Introduction
Malignant melanoma, accounting for the greater
percentage of skin cancer cases is a highly lethal cutaneous tumor
which has shown an increasing incidence in the last few decades
(1). The conventional therapy for
malignant melanoma includes surgery, chemotherapy, immunotherapy
and radiation therapy (2). However,
these therapeutic strategies are typically self-limited and far
from satisfactory. The urgent task on hand is to seek an effective
drug to improve the comprehensive treatment, reduce the side
effects and reduce the mortality of melanoma.
It has been demonstrated that flavonoids have
cytotoxic activities toward multiple types of human cancer cells,
yet have little or no effect on normal cells. Numerous flavonoids
in traditional Chinese herbs may be promising candidates for the
development of novel anticancer drugs (3). Previously, we found that Licochalcone
C induced apoptosis via B-cell lymphoma 2 family proteins in T24
cells (4). Licochalcone A induced
T24 bladder cancer cell apoptosis by increasing intracellular
calcium levels (5).
Isoliquiritigenin inhibited proliferation and induced apoptosis via
alleviating hypoxia and reducing glycolysis in mouse melanoma
B16F10 cells (6). Recently, we
demonstrated that Licochalcone B arrested cell cycle progression
and induced apoptosis in human breast cancer MCF-7 cells (7). Taken together, these studies indicate
the application potential of flavonoids for cancer therapy.
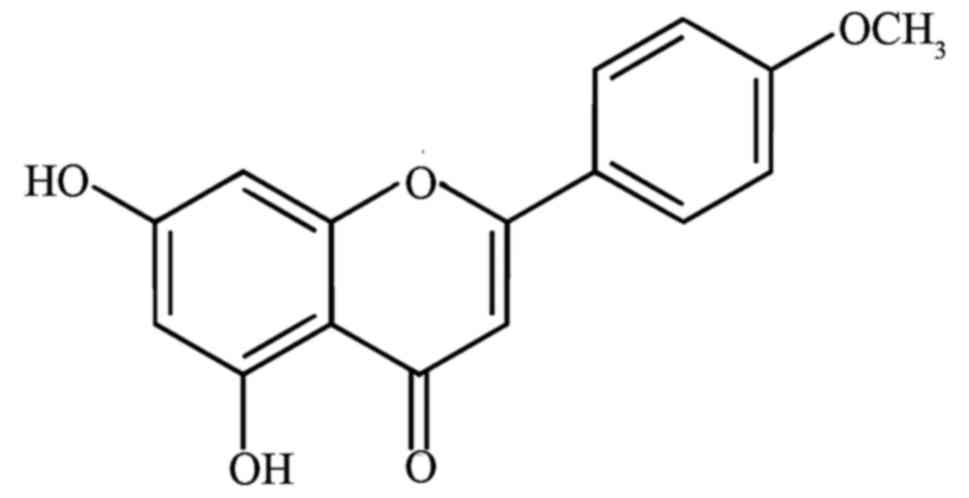
Licochalcone D (LD, Fig. 1) is a
flavonoid compound mainly existing in the root of Glycyrrhiza
inflata, commonly named Chinese licorice which possesses
antioxidant and anti-inflammatory properties (8–10).
However, the effect of LD on the metastasis and apoptosis of A375
cells has never been reported. The present study was designed to
evaluate the anticancer, metastatic and apoptotic effects of LD on
A375 cells in vitro.
Materials and methods
Cell culture and treatment
A375 cells were purchased from the Cell Bank of the
Committee on Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). B16F0 cells were obtained from the
China Center for Type Culture Collection (Wuhan, China). SK-MEL-5
cells were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). A375 cells were cultured in Gibco™ DMEM,
while SK-MEL-5 and B16F0 cells were cultured in Gibco™ RPMI-1640
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
HyClone™ 10% fetal bovine serum (FBS; GE Healthcare Life Sciences),
2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin.
All cell lines were cultured at 37°C in 5% CO2. Cells
were allowed to attach for 24 h before treatment. LD was dissolved
in dimethyl sulfoxide (DMSO) (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and diluted with fresh medium to achieve the desired
concentration. The final concentration of DMSO did not exceed 0.2%
in the fresh medium, and DMSO at this concentration has no
significant effect on cell viability.
Cell viability assay
A375 cells were trypsinized and seeded into 96-well
plates at 105 cells/well. Thereafter, cells were exposed
to LD (0, 1, 2.5, 5, 15, 30, 45, 60, 75 and 90 µmol/l) for 24 h.
SK-MEL-5 cells were trypsinized and seeded into 96-well plates at
104 cells/well, and exposed to LD (0, 20, 40, 60 and 80
µmol/l) for 24 h, followed by extra incubation in fresh medium for
another 24 h. The effect of LD-induced cytotoxicity was evaluated
using sulforhodamine B (SRB) (Tokyo Chemical Industry Co., Ltd.,
Tokoy, Japan) assay as previously described (6). The optical density was detected at a
wavelength of 490 nm.
Observation of morphological
changes
A375 cells were trypsinized and seeded into 6-well
plates at 2×105 cells/well. After being treated with LD
(0, 30, 60 and 90 µmol/l) for 24 h, the cells were fixed with
Carnoy's fixative consisting of methanol (Kaixin, Tianjin, China)
and glacial acetic acid (3:1, v/v). Hoechst 33258 (5 µg/ml)
(Solarbio, Beijing, China) was applied for visualization of the
nucleus. The changes in nuclear morphology of apoptotic cells were
observed by labeling the cells with the nuclear stain Hoechst 33258
and examining them under fluorescence microscopy at ×200
magnification (MIC00266; Carl Zeiss, Oberkochen, Germany). Cells
that exhibited reduced nuclear size, chromatin condensation,
intense fluorescence, and nuclear fragmentation were considered as
apoptotic.
Trypan blue exclusion test
The lethality of LD on A375 cells was determined by
the trypan blue exclusion test as previously described (11). After 24 h of incubation with LD (0,
30, 60 and 90 µmol/l), A375 cells were removed from the culture
medium and cells that excluded trypan blue (Sigma-Aldrich; Merck
KGaA) were counted in a Neubauer chamber.
Annexin V/PI staining assay
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide kit (BD Pharmingen, San Diego, CA, USA) was
used to assess cell apoptosis according to the supplier's
instructions (12). After treatment
with LD (0, 30, 60 and 90 µmol/l) for 24 h, cells were collected,
counted, centrifuged and resuspended in 500 µl of 1X binding
buffer. Then Annexin V-FITC (5 µl) and PI (5 µl) were added to each
sample. The samples were incubated in the dark at room temperature
for 15 min and examined immediately on a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). The assays were repeated at
least three times.
RNA isolation, RT-PCR and qPCR
Semi-quantitative reverse transcription-PCR (RT-PCR)
(13) and quantitative real-time
PCR (qPCR) (14) were performed to
examine the mRNA expression of Bax, Bcl-2, caspase-3, caspase-9,
MMP-2 and MMP-9. The primer sequences (synthesized by Sangon
Biotech Co., Ltd., Shanghai, China) are shown in Table I. Total cellular RNA was extracted
using TRIzol reagent (Sangon Biotechnology Co., Ltd.), and then
cDNA was synthesized with the use of 3 µl total RNA primed with
oligo(dT) (deoxy-thymidine) (Fermentas, Vilnius, Lithuania) in 25
µl reaction solution. The resulting total cDNA was used in the
polymerase chain reaction. The reaction conditions were established
by 12.5 µl 2X PCR Master (Tiangen Biotech, Beijing, China), 3 µl
cDNA template, and 0.5 µl of each primer. RT-PCR products were
resolved on a 1.5% agarose gel, stained with ethidium bromide, and
the intensity was quantified by Gel-Pro analysis software (ImageJ
software, version 1.49n; National Institutes of Health, Bethesda,
MD, USA). qPCR amplification was conducted using SYBR-Green
q-RT-PCR kit reagents (Fermentas) according to the manufacturer's
instructions. The cycling conditions were as follows: 95°C for 5
min, followed by 40 cycles of 95°C for 5 min, Tm (C) for 30 sec,
and 72°C for 30 sec. For quantification, the relative gene
expression was calculated using Ct methods.
 | Table I.Sequences of the primer pairs for
real-time RT-PCR and qPCR. |
Table I.
Sequences of the primer pairs for
real-time RT-PCR and qPCR.
| Gene | Primer | Size (bp) |
|---|
| Bax | F:
5′-ACGAACTGGACAGTAACATGGAG-3′ |
839 |
|
| R:
5′-CAGTTTGCTGGCAAAGTAGAAAAG-3′ |
|
| Bcl-2 | F:
5′-ATGTGTGTGGAGAGCGTCAA-3′ | 6492 |
|
| R:
5′-GAGACAGCCAGGAGAAATCAA-3′ |
|
| Caspase-3 | F:
5′-CTGGACTGTGGCATTGAGAC-3′ | 2689 |
|
| R:
5′-ACAAAGCGACTGGATGAACC-3′ |
|
| Caspase-9 | F:
5′-AGGGTCGCTAATGCTGTTTC-3′ | 1848 |
|
| R:
5′-GCAAGATAAGGCAGGGTGAG-3′ |
|
| MMP-2 | F:
5′-ACCTACACCAAGAACTTCCG-3′ | 3152 |
|
| R:
5′-TTGGTTCTCCAGCTTCAGGT-3′ |
|
| MMP-9 | F:
5′-TGACAGCGACAAGAAGTG-3′ | 2387 |
|
| R:
5′-CAGTGAAGCGGTACATAGG-3′ |
|
| GAPDH | F:
5′-CAAGGTCATCCATGACAACTTTG-3′ | 1513 |
|
| R:
5′-GTCCACCACCCTGTTGCTGTAG-3′ |
|
Detection of ΔΨm by JC-1
A375 cells were incubated with LD (0, 30, 60 and 90
µmol/l) for 24 h, then washed with cold PBS, exposed to 500 µl JC-1
dye solution (KeyGen Biotech. Inc., Nanjing, China) and incubated
at 37°C for 20 min in the dark. After washed three times with
incubation buffer, the cells were diluted in 500 µl incubation
buffer and the fluorescence intensity of the cells was analyzed
using a FACScan flow cytometer (BD Biosciences). The wavelength at
excitation/emission 485/580 nm (red), and then at
excitation/emission 485/530 nm (green) was read by a fluorescent
plate reader (Varioskan Flash 3001; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Detection of intracellular ROS
levels
ROS in A375 cells were detected using a fluorescent
probe-2,7-dichlorofluorescein diacetate (DCFH-DA) (Sigma-Aldrich;
Merck KGaA) as previously described (15). When DCFH-DA penetrates cells, it is
hydrolyzed to DCFH by an esterase which is oxidized to fluorescent
DCF by intracellular ROS. Thus, 2×105 cells/bottle were
incubated with the indicated concentration of LD (0, 30, 60 and 90
µmol/l) with or without ROS scavengers (N-acetyl-cysteine;
NAC) (Sigma-Aldrich; Merck KGaA) for 24 h, then cells were
incubated with DCFH-DA (30 µmol/l) at 37°C for 30 min and washed
with PBS, and the fluorescence intensity of the cells was analyzed
using a FACScan flow cytometry (BD Biosciences). In parallel, the
wavelength at 495 nm excitation and 529 nm emission for DCF was
read by a fluorescent plate reader (Varioskan Flash 3001; Thermo
Fisher Scientific, Inc.).
Wound healing assays
A375 cells were trypsinized and seeded into 24-well
plates at 105 cells/well, and resuspended in serum-free
DMEM with 0.1% DMSO (vehicle control). When the cells reached 90%
confluence, the cells were wounded by scratching with a sterile
pipette tip (16). The cells were
treated with LD (0, 1, 2.5, 5, 15 and 25 µmol/l) at 37°C with 5%
CO2 for 24 h. Until normal (control) group healing,
images of cell migration were captured at the indicated time
(MIC00266; Carl Zeiss).
Assay for gelatin zymography
A375 cells were seeded in a 96-well (104
cells/well) dish and stabilized in DMEM with 10% FBS at 37°C for 24
h. Then, the cells were treated with LD (0, 5, 15 and 25 µmol/l)
for 24 h. The conditioned medium was harvested and then
concentrated by a Thermo centrifugal filter device (1,500 × g, 5
min). The conditioned media with the same amount of protein were
mixed with an equal volume of sodium dodecyl sulfate (SDS)-gel
loading buffer without boiling and then subjected to 10% SDS
polyacrylamide gels containing 0.1% gelatin under non-reducing
conditions. After electrophoresis at 4°C, the gels were soaked in
zymogen renaturation buffer for 1 h to remove residual SDS and
rinsed in distilled water. The gels were incubated in a developing
buffer (50 mmol/l Tris-HCl, 150 mmol/l NaCl, 5 mmol/l
CaCl2, 2 µmol/l ZnCl2, pH 7.5) for 18 h,
stained with 0.2% Coomassie blue R-250 (Beyotime Institute of
Biotechnology, Haimen, China), and visualized by destaining
solution (35% methanol, 10% acetic acid). The gelatinase activities
of MMP-2 and MMP-9 were determined by analyzing the signal
intensity using Gel-PRO Analyzer (ImageJ software version 1.49n;
National Institutes of Health) (17).
Assay of MMP-2 and MMP-9
activities
A375 cells (2×105 cells/bottle), in the
log phase of proliferation, were incubated at 37°C with 5%
CO2. After treatment with LD (0, 5, 15 and 25 µmol/l)
for 24 h, MMP-2 and MMP-9 activities were quantitatively measured
by enzyme-linked immunosorbent assay (ELISA) (Shanghai Westang
Bio-Tech Co., Shanghai, China) according to the manufacturer's
instructions. In brief, 100 µl of each sample was dispensed into a
96-well microtitre plate coated with polyclonal anti-MMP-2 or MMP-9
followed by 37°C for 40 min. After washing, 50 µl of detection
reagent was applied into each well at 37°C for 30 min. Chromogenic
agent A and B (50 µl) were added and mixed for 30 sec and then put
at 37°C for 30 min. Absorbance was read at 450 nm on a microplate
reader (Varioskan Flash 3001; Thermo Fisher Scientific, Inc.).
Transwell assays
Transwell migration assays were performed to assess
cancer cell migration upon treatments in Transwell chambers with a
non-coated membrane using a 24-well insert (Corning, Inc., Corning,
NY, USA). A375 cells (105 cells/well) were seeded in the
top of the chambers and incubated overnight. For invasion assays,
A375 cells were plated in the top chamber with a Matrigel-coated
membrane. Medium (without serum) was added to the upper chamber.
The medium (containing 10% FBS) was added in the lower chamber.
After 24 h, the cells were fixed in 10% neutral buffered formalin
solution for 30 min and stained with Giemsa. Cells on each insert
were calculated using a light microscope (Axioskop; Carl
Zeiss).
In vivo antitumor activity
All experimental protocols in the present study were
performed after the approval by the Institutional Animal Care and
Use Committee of Shihezi University. B16F0 cells (200 µl)
(105 cells/ml) were injected subcutaneously into the
right flank of C57BL/6 mice, and then tumor formation in the C57
mice was monitored. Subcutaneous tumors induced by B16F0 cells in
C57 mice were randomly divided into three treatment groups (6 in
each group). One week after inoculation, the mice were administered
25 and 50 mg/kg of LD by intragastric administration (i.g.) daily,
respectively. Control mice received the same volume of normal
saline. The mice were observed for body weight changes every two
days. A week later after the first treatment with LD, the mice in
each group were anesthetized with 3% sodium pentobarbital via
intraperitoneal injection. The implanted melanomas were separated
and weighed, and the tumor inhibition rate (TIR) was calculated
according to the following formulate: TIR (%) = (WC - WE)/WE ×
100%. WC is the mean tumor weight in the control group; WE is the
mean tumor weight in the tested groups, respectively. More than 30%
was regarded as having an inhibitory effect. Mice with signs of
severe distress or pain were euthanized before the end of the
study.
Statistical analysis
All values are expressed as the mean ± SD. All
experiments were repeated at least three times. Statistical
differences between two groups were determined using the Student's
t-test. Two-way analysis of variance (ANOVA) with general linear
model procedures using a univariate approach was applied for more
than two groups. The results were considered significantly
different at P<0.05 and highly significantly different at
P<0.01.
Results
LD treatment inhibits the
proliferation of human melanoma cells
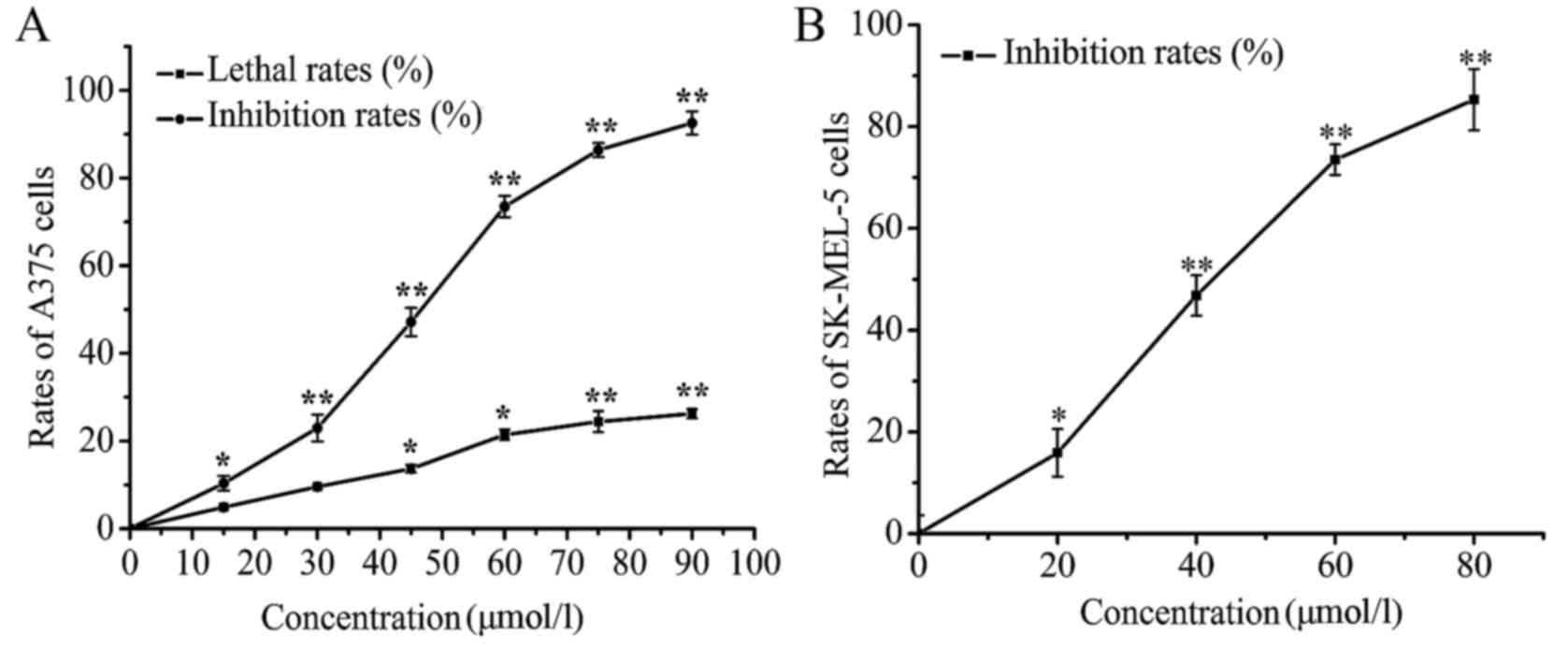
Cell viability in vitro was assessed using
SRB assay to show the inhibitory effect of LD on cell
proliferation. After treatment with LD (0, 15, 30, 45, 60, 75 and
90 µmol/l) for 24 h, the inhibition rate of A375 cells increased
with an increase in the concentration of LD, and the
IC50 value was ~48.61 µmol/l. LD (<30 µmol/l) did not
significantly affect the lethality rate of the A375 cells (Fig. 2A), which indicated that the
inhibitory effect of LD on cell proliferation was not due to the
direct killing of the A375 cells. In addition, the effect of LD on
another human melanoma cell line SK-MEL-5 also be examined. The
SK-MEL-5 cells were treated with different concentrations (20, 40,
60 and 80 µmol/l) of LD. The data from the cell viability assay
indicated that LD inhibited the proliferation of SK-MEL-5 cells in
a concentration-dependent manner (Fig.
2B).
LD induces the apoptosis of A375
cells
We explored whether LD could induce apoptosis in
A375 cells. After treatment with LD for 24 h, a fewer number of
cells and smaller circular morphology of the A375 cells were
observed by microscopy (Fig. 3A).
As shown in Fig. 3B, cells
exhibited obvious apoptotic characteristics after treatment with LD
(0, 30, 60 and 90 µmol/l) for 24 h; nuclei were condensed and
fragmented in the apoptotic cells. Moreover, we confirmed the ell
apoptosis rate using an Annexin V-PI apoptosis detection kit, and
the percentages of apoptotic cells were calculated. As shown in
Fig. 3C and D, the cell apoptosis
rates in the LD-treated cells (0, 30, 60 and 90 µmol/l) were
1.94±4.39, 11.26±2.35, 31.65±5.60 and 52.10±4.79%, respectively.
Clearly, with the increasing concentration of LD, the percentage of
apoptotic cells also increased. As shown in Fig. 3E and F, LD downregulated the mRNA
level of Bcl-2 and upregulated the mRNA levels of caspase-3,
caspase-9 and Bax.
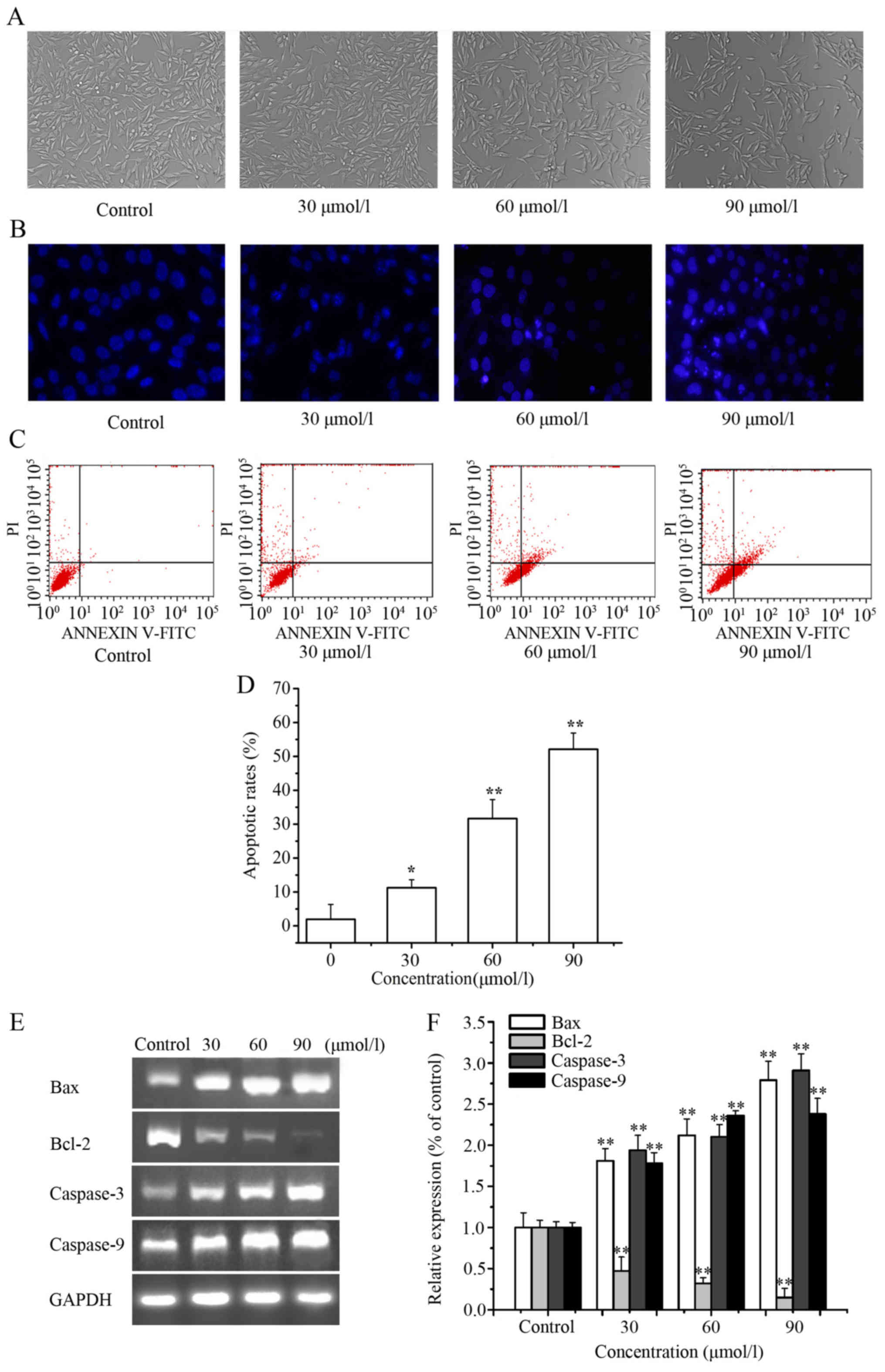 | Figure 3.Induction of apoptosis in A375 cells
by Licochalcone D (LD) treatment. (A) Cell morphological changes
were observed by phase-contrast microscopy (magnification, ×200)
after treatment with LD (0, 30, 60 and 90 µmol/l) for 24 h. (B)
Apoptosis was visualized by the appropriate changes in nuclei
stained with Hoechst 33258 (blue) (magnification, ×200). (C) The
effects of LD on the induction of A375 cell apoptosis were analyzed
by FCM analysis. (D) The apoptosis rate as statistically analyzed.
(E) RT-PCR analyses of A375 cells to evaluate mRNA expression of
Bcl-2, Bax, caspase-3 and caspase-9. (F) qPCR analyses of A375
cells to evaluate mRNA expression of Bcl-2, Bax, caspase-3 and
caspase-9, and relative intensities were normalized by levels of
GAPDH. The untreated group level was considered as ‘1.0’. Data are
presented as means ± SD of at least three independent experiments.
*P<0.05, **P<0.01 as compared with the untreated control
group. |
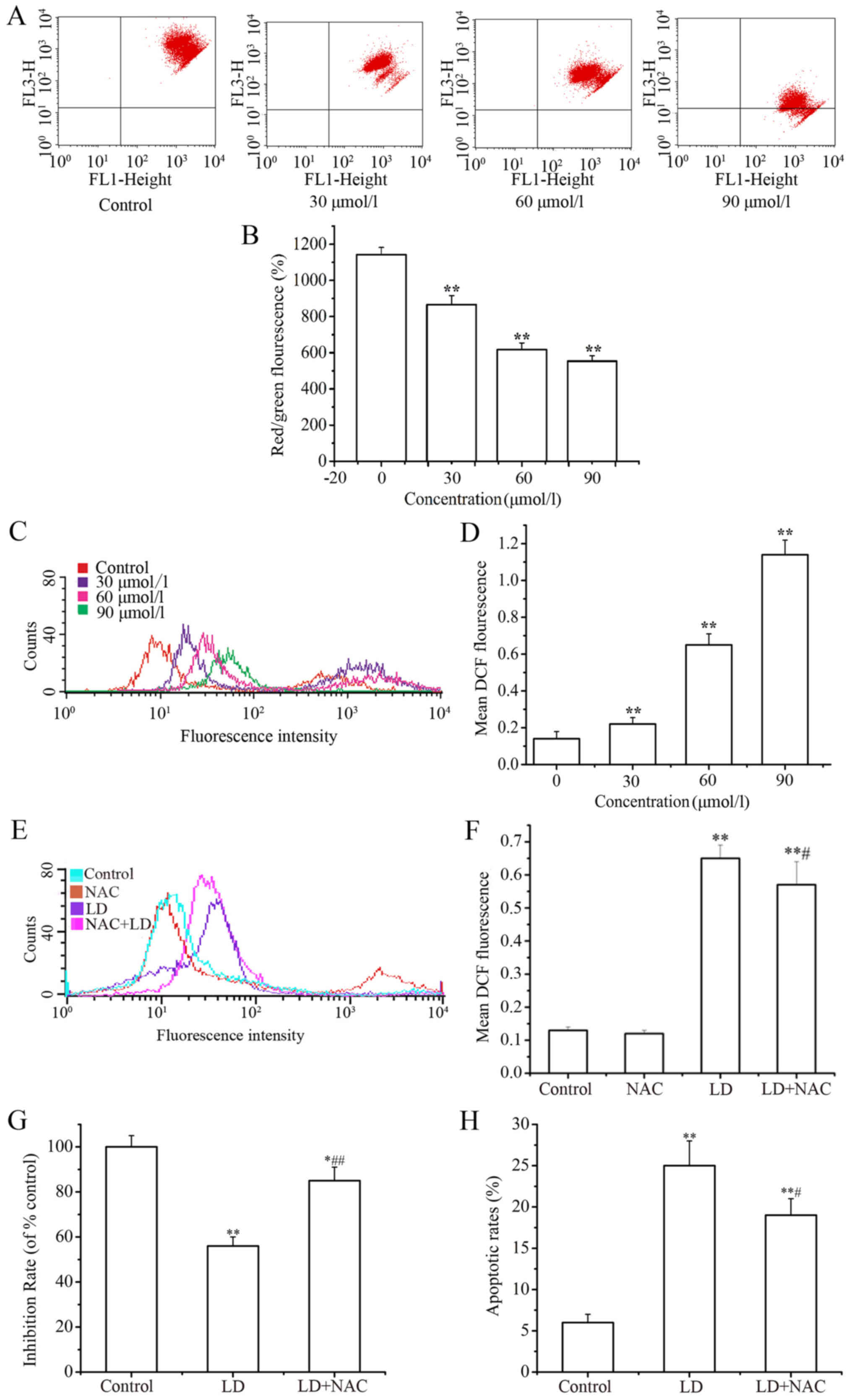
Effects of LD on ΔΨm and ROS in A375
cells
(Fig. 4A and B) The
changes in mitochondrial membrane potential ΔΨm in A375 cells were
tested by staining with JC-1 dye solution after treatment with LD,
and the staining was detected by flow cytometry and fluorescence
plate reader. A decrease in the intensity of JC-1 dye solution
staining reflects loss of the ΔΨm. A concentration-dependent
reduction in ΔΨm was observed in the LD-treated cells. (Fig. 4C and D) DCF-DA was used as a
fluorescence indicator to measure the intracellular ROS level. The
ROS levels in the LD-treated cells were significantly higher than
that noted in the control. (Fig. 4E and
F) Moreover, ROS scavengers (NAC) were used to determine
whether ROS exerted an interference effect against LD-induced A375
cell proliferation. ROS production was inhibited obviously with the
co-addition of NAC (300 µM). Meanwhile, the cell proliferation
inhibition ratio increased (Fig.
4G) and the percentage of apoptotic cells decreased (Fig. 4H) in the NAC co-treatment
groups.
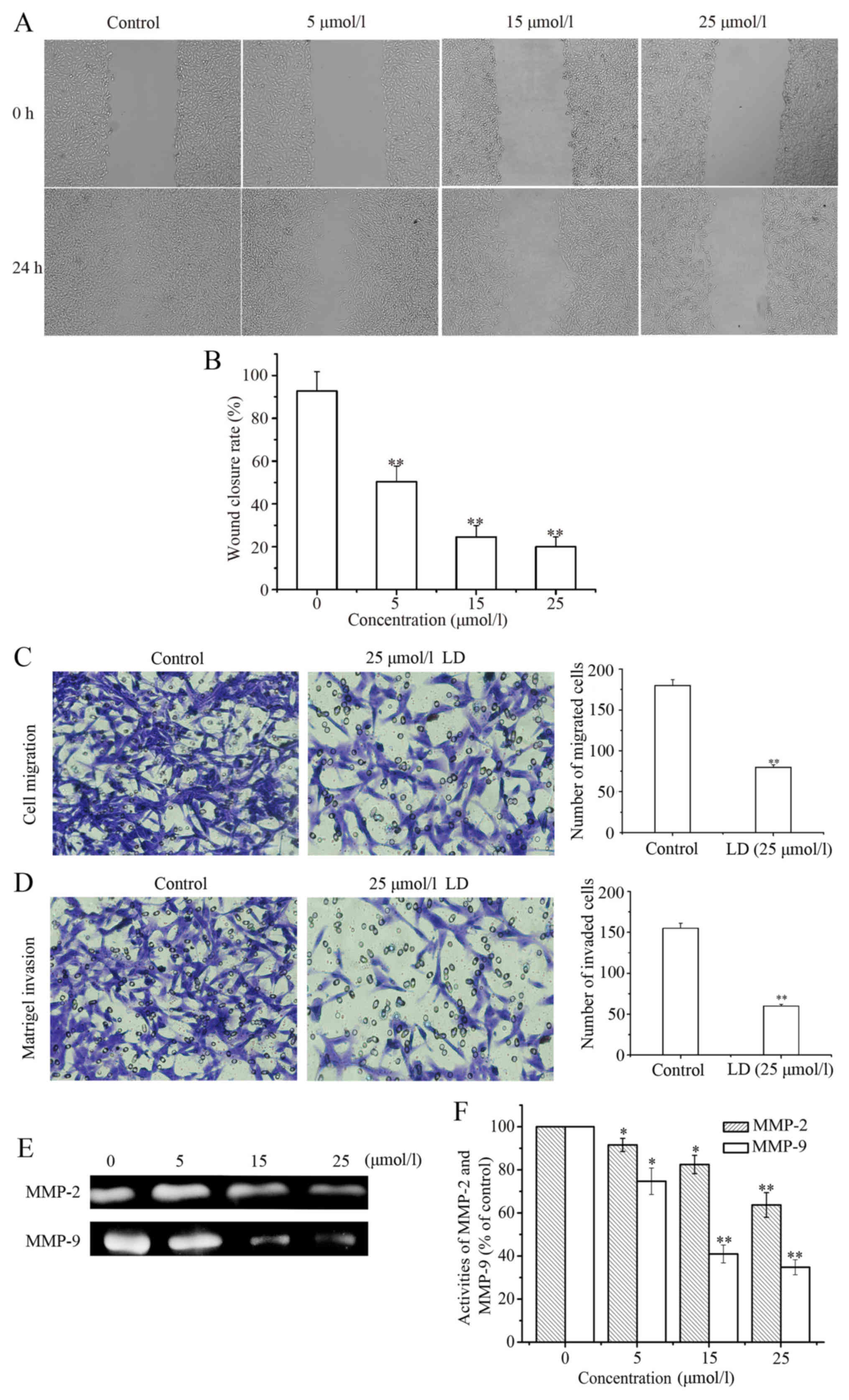
LD decreases the migration and
invasion ability of the A375 cells
After treatment with LD (0, 5, 15 and 25 µmol/l) for
24 h, we assessed the effect of LD on A375 cell migration. As shown
in Fig. 5A, wound healing was
observable with a low magnification objective (200×) after 24 h
wounding with a pipette tip. The wound closure rate was 92.67±9.08%
in the absence of LD. However, the closure rates were 50.31±7.39%
(P<0.01), 24.53±5.32% (P<0.01) and 20±4.55% (P<0.01) after
treatment with LD, which indicated that the healing rate
significantly decreased in a concentration-dependent manner
(Fig. 5B). Next we performed
Transwell assays to investigate whether LD affects the invasion and
migration of A375 cells. The results showed that LD significantly
suppressed cell migration in the A375 cells (Fig. 5C). Similar results were observed
with cell invasion (Fig. 5D). Taken
together, LD inhibited cell motility and invasiveness in
vitro. The activities of MMP-2 and MMP-9 in conditioned media
were determined via gelatin zymography assay. As shown in Fig. 5E and F, after treatment with LD for
24 h, the activities of MMP-2 and MMP-9 were significantly
decreased in a concentration-dependent manner. In order to evaluate
the effect of LD on the mRNA expression of MMP-2 and MMP-9,
validated mRNA target genes were evaluated with the RT-PCR and qPCR
methods. Compared with the untreated group, LD treatment
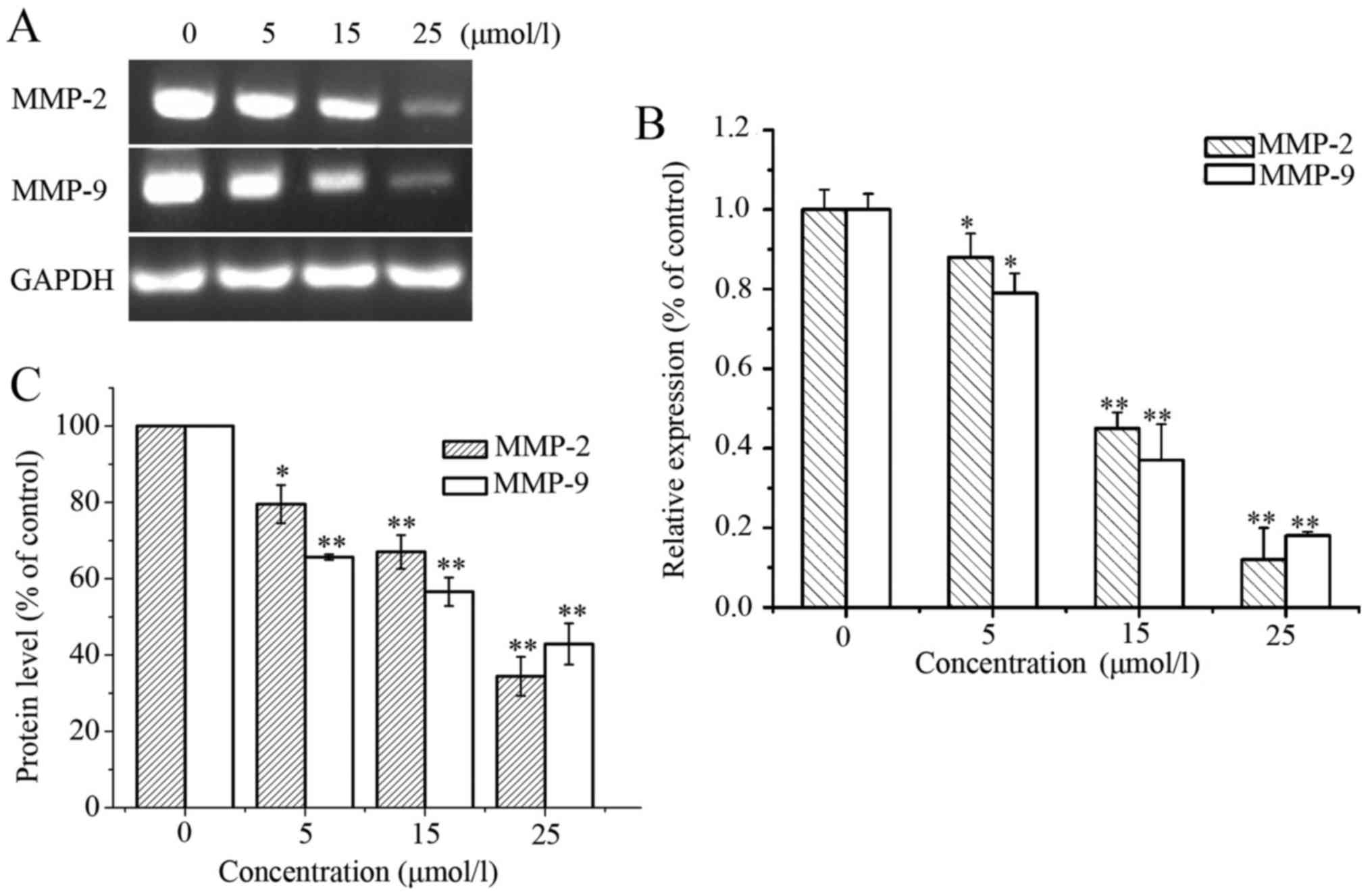
downregulated the mRNA expression of MMP-2 and MMP-9 (Fig. 6A and B). Similar to the mRNA
expression, the protein levels of MMP-2 and MMP-9 also decreased in
the LD-treated cells (Fig. 6C). In
order to exclude the possibility of cell motility leading to the
decrease in expression and secretion of MMPs, we measured the
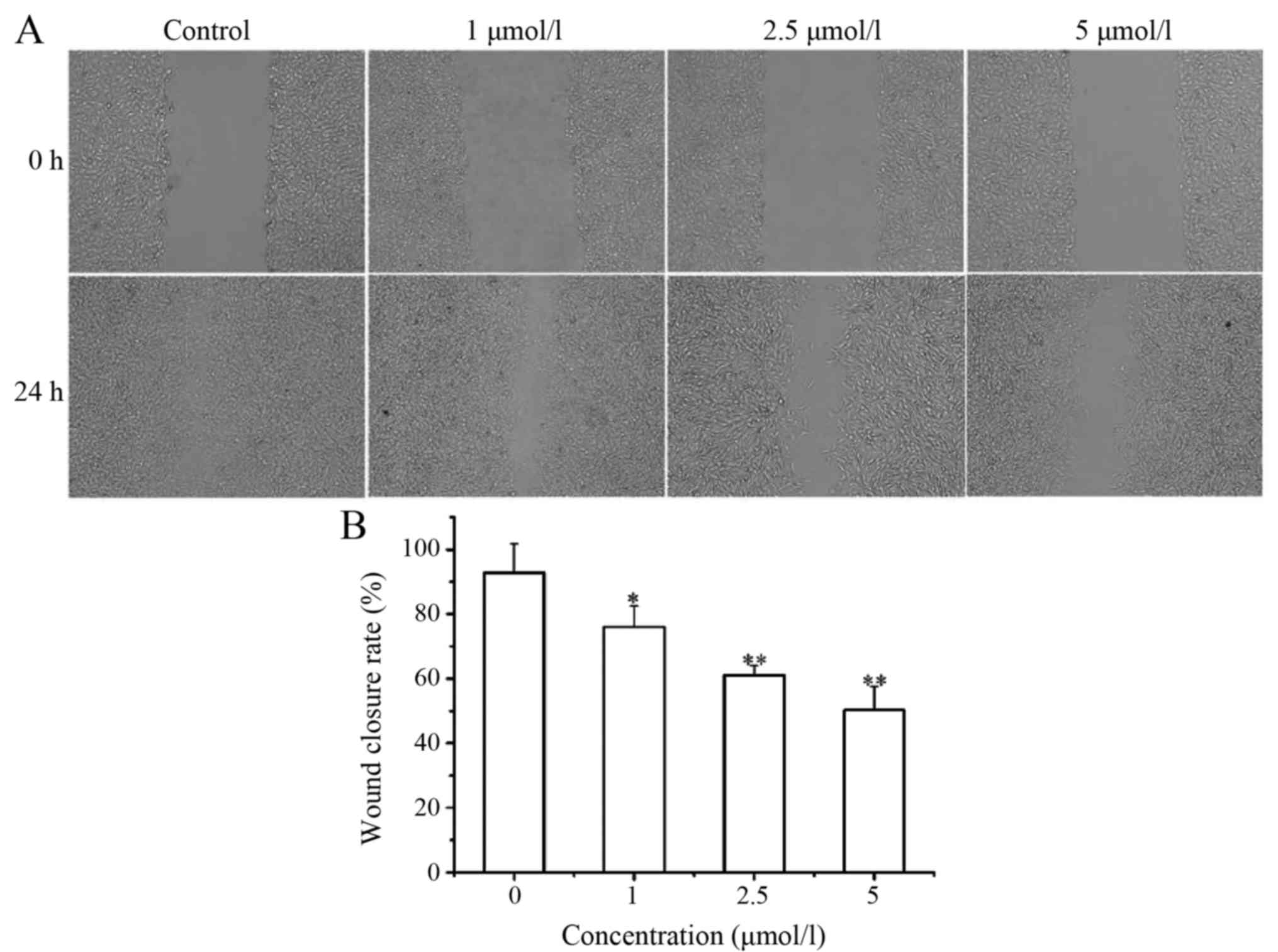
effect of LD at lower concentrations (1, 2.5 and 5 µmol/l) on A375
cell migration. Lower concentrations of LD significantly decreased
the wound healing rate (Fig. 8A and
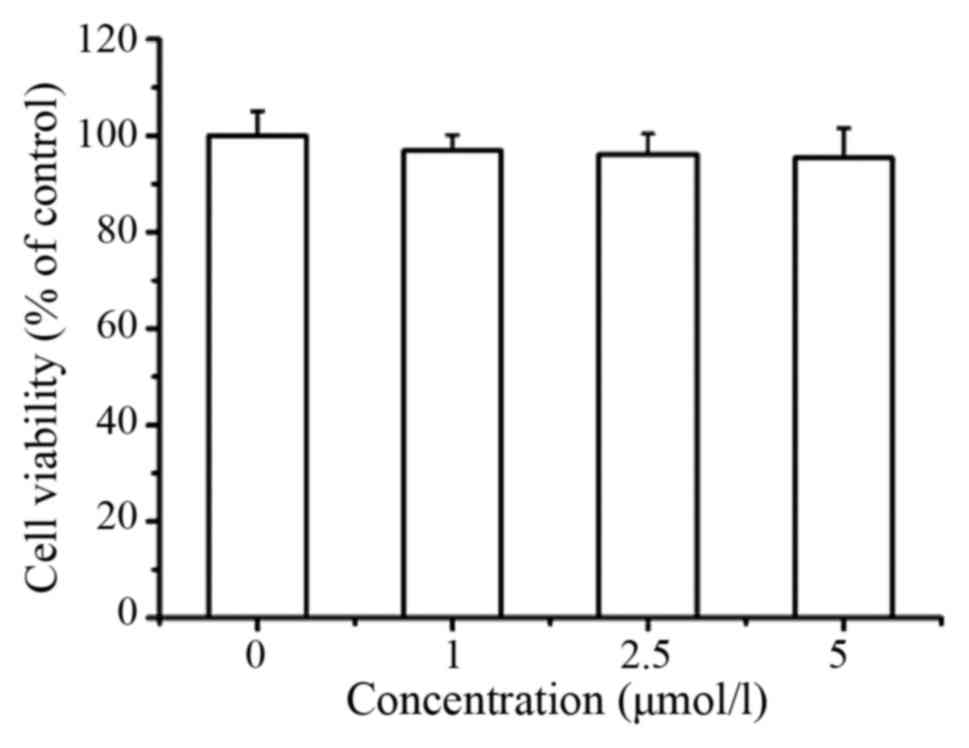
B). In addition, LD at lower concentrations (1, 2.5 and 5
µmol/l) had no apparent effect on the proliferation of A375 cells
(Fig. 7). These results implied
that LD treatment inhibited the migration of melanoma cells which
was associated with downregulation of MMP-2 and MMP-9.
LD inhibits the tumor growth in a
mouse xenograft model of murine melanoma B16F0 cells
Based on the findings that LD induced A375 cell
apoptosis in vitro, we used B16F0 tumor models to measure
whether LD could suppress tumor progression in vivo. C57BL/6
mice bearing melanoma B16F0 cell-derived tumors were used as an
in vivo model to evaluate the effects of LD. Compared with
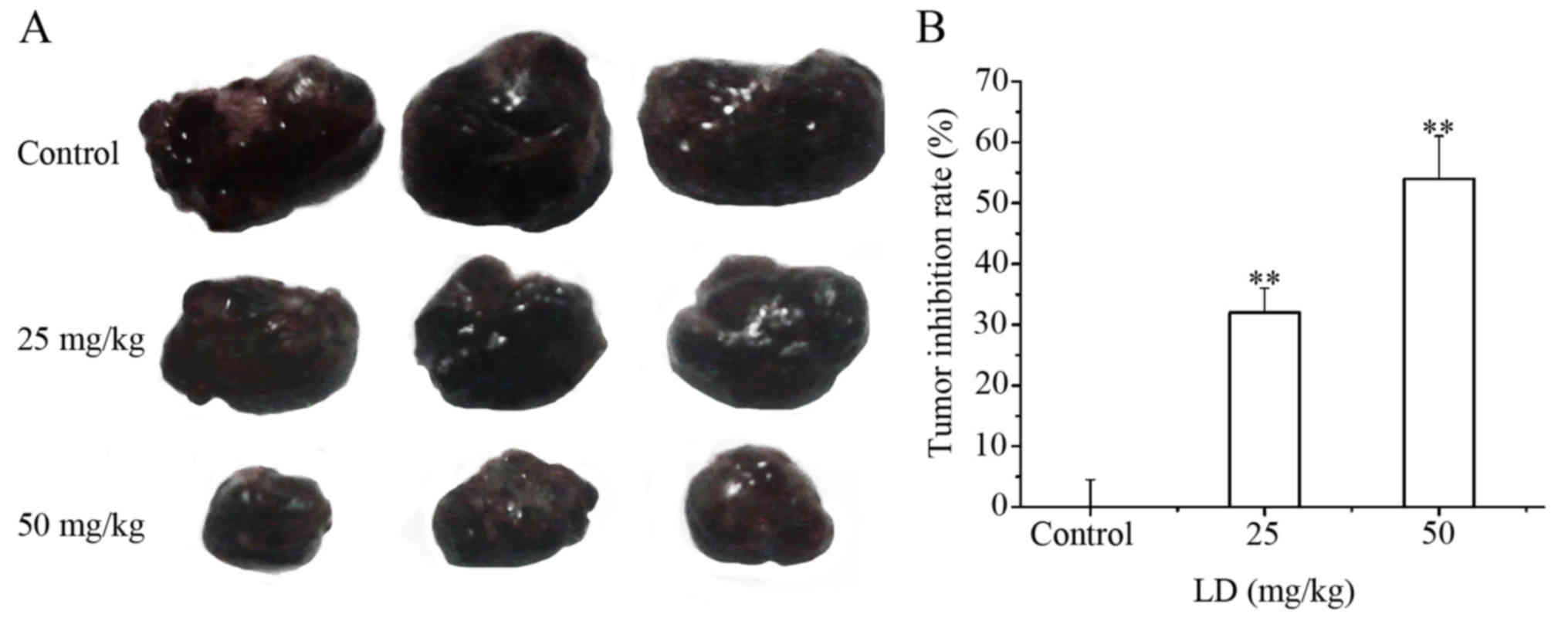
the control group, the tumor growth rates were obviously lower in
mice treated with LD. The tumor growth inhibition rates were
calculated to be 32.0 and 54.1% in the LD-treated groups (25 and 50
mg/kg), respectively (Fig. 9A and
B).
Discussion
In recent decades, the incidence of malignant
melanoma has rapidly increased, and it is urgent to develop new
drugs to treat melanoma. In the present study, we found that
Licochalcone D (LD). a flavonoid compound mainly existing in the
root of Glycyrrhiza inflata, was a potent inhibitor of the
proliferation of human melanoma cells.
Our results demonstrated that LD inhibited the
proliferation of A375 cells through apoptosis induction. Apoptosis
plays an important role in the development and maintenance of
tissue homeostasis and the elimination of unwanted or damaged cells
(18). Therefore, inducing
apoptosis is a promising strategy with which to treat cancers
(19). There are two classic
apoptotic pathways. Firstly, caspase-3 and caspase-9 play a central
role (20). Secondly, Bcl-2 family
protein is involved in the induction of intrinsic apoptosis,
including pro-apoptotic protein Bax and anti-apoptotic protein
Bcl-2 (21). In our study, the
results showed that the occurrence of apoptosis induced by LD was
confirmed.
Moreover, evidence has shown that the ROS
concentration plays a role in cell apoptosis or cell cycle arrest
activated by anticancer agents (22,23).
The role of ROS in cancer biology is rather complex. A modest level
of ROS is required for tumor promotion, while excessive or low
levels of ROS may disrupt mitochondrial membrane potential and
induce apoptosis (24,25). In the present study, the
mitochondrial membrane potential assay results demonstrated that LD
significantly triggered dissipation of mitochondrial membrane
potential (ΔΨm) of A375 cells, which suggested that the
mitochondrial-mediated pathway may be involved in LD-induced
apoptosis. The loss of ΔΨm could induce the increase in
intracellular ROS, which is strongly supported by the result of the
ROS detection assay. Therefore, the intrinsic apoptotic pathway may
be involved in the apoptotic death of A375 cells induced by LD.
The metastatic process of melanoma is complex. Tumor
cell invasion and migration are key steps during tumor metastasis.
Migration plays an important role in the transport of cancer cells
into the blood vessels as well as the extravasation to secondary
organs (26,27). Therefore, it is indispensable to
examine whether LD inhibits A375 cell migration and invasion. In
this research, we utilized assays to confirm the anti-metastatic
ability of LD by targeting tumor cell migration and invasion. We
observed that treatment of metastatic A375 cells with LD markedly
inhibited tumor cell migration and invasion
In addition, MMPs play a fundamental role in
extracellular matrix degradation related to cancer cell invasion
and metastasis. MMP-2 and MMP-9 have been reported to be most
closely associated with metastatic potential. Cancer cell invasion
and migration into surrounding tissues are mediated by MMP-2 and
MMP-9 (28). MMP-2 and MMP-9 can
degrade extracellular matrix and regulate the ability of cells to
migrate. Overexpression of MMP-2 and MMP-9 promotes cancer
progression and is highly correlated with poor prognosis of cancer
patients (29). Targeting of MMP-2
and MMP-9 represents a promising strategy for cancer treatment
(30). Therefore, regulation of
MMP-2 and MMP-9 are essential for preventing cancer invasion and
metastasis. In the present study, we examined the effect of LD on
the secretion and expression of MMP-2 and MMP-9, and the results
indicated that LD decreased MMP-2 and MMP-9 activity and expression
levels. In addition, intragastric injection of LD into our mouse
model of melanoma suppressed tumor growth in vivo.
Based on these observations, we propose that LD may
be a potential drug for human melanoma treatment by inhibiting
proliferation, inducing apoptosis via the mitochondrial pathway and
blocking cell migration.
Acknowledgements
Not applicable.
Glossary
Abbreviations
Abbreviations:
|
LD
|
Licochalcone D
|
|
SRB
|
sulforhodamine B
|
|
FITC
|
Annexin V-fluorescein
isothiocyanate
|
|
PI
|
propidium iodide
|
|
RT-PCR
|
semi-quantitative reverse
transcription-polymerase chain reaction
|
|
qPCR
|
quantitative real-time PCR methods
|
|
ΔΨm
|
mitochondrial membrane potential
|
|
JC-1
|
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazole
carbocyanine iodide
|
|
ROS
|
reactive oxygen species
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Franklin C, Livingstone E, Roesch A,
Schilling B and Schadendorf D: Immunotherapy in melanoma: Recent
advances and future directions. Eur J Surg Oncol. 43:604–611. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reuland SN, Goldstein NB, Partyka KA,
Cooper DA, Fujita M, Norris DA and Shellman YG: The combination of
BH3-mimetic ABT-737 with the alkylating agent temozolomide induces
strong synergistic killing of melanoma cells independent of p53.
PLoS One. 6:e242942011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan H, Lu X, Ma Q, Li D, Xu G and Piao G:
Flavonoids from Artemisia sacrorum Ledeb. and their
cytotoxic activities against human cancer cell lines. Exp Ther Med.
12:1873–1878. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang P, Yuan X, Wang Y, Zhao H, Sun X and
Zheng Q: Licochalcone C induces apoptosis via B-cell lymphoma 2
family proteins in T24 cells. Mol Med Rep. 12:7623–7628. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Jiang J, Yang X, Han J and Zheng
Q: Licochalcone A induces T24 bladder cancer cell apoptosis by
increasing intracellular calcium levels. Mol Med Rep. 14:911–919.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Ma J, Yan X, Chen X, Si L, Liu Y,
Han J, Hao W and Zheng Q: Isoliquiritigenin inhibits proliferation
and induces apoptosis via alleviating hypoxia and reducing
glycolysis in melanoma B16F10 cells. Recent Pat Anticancer Drug
Discov. 11:215–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu L, Ma J, Han J, Wang B, Chen X, Gao C,
Li D and Zheng Q: Licochalcone B arrests cell cycle progression and
induces apoptosis in human breast cancer MCF-7 cells. Recent Pat
Anticancer Drug Discov. 11:444–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haraguchi H, Ishikawa H, Mizutani K,
Tamura Y and Kinoshita T: Antioxidative and superoxide scavenging
activities of retrochalcones in Glycyrrhiza inflata. Bioorg
Med Chem. 6:339–347. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong GP, Bak EJ, Woo GH, Kim JM, Quan Z,
Kim JM, Yoon HK, Cheon SH, Yoon G, Yoo YJ, et al: Licochalcone E
has an antidiabetic effect. J Nutr Biochem. 23:759–767. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SJ, Kim CG, Yun SR, Kim JK and Jun JG:
Synthesis of licochalcone analogues with increased
anti-inflammatory activity. Bioorg Med Chem Lett. 24:181–185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esmaeli A, Moshrefi M, Shamsara A,
Eftekhar-Vaghefi SH and Nematollahi-Mahani SN: Xeno-free culture
condition for human bone marrow and umbilical cord matrix-derived
mesenchymal stem/stromal cells using human umbilical cord blood
serum. Int J Reprod Biomed. 14:567–576. 2016. View Article : Google Scholar
|
|
12
|
Hung CM, Lin YC, Liu LC, Kuo SC, Ho CT and
Way TD: CWF-145, a novel synthetic quinolone derivative exerts
potent antimitotic activity against human prostate cancer:
Rapamycin enhances antimitotic drug-induced apoptosis through the
inhibition of Akt/mTOR pathway. Chem Biol Interact. 260:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Magro AM, Magro AD, Cunningham C and
Miller MR: Down-regulation of vinculin upon MK886-induced apoptosis
in LN18 glioblastoma cells. Neoplasma. 54:517–526. 2007.PubMed/NCBI
|
|
14
|
Fu Q, Dai S, Zhou Y, Zheng H, Xiang H,
Tian X, Gao F, Manyande A, Cao F, Tian Y and Ye D: MHC-I promotes
apoptosis of GABAergic interneurons in the spinal dorsal horn and
contributes to cancer induced bone pain. Exp Neurol. 286:12–20.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang HT, Chou CT, Chen IS, Yu CC, Lu T,
Hsu SS, Shieh P, Jan CR and Liang WZ: Mechanisms underlying effect
of the mycotoxin cytochalasin B on induction of cytotoxcity,
modulation of cell cycle, Ca2+ homeostasis and ROS
production in human breast cells. Toxicology. 370:1–19. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Y, Lin S, Tseng KF, Han K, Wang Y,
Gan ZH, Min DL and Hu HY: Selumetinib suppresses cell
proliferation, migration and trigger apoptosis, G1 arrest in
triple-negative breast cancer cells. BMC Cancer. 16:8182016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yun J, Kim BG, Kang JS, Park SK, Lee K,
Hyun DH, Kim HM, In MJ and Kim DC: Lipid-soluble ginseng extract
inhibits invasion and metastasis of B16F10 melanoma cells. J Med
Food. 18:102–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, −6, and −7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Autret A and Martin SJ: Emerging role for
members of the Bcl-2 family in mitochondrial morphogenesis. Mol
Cell. 36:355–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Apel K and Hirt H: Reactive oxygen
species: Metabolism, oxidative stress, and signal transduction.
Annu Rev Plant Biol. 55:373–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Cao W, Zheng W, Fan C and Chen T:
Ruthenium complexes containing 2,6-bis(benzimidazolyl)pyridine
derivatives induce cancer cell apoptosis by triggering DNA
damage-mediated p53 phosphorylation. Dalton Trans. 41:12766–12772.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Halliwell B: Oxidative stress and cancer:
Have we moved forward? Biochem J. 401:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu X and Hua X: Targeting ROS: Selective
killing of cancer cells by a cruciferous vegetable derived
pro-oxidant compound. Cancer Biol Ther. 6:646–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nguyen DX and Massagué J: Genetic
determinants of cancer metastasis. Nat Rev Genet. 8:341–352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fietz S, Einspanier R, Hoppner S, Hertsch
B and Bondzio A: Determination of MMP-2 and −9 activities in
synovial fluid of horses with osteoarthritic and arthritic joint
diseases using gelatin zymography and immunocapture activity
assays. Equine Vet J. 40:266–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Ping W, Zu Y and Sun W:
Correlations of lysyl oxidase with MMP2/MMP9 expression and its
prognostic value in non-small cell lung cancer. Int J Clin Exp
Pathol. 7:6040–6047. 2014.PubMed/NCBI
|
|
30
|
Jacob A and Prekeris R: The regulation of
MMP targeting to invadopodia during cancer metastasis. Front Cell
Dev Biol. 3:42015. View Article : Google Scholar : PubMed/NCBI
|