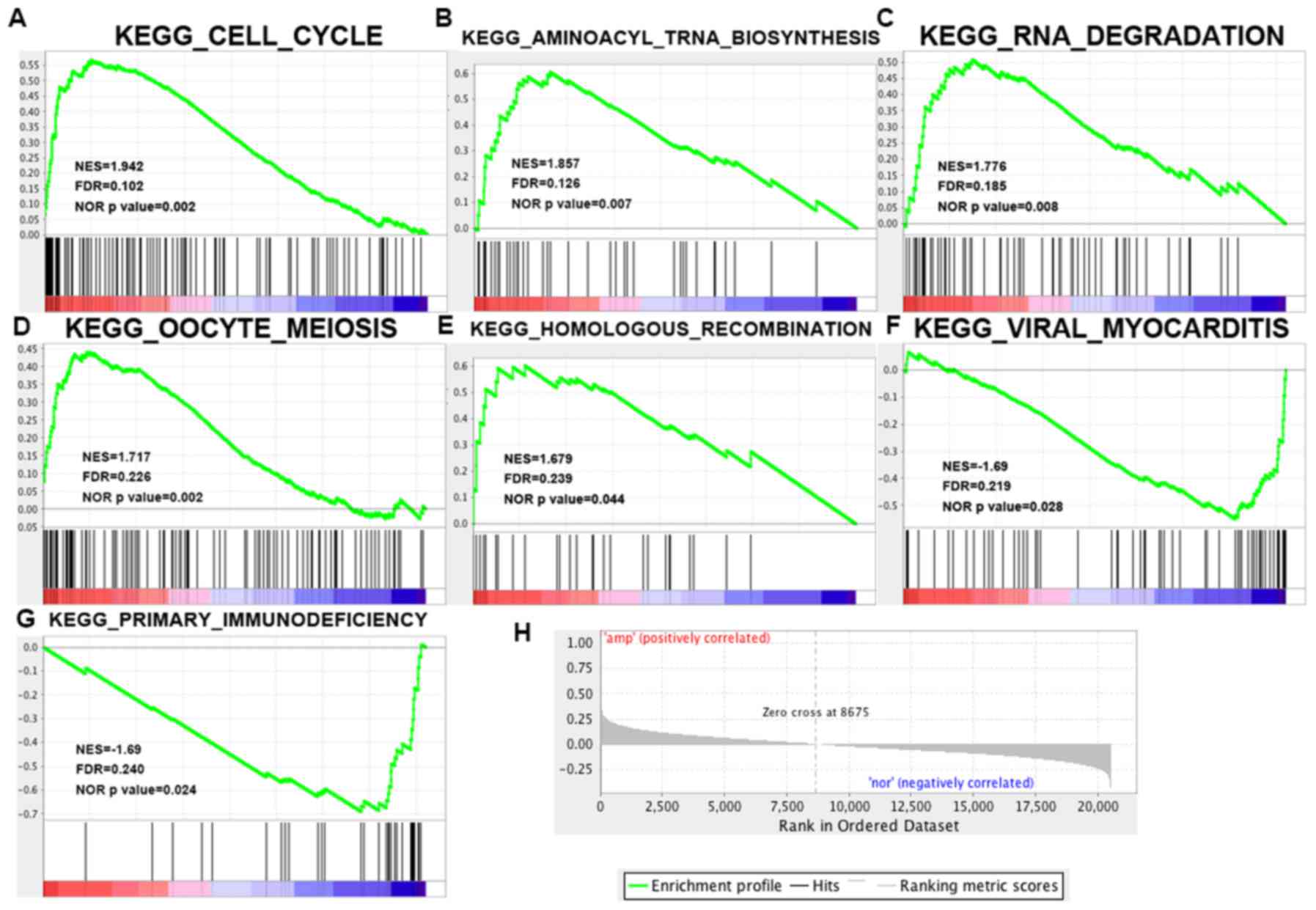
YWHAZ may exert its function through
the cell cycle pathway
GSEA was performed to determine the associations
between YWHAZ expression and cancer-related pathways based
on KEGG gene sets. As a result, 60/178 gene sets were found to be
upregulated in the YWHAZ overexpression phenotype with 5
gene sets significant at FDR <25% and 4 gene sets significantly
enriched at nominal P<0.01. Of the YWHAZ
normal-expression phenotype, 118/178 gene sets were upregulated
with 2 gene sets significantly enriched at FDR <25% and 2 gene
sets significantly enriched at nominal P<0.01. The enrichment
plot of 7 upregulated gene sets is shown in Fig. 6. Of these, the cell cycle pathway
shows the most relative correlation with the smallest normal
P-value 0.002 and FDR 0.102.
Discussion
In the present study, we found that YWHAZ
downregulation dramatically promoted tumorigenesis both in
vitro and in vivo. This finding suggests that
YWHAZ functions as a tumor-suppressor gene and it could be a
potential biomarker for bladder cancer. To the best of our
knowledge, scarce studies have focused on YWHAZ and its
coding protein, 14-3-3ζ on bladder cancer. Thus, this study was an
innovative attempt and these findings were also in accordance with
the results of our previous study that YWHAZ amplification
predicts better survival, under the background of some
anti-oncogene loss such as TP53 and CDKN2A (10).
However, previous studies have reported that in some
other types of cancer, 14-3-3ζ may serve as a pro-tumor molecule
(6,7,15,16).
Thus, in our first step, we verified the role of YWHAZ in
three typical cancers that have been commonly reported before,
breast, lung and prostate cancer. Our results in these three types
of cancer showed that although some differences did not reach
statistically significance, there was always a trend toward worse
survival outcomes when YWHAZ was overexpressed. These
demonstrated that 14-3-3ζ may function differentially in bladder
cancer than in other types of cancer.
A previous study demonstrated that 14-3-3 protein
exerts its function through binding ability. The structure of
14-3-3 contains two subunits that are strongly associated with each
other to form C-shaped dimers. In the dimers, the N-terminal
helices of two subunits contact one another and form the floor of a
central groove. Phosphorylated sites on target peptides and
proteins dock directly into the groove (17). The binding partner decides the
functions that 14-3-3 may play in multiple cellular process. The
majority of studies have demonstrated that 14-3-3 could bind to
multiple pro-apoptosis proteins, such as Bax, FOXO3 and AP-1, and
thus may affect cell apoptosis (18,19).
However, in the present study, we found that YWHAZ
downregulation had no effects on cell apoptosis. This suggests that
in bladder cancer 14-3-3 may not mainly function in cell
apoptosis.
Our results indicated that both in 5637 and T24
cells, YWHAZ knockdown promoted cell cycle transition from
G1 to S phase. This suggest that 14-3-3ζ may exert its antitumor
function through blocking the cell cycle. Further in silico
GSEA analysis also confirmed the hypothesis. Studies have also
shown that 14-3-3 family protein could bind to CDC25 family
protein, which plays an important role in transitions between cell
cycle phases by dephosphorylating and activating CDKs (20). CDC25B and CDC25C play a major role
in G2/M progression, whereas CDC25A assists in G1/S transition
(21,22). Combining the established facts and
our findings, we speculated that 14-3-3 could arrest cell cycle by
sequestering the critical stage, thus exerting a tumor-suppressor
function. One study has shown that high expression of CDC25B and
low expression of 14-3-3σ is associated with the development and
poor prognosis in urothelial carcinoma of bladder (23). Since CDC25 have varieties of
isoforms, the affinity of 14-3-3ζ to different CDC25 isoforms
remains to be explored. Moreover, this could explain our findings
in a previous study that YWHAZ amplification significantly
benefited survival outcomes under the background of CDKN2A
or TP53 loss, both critical factors in cell cycle regulation
(10). The malfunction of
CDKN2A or TP53 causes disruption of cell cycle
control and enhancement of proliferation. Thus, YWHAZ
amplification could offset the cell cycle acceleration effect by
the reason of critical cell cycle regulator disorder.
Since 14-3-3 proteins have seven isoforms, the
affinity of their binding ability to CDC25 protein should be
distinct. A study has shown that CDC25B has a strong interaction
with 14-3-3ζ and 14-3-3η, but poor with 14-3-3ε (24). The different binding ability may
contribute to the contrasting role of different isoforms in a
certain cancer type. As an instance, 14-3-3ζ promoted tumor
invasion, while 14-3-3σ serves as a tumor-suppressor gene in
non-small cell lung cancer (16,25).
In addition, the expression level of 14-3-3 isoforms in tissue may
be different (26). This may
explain why 14-3-3ζ plays a pro-tumor role in various types of
cancer, but exerts antitumor function in bladder cancer.
In conclusion, our results indicated that
YWHAZ knockdown promoted the tumorigenesis of bladder cancer
both in vitro and in vivo, and revealed its
correlation with the cell cycle pathway. Importantly, our study
highlighted for the first time that YWHAZ may be a novel
beneficial prognostic factors in bladder cancer warranting further
study.
Acknowledgements
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abdollah F, Gandaglia G, Thuret R,
Schmitges J, Tian Z, Jeldres C, Passoni NM, Briganti A, Shariat SF,
Perrotte P, et al: Incidence, survival and mortality rates of
stage-specific bladder cancer in United States: A trend analysis.
Cancer Epidemiol. 37:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matta A, Siu KW and Ralhan R: 14-3-3 zeta
as novel molecular target for cancer therapy. Expert Opin Ther
Targets. 16:515–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aghazadeh Y and Papadopoulos V: The role
of the 14-3-3 protein family in health, disease, and drug
development. Drug Discov Today. 21:278–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT,
Lu J, Danes CG, Guo H, Lan KH, Ensor J, et al: 14-3-3zeta
overexpression defines high risk for breast cancer recurrence and
promotes cancer cell survival. Cancer Res. 69:3425–3432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murata T, Takayama K, Urano T, Fujimura T,
Ashikari D, Obinata D, Horie-Inoue K, Takahashi S, Ouchi Y, Homma
Y, et al: 14-3-3ζ, a novel androgen-responsive gene, is upregulated
in prostate cancer and promotes prostate cancer cell proliferation
and survival. Clin Cancer Res. 18:5617–5627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao GY, Ding JY, Lu CL, Lin ZW and Guo J:
The overexpression of 14-3-3ζ and Hsp27 promotes non-small cell
lung cancer progression. Cancer. 120:652–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin LM, Han XH, Jie YQ and Meng SS:
14-3-3ζ silencing retards tongue squamous cell carcinoma
progression by inhibiting cell survival and migration. Cancer Gene
Ther. 23:206–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Wu Y, Yang T, Feng C and Jiang H:
Coexistence of YWHAZ amplification predicts better prognosis in
muscle-invasive bladder cancer with CDKN2A or TP53 loss.
Oncotarget. 7:34752–34758. 2016.PubMed/NCBI
|
|
11
|
Feng C, Xiong Z, Jiang H, Ding Q, Fang Z
and Hui W: Genetic alteration in notch pathway is associated with
better prognosis in renal cell carcinoma. Biofactors. 42:41–48.
2016.PubMed/NCBI
|
|
12
|
Feng C, Ding G, Jiang H, Ding Q and Wen H:
Loss of MLH1 confers resistance to PI3Kβ inhibitors in renal clear
cell carcinoma with SETD2 mutation. Tumour Biol. 36:3457–3464.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng C, Sun Y, Ding G, Wu Z, Jiang H, Wang
L, Ding Q and Wen H: PI3Kβ inhibitor TGX221 selectively inhibits
renal cell carcinoma cells with both VHL and SETD2 mutations and
links multiple pathways. Sci Rep. 5:94652015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Subramanian A, Kuehn H, Gould J, Tamayo P
and Mesirov JP: GSEA-P: A desktop application for Gene Set
Enrichment Analysis. Bioinformatics. 23:3251–3253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Li Y, Lin C, Ding J, Liao G and
Tang B: Aberrant upregulation of 14-3-3σ and EZH2 expression serves
as an inferior prognostic biomarker for hepatocellular carcinoma.
PLoS One. 9:e1072512014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun N, Wu Y, Huang B, Liu Q, Dong Y, Ding
J and Liu Y: Decreased expression of 14-3-3σ, an early event of
malignant transformation of respiratory epithelium, also
facilitates progression of squamous cell lung cancer. Thorac
Cancer. 6:715–721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mackintosh C: Dynamic interactions between
14-3-3 proteins and phosphoproteins regulate diverse cellular
processes. Biochem J. 381:329–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sunayama J, Tsuruta F, Masuyama N and
Gotoh Y: JNK antagonizes Akt-mediated survival signals by
phosphorylating 14-3-3. J Cell Biol. 170:295–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshida K, Yamaguchi T, Natsume T, Kufe D
and Miki Y: JNK phosphorylation of 14-3-3 proteins regulates
nuclear targeting of c-Abl in the apoptotic response to DNA damage.
Nat Cell Biol. 7:278–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen MS, Ryan CE and Piwnica-Worms H: Chk1
kinase negatively regulates mitotic function of Cdc25A phosphatase
through 14-3-3 binding. Mol Cell Biol. 23:7488–7497. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lavecchia A, Di Giovanni C and Novellino
E: Inhibitors of Cdc25 phosphatases as anticancer agents: A patent
review. Expert Opin Ther Pat. 20:405–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sur S and Agrawal DK: Phosphatases and
kinases regulating CDC25 activity in the cell cycle: Clinical
implications of CDC25 overexpression and potential treatment
strategies. Mol Cell Biochem. 416:33–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Zhang G and Kong C: High
expression of Cdc25B and low expression of 14-3-3σ is associated
with the development and poor prognosis in urothelial carcinoma of
bladder. Tumour Biol. 35:2503–2512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mils V, Baldin V, Goubin F, Pinta I, Papin
C, Waye M, Eychene A and Ducommun B: Specific interaction between
14-3-3 isoforms and the human CDC25B phosphatase. Oncogene.
19:1257–1265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tong S, Xia T, Fan K, Jiang K, Zhai W, Li
JS, Wang SH and Wang JJ: 14-3-3ζ promotes lung cancer cell invasion
by increasing the Snail protein expression through atypical protein
kinase C (aPKC)/NF-κB signaling. Exp Cell Res. 348:1–9. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
MacKay RK, Colson NJ, Dodd PR and Lewohl
JM: Differential expression of 14-3-3 isoforms in human alcoholic
brain. Alcohol Clin Exp Res. 35:1041–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|