Introduction
Oral squamous cell carcinoma (OSCC) is one of the
most common types of head and neck cancer in the world and accounts
for ~95% of all cases of head and neck cancer (1,2).
Patients with OSCC have a five-year survival rate of ~50%, with the
proportion of men with oral squamous cell carcinoma being higher
than that of women (2,3). The symptoms of OSCC include
erythroplakia, leukoplakia, ulcers in the mouth, necrosis of the
surface tissue, formation of an intermediate cavity or an uneven
surface mass, bleeding, and swelling of the submandibular and
cervical lymph nodes (4,5). OSCC is mainly caused by chemical
factors, including nicotine, alcohol and betel nut juice; physical
factors, including the consumption of high-temperature foods over a
long period; and viral infections, including human papillomavirus,
Epstein-Barr virus and human immunodeficiency virus (6–8).
Histologically, when a tumor has not invaded other organs in its
formation, it can be classified via the symptoms of epithelial
tissue according to reactive epithelial or pre-neoplasia
preneoplastic changes (9,10). The current chemotherapeutic agents
for OSCC are cisplatin, 5-fluorouracil, bleomycin, mitomycin-c,
methotrexate, oxaliplatin and tegafur/uracil (11,12).
Despite scientific investigations and advanced medical
technological achievements, the prognosis of OSCC has remained poor
over the last 10 years (13,14).
The focus of current investigations in novel drug identification is
on the development of low-side-effect and high-efficacy treatments
against chemoresistant cancer cells.
Ursolic acid (3β-hydroxy-urs-12-ene-28-oic acid) is
a lipophilic and pentacyclic triterpenoid compound. The molecular
weight of ursolic acid is 456.68 g/mole, and is a white powder that
was first identified from the epicuticular wax of apples in 1920
(15). Ursolic acid is usually
found in leaves, stem bark and fruit peel, and is present largely
in specific plants and dietary foods, including basil, apples,
peppermint, cranberries, rosemary, lavender, thyme, hawthorn,
oregano, prunes, bilberries and elderflower (16–18).
Ursolic acid has been used for its health-promoting activities via
the composition of herb extracts applied in popular medicines for
centuries (19–21). As ursolic acid exists in common
edible plants, it is considered to exhibit almost no toxicity
towards humans (22,23). With the rapid developments in our
understanding of traditional medicines, ursolic acid has been found
to have pharmacological and biological effects, including
antioxidant, anti-inflammatory, antidiabetic, antibacterial and
antitumor activities. Furthermore, it is used in protection and
prevention against cancer (19–23).
Ursolic acid may be a potential natural compound for
cancer therapy (19–23). In previous years, ursolic acid has
been found to possess pharmacological effects in the prevention and
treatment of cancer (24,25). The pharmacological activities of
ursolic acid not only destroy cancer cells but also regulate cancer
cell metabolism, prevent angiogenesis and metastasis, enhance cell
differentiation, and protect healthy tissues from the oxidative and
inflammatory stimulation that lead to the process of cancer cell
metastasis (24–26). The present study aimed to examine
the anti-growth effects of ursolic acid and the underlying
mechanisms of apoptotic cell death in cisplatin-resistant human
oral cancer CAR cells.
Materials and methods
Cell culture
The CAL 27 parental human oral cancer cell line was
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA), which is a cell line identified as tongue squamous cell
carcinoma. The cisplatin-resistant oral cancer CAR cells were
established by gradient induction of increasing concentrations of
cisplatin up to 80 µM in CAL 27 cells, as previously described
(1,8,27). In
brief, the CAL 27 cells were initially incubated with 10 µM
cisplatin for 24 h, and the culture media was replaced by
cisplatin-free fresh culture medium until the CAL 27 cells reached
a confluence of 80–90%. The procedure was repeated with increasing
concentrations of cisplatin, and the CAL 27 cells were cultured
with each concentration (10–80 µM) of cisplatin for five cycles to
obtain the cisplatin-resistant CAR cells. The CAR cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) with 10%
fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin and
100 µg/ml streptomycin. Normal human primary gingival fibroblast
(HGF) was obtained from CLS Cell Lines Service GmbH (Eppelheim,
Germany) and cultivated in DMEM/F12 1:1 medium (HyClone
Laboratories; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% FBS, 100 µg/ml streptomycin, 100 U/ml
penicillin and 2 mM L-glutamine. All cells were cultured in a 37°C
humidified incubator with 5% CO2.
Chemicals, reagents and
antibodies
DMEM, DMEM/F12 1:1 medium, FBS, L-glutamine, and
penicillin/streptomycin were purchased from HyClone Laboratories;
GE Healthcare Life Sciences. Ursolic acid,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
and other chemicals were of analytical grade from Sigma-Aldrich;
EMD Millipore (Billerica, MA, USA) unless otherwise specified. The
pan-caspase inhibitor z-VAD-fmk was purchased from EMD Millipore.
Caspase-3 and Caspase-9 Colorimetric Assay kits were from R&D
Systems, Inc. (Minneapolis, MN, USA). The anti-caspase-3 (cat. no.
GTX110543), anti-caspase-9 (cat. no. GTX112888),
anti-phosphorylated (p)-AKTSer473 (cat. no. GTX28932),
anti-AKT (cat. no. GTX121937), anti-p-BADSer136 (cat.
no. GTX50136), anti-BAD (cat. no. GTX130108), anti-Bax (cat. no.
GTX109683), anti-Bcl-2 (cat. no. GTX100064), anti-Bcl-xL (cat. no.
GTX84834), and anti-β-actin (cat. no. GTX109639) antibodies, and
the anti-rabbit (cat. no. GTX213110-01) and anti-mouse (cat. no.
GTX213111-01) IgG horseradish peroxidase (HRP)-linked antibodies
were all purchased from GeneTex, Inc. (Hsinchu, Taiwan). The
reactive oxygen species (ROS) indicator H2DCFDA and the
mitochondrial membrane potential (ΔΨm) detector
DiOC6(3) were obtained
from Molecular Probes; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA).
Cell viability assay
Cell viability was evaluated with an MTT method, as
previously described (23,28). Briefly, the CAR or HGF cells were
seeded in a 96-well plate at a density of 1×104
cells/well and treated with different concentrations (50, 100, 150
or 200 µM) of ursolic acid prior to pre-incubation with or without
10 µM z-VAD-fmk (pan-caspase inhibitor) for 1.5 h at 37°C. After 24
h, the medium was removed, and the cells were cultured with 0.5
mg/ml MTT solution for an additional 2 h at 37°C. Subsequently, 100
µl DMSO was used to dissolve the blue formazan product, and cell
viability was spectrophotometrically measured at the absorbance of
570 nm, as previously described (29,30).
Kinetic cell confluence assay
The cell confluence experiment was monitored using
the IncuCyte ZOOM system (Essen BioScience, Ann Arbor, MI, USA), as
previously described (1,31). In brief, the CAR cells at a density
of 1×104 cells per well were seeded in a 96-well plate
and then exposed to 0, 50, 100 and 200 µM of ursolic acid for 48 h.
Data collection was performed every 2 h until 48 h, and images of
the morphological changes were captured and collected every 12 h
using the IncuCyte ZOOM system (Essen BioScience).
In vitro caspase activity assay
The activities of caspase-3 and caspase-9 were
detected using Caspase-3 and Caspase-9 Colorimetric Assay kits
(R&D Systems Inc.) with synthetic tetrapeptides
[Asp-Glu-Val-Asp (DEAD) for caspase-3; Leu-Glu-His-Asp (LEHD) for
caspase-9] labeled with p-nitroaniline (pNA) to link to the
caspase-specific substrate. The CAR cells (5×106 cells
per 75T flask) were treated with or without 50, 100, 150 and 200 µM
of ursolic acid for 24 h. The cell lysates were then harvested, and
the supernatants were incubated with the supplied reaction buffer
with dithiothreitol and DEAD-pNA or LEHD-pNA as substrates at 37°C
for 2 h in the dark according to the manufacturer's protocols.
Immunoblotting analysis
The CAR cells (5×106 cells per 75T flask)
were treated with or without 100, 150 and 200 µM of ursolic acid
for 12 h. The cells were then harvested and lysed with Trident RIPA
lysis buffer (GeneTex, Inc.). The Pierce BCA protein assay kit was
used to detect the protein concentration, following which an equal
quantity of the protein sample (40 µg) was subjected to
electrophoresis on a 10–12% sodium dodecyl sulfate-polyacrylamide
gel, as previously described (32,33).
The separated protein was transferred onto the Immobilon-P Transfer
membrane (Merck Millipore, Darmstadt, Germany) via use of
electroblotting. Thereafter, the membranes were soaked in 5% skim
milk and individually incubated overnight with primary antibodies,
including caspase-3, caspase-9, p-AKTSer473, AKT,
p-BADSer136, BAD, Bax, Bcl-2, Bcl-xL (all 1:1,000
dilution) and β-actin (1:5,000 dilution) at 4°C, followed by
incubation with the appropriate HRP-conjugated secondary antibodies
(1:10,000 dilution) for 1 h at room temperature to hybridize
targeted protein using Immobilon Western Chemiluminescent HRP
substrate (Merck Millipore), as previously described (34,35).
All bands of immunoblots were normalized to β-actin, and their
densitometric quantification was performed using NIH ImageJ 1.47
software (National Institutes of Health, Bethesda, MD, USA).
Measurements of ΔΨm and ROS production
using flow cytometry
The CAR cells (2×105 cells/ml) in 12-well
plates were exposed to 0, 50, 100, 150 and 200 µM of ursolic acid
for 12 h. The cells were then collected and incubated with 500 µl
of H2DCF-DA (ROS detector dye, 10 µM) and 50 nM of the
cell-permeant ΔΨm probe, DiOC6(3), at 37°C for 30 min using flow
cytometry, as previously described (33,36).
Statistical analysis
The values are expressed as the mean ± standard
deviation from at least three separate experiments. Data analysis
was performed using SPSS software version 16.0 (SPSS, Inc.,
Chicago, IL, USA). The differences were analyzed using one-way
analysis of variance followed by Dunnett's test. P<0.001 was
considered indicate a statistically significant difference.
Results
Effects of ursolic acid on the
viability of cisplatin-resistant human oral cancer CAR cells
The cytotoxicity of ursolic acid towards CAR cells
was first investigated. The cells were cultured with various
concentrations of ursolic acid (50, 100, 150 and 200 µM) for 24 h.
Cell viability was evaluated using the MTT assay. The results
demonstrated that ursolic acid at 100, 150 and 200 µM significantly
reduced the viability of CAR cells in a concentration-dependent
manner (Fig. 1A). By contrast,
ursolic acid exerted no toxicity towards the normal HGF cells
(Fig. 1B). Similarly, the cell
confluence of the cultured CAR cells following exposure to the
different concentrations (0, 50, 100 and 200 µM) of ursolic acid
was monitored using the IncuCyte ZOOM system instrument at the 2-h
period. The data showed that the inhibition of CAR cell confluence
appeared following incubation with 200 µM ursolic acid, compared
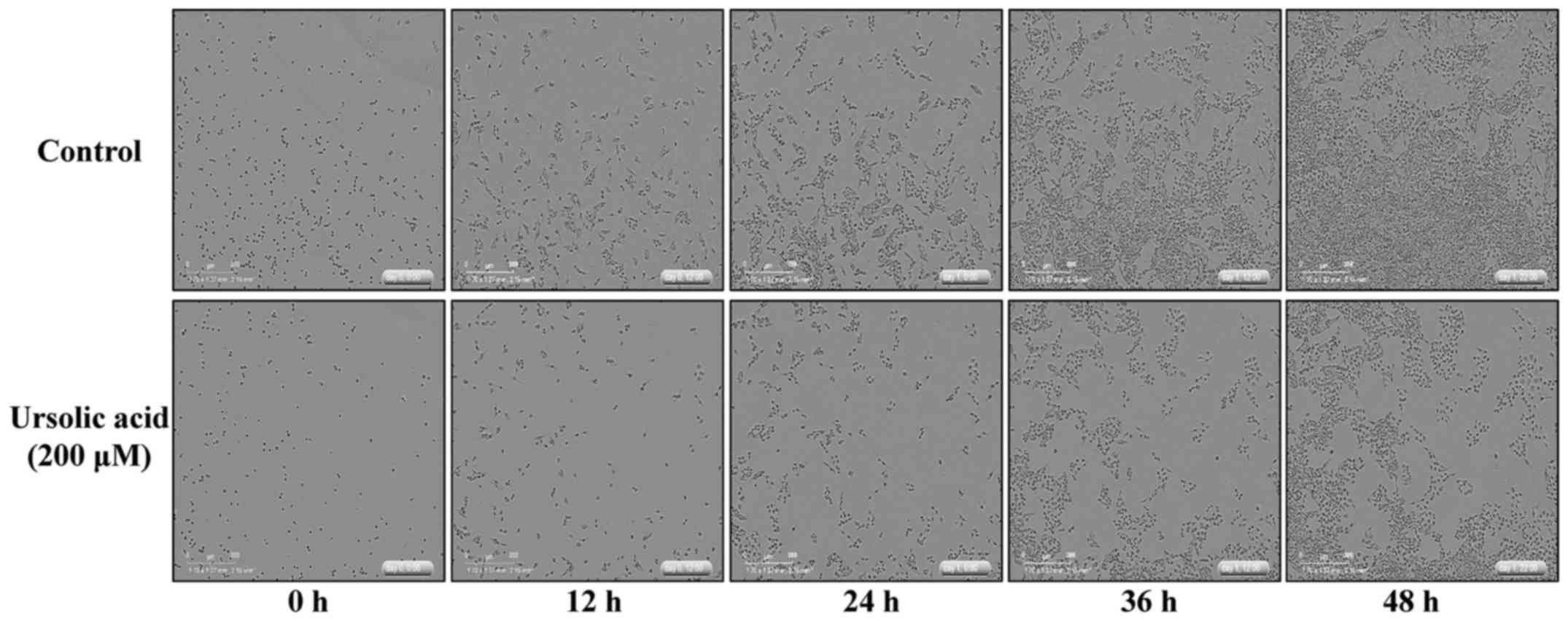
with the control, when incubated for up to 48 h (Fig. 2). In addition, images of the
cultured CAR cells captured at 12-h intervals demonstrated that
ursolic acid at 200 µM induced cell morphology changes and a
decrease of cell confluence, and triggered CAR cell death (Fig. 3). Therefore, these finding suggested
that ursolic acid at 200 µM produced a marked reduction in the
viability of CAR cells.
Effects of the pan-caspase inhibitor
z-VAD-fmk against ursolic acid-induced caspase-dependent apoptosis
of CAR cells
To further examine whether the observed suppression
of cell viability involved apoptotic machinery, the cells were
pretreated with 10 µM z-VAD-fmk and then exposed to 200 µM ursolic
acid for 24 h. The results showed that, without prior incubation of
the CAR cells with 10 µM z-VAD-fmk, the inhibition of cell
viability was significantly inhibited by ursolic acid at 200 µM
(Fig. 4). Therefore, ursolic acid
inhibited CAR cell viability via the caspase pathway.
Effects of ursolic acid on
caspase-3/-9-dependent apoptosis of CAR cells
To investigate whether the ursolic acid-induced
apoptosis in CAR cells is associated with the intrinsic pathway
(caspase-3 and caspase-9) following treatment with various
concentrations (0, 50, 100, 150 and 200 µM) of ursolic acid, the
activities and protein levels of caspase-3 and −9 were individually
assayed using a colorimetric assay and western blot analysis. The
results indicated that ursolic acid significantly promoted the
activities of caspase-3 at 100, 150 and 200 µM concentrations in a
concentration-dependent manner (Fig.
5A). Similarly, the activity of caspase-9 was markedly enhanced
in the CAR cells exposed to ursolic acid (150 and 200 µM; Fig. 5B). Ursolic acid markedly increased
the protein level of the active form of caspase-3 (Fig. 5C). In addition, it promoted an
increase in the protein level of cleaved caspase-9 (Fig. 5C). On the basis of these results, it
was suggested that the apoptotic mechanism of ursolic acid in CAR
cells was mediated via caspase-3/-9-dependent signaling.
Effects of ursolic acid on the levels
of ROS production and the ΔΨm of CAR cells
As ursolic acid affected the activation of
caspase-9, it was hypothesized that ursolic acid-induced apoptosis
may be regulated via the mitochondria-dependent pathway. The CAR
cells were treated with ursolic acid at various concentrations for
12 h. The levels of ROS production and ΔΨm were measured by flow
cytometric assays. The results indicated that ursolic acid promoted
the production of ROS (Fig. 6A),
but decreased the level of ΔΨm (Fig.
6B) in the CAR cells, and these effects were
concentration-dependent. Based on these findings, mitochondrial
dysfunction may be required for the ursolic acid-induced apoptosis
of CAR cells.
Effects on apoptosis-related protein
levels of CAR cells treated with ursolic acid
To further understand the mechanism of apoptosis in
CAR cells, the protein signals of AKT, BAD, Bax, Bcl-2 and Bcl-xL
were determined in the ursolic acid-treated cells. Ursolic acid at
100, 150 and 200 µM for 12 h decreased the phosphorylation of AKT
on Ser473 (p-AKT) and BAD on Ser136 (p-BAD), decreased the protein
levels of Bcl-2 and Bcl-xL, and increased the expression of BAD and
Bax (Fig. 7A and B). These findings
showed that ursolic acid induced apoptotic CAR cell death through a
mitochondria-dependent pathway.
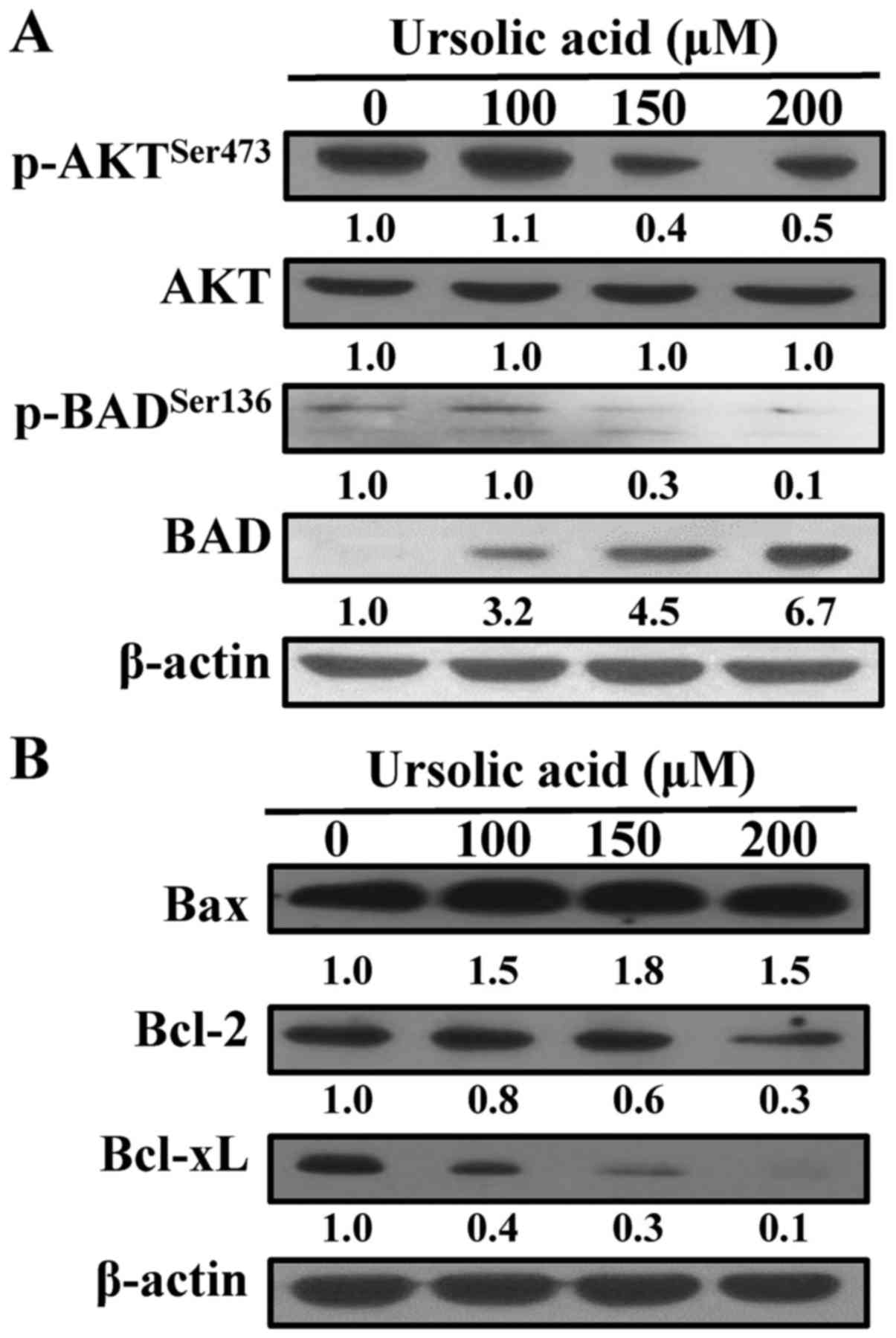 | Figure 7.Effects of ursolic acid on apoptotic
signaling of CAR cells. Cells were treated without or with 100, 150
and 200 µM ursolic acid for 12 h, and cell lysates were collected
and blotted using specific antibodies, including (A)
p-AKTSer473, AKT, p-BADSer136 and BAD, and
(B) Bax, Bcl-2 and Bcl-xL, by immunoblot analysis. Each lane of
protein signaling is normalized to β-actin. p-, phosphorylated;
Bcl-2, B-cell lymphoma 2; BAD, Bcl-2-associated agonist of cell
death; Bax, Bcl-2-associated × protein; Bcl-xL, Bcl-extra
large. |
Discussion
Cisplatin, including oxaliplatin and carboplatin, is
a member of platinum-containing chemotherapeutic agents (37,38).
Unfortunately, cisplatin resistance remains the major cause of
treatment failure in OSCC (39).
Therefore, the identification of novel drugs that can enhance the
inhibition of cell proliferation or target cisplatin-resistant
cancer cells is of paramount importance. In our previous studies
(8,40), human cisplatin-resistant oral cancer
CAR cells were established and the differences between the parental
cell line (CAL 27) and CAR cells were investigated. The preliminary
aim was to examine the effects on cell morphology, viability
(40) and the expression of
ATP-binding cassette B1, a multidrug resistance protein 1 (MDR1) in
CAL 27 and CAR cells prior to cisplatin treatment. It was found
that CAR cells were resistant to 80 µM cisplatin compared with the
parental CAL 27 cells. The protein expression of MDR1 was higher in
CAR cells than in CAL 27 cells. To date, CAR cells have been
applied as a cell platform to assess various photochemicals, novel
compounds and cell conditioned media (1,6–8,40,41).
Ursolic acid is a potent phytochemical and its use
is popular in natural medicinal plants (16–21).
It has been reported that ursolic acid has anticancer effects on
chemoresistant cells. For example, ursolic acid attenuates
temozolomide resistance in glioblastoma cells by downregulating the
expression of O6-methylguanine-DNA
methyltransferase in vitro and in vivo (42). Ursolic acid also inhibits the growth
of gemcitabine-resistant MIA PaCa-2 human pancreatic cancer cells
and induces apoptosis through c-Jun N-terminal kinase and
phosphoinositide 3-kinase/AKT/nuclear factor-κB pathways (43). Ursolic acid enhances the
cytotoxicity in adriamycin-resistant HL60/ADR, K562/ADR, and
MCF-7/ADR cells (44), and induces
doxorubicin-resistant HepG2 cell death via the apoptosis-inducing
factor-dependent pathway (45). In
the present study, it was shown that 200 µM ursolic acid
significantly inhibited the cell viability (Fig. 1A) and cell confluence (Fig. 2; http://goo.gl/zytqBi) of CAR cells. Ursolic acid was
relatively non-toxic to normal HGF cells (Fig.1B), and this result is in agreement
with previous studies on non-tumorigenic cells, including human
normal CCD841 and LO2 cell lines (46) and normal bone marrow mononuclear
cells (47). Ursolic acid led to
the selective cell death of human cisplatin-resistant CAR cells,
rather than normal cells. These results provide novel information
on the oral anticancer activity of ursolic acid in
cisplatin-resistant CAR cells.
It has been demonstrated that ursolic acid inhibits
cell proliferation and apoptosis in human oral cancer KB cells
(48). Ursolic acid also suppresses
the transcription of cyclooxygenase-2 in human oral epithelial
cells (49). However, the molecular
mechanism involved in the effect of ursolic acid on apoptosis in
drug-resistant OSCC remains to be fully elucidated. In the present
study, ursolic acid was investigated for its antitumor effects and
signaling transduction in apoptosis of CAR cells. Ursolic acid
significantly inhibited the proliferation of the CAR cells
(Figs. 1 and 3). Ursolic acid-induced apoptosis was
confirmed by the pan-caspase inhibitor, z-VAD-fmk, which reversed
the reduction in cellular viability in the ursolic acid-treated CAR
cells (Fig. 4). Ursolic acid
increased the activities of caspase-3/caspase-9 and the protein
levels of cleavage-activated caspase-3 and caspase-9 in the CAR
cells (Fig. 5). Ursolic acid also
increased ROS production and decreased ΔΨm in CAR cells (Fig. 6). These results suggested that
ursolic acid induced apoptosis through a mitochondria-dependent
pathway in CAR cells.
BAD is a member of the Bcl-2 family. BAD has a
pro-apoptotic role in the process of apoptosis (50,51).
Dephosphorylated BAD promotes apoptosis and inactivates other
anti-apoptotic Bcl-2 family proteins, including Bcl-2, Bcl-xL and
Bcl-w (50). The BAD protein is
phosphorylated on Serine 99 and Serine 134 sites (Serine 136 site
in mice) by AKT; the BAD protein dissociates from the heterodimer
of Bcl-2/Bcl-xL and then binds to the 14-3-3 protein in the
cytoplasm with an inactive form. Therefore, the free Bcl-2 and
Bcl-xL can inhibit apoptosis (52).
AKT signal transduction is involved in anti-apoptotic effects and
cell proliferation. Piticlisib (GDC-0941), a PI3K inhibitor, has
been demonstrated to inhibit the phosphorylation of BAD on Serine
75 and Serine 99 sites and to induce glioblastoma cell apoptosis in
clinical trials (53). Burpalisib
(BKM120) has also been shown to inhibit the phosphorylation of BAD
on Serine 99 site via the PI3K/AKT pathway in T and B cell acute
lymphoblastic leukemia (54). It
has been reported that ursolic acid suppresses the phosphorylation
and activation of AKT, and then induces apoptosis in leukemia cells
(55,56), colon cancer cells (46,57),
gemcitabine-resistant human pancreatic cancer cells (43), human bladder cancer cells (58), prostate cancer cells (59,60),
and hepatocellular carcinoma cells (61,62).
The results of the present study showed that ursolic acid
suppressed the phosphorylation of AKT and then inhibited the level
of phosphorylated BAD downstream. In addition, ursolic acid
decreased the protein levels of Bcl-2 and Bcl-xL in CAR cells
(Fig. 7). These findings suggested
that ursolic acid suppressed CAR cell growth and induced
mitochondria-dependent apoptosis through suppressing the
phosphorylation of the AKT/BAD pathway.
In conclusion, the results of the present study
supported the hypothesis that ursolic acid-induced apoptosis may
involve the AKT/BAD pathway. The suggested integrated model of the
molecular signaling induced by ursolic acid in CAR cells is
summarized in Fig. 8. The present
study is the first, to the best of our knowledge, to demonstrate
that ursolic acid represents a promising candidate as an oral
anticancer drug, and it may be used as an agent for treating
drug-resistant oral cancer in the future.
Acknowledgements
The authors would like to thank Mr. Meng-Jou Liao
(Tekon Scientific Corporation, Taipei, Taiwan), Mr. Chin-Chen Lin
(Tekon Scientific Corporation) and Mr. Chang-Wei Li (AllBio Science
Inc., Taichung, Taiwan) for their support with techniques and
equipment.
Funding
The present study was supported by the China Medical
University Hospital (grant no. DMR-107-123).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
CFC, HJH and TDW conceived and designed the
experiments; CFC, JSY, WKC, CCL and JHC performed the experiments.
CFC, HYC, SCT and YNJ analyzed the data; CFC, HJH and TDW wrote and
modified the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee MR, Lin C, Lu CC, Kuo SC, Tsao JW,
Juan YN, Chiu HY, Lee FY, Yang JS and Tsai FJ: YC-1 induces G0/G1
phase arrest and mitochondria-dependent apoptosis in
cisplatin-resistant human oral cancer CAR cells. Biomedicine.
7:122017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang ZX, Bian HB, Yang JS, De W and Ji XH:
Adenovirus-mediated suicide gene therapy under the control of Cox-2
promoter for colorectal cancer. Cancer Biol Ther. 8:1480–1488.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferguson BL, Barber S, Asher IH and Wood
CR: Role of oral microbial infections in oral cancer. Dent Clin
North Am. 61:425–434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen S, Hu H, Miao S, Zheng J, Xie Z and
Zhao H: Anti-tumor effect of cisplatin in human oral squamous cell
carcinoma was enhanced by andrographolide via upregulation of
phospho-p53 in vitro and in vivo. Tumour Biol.
39:10104283177053302017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumari K, Haragannavar VC, Kumar KV,
Prasad K and Nambiar S: Basaloid Squamous Cell Carcinoma of Tongue:
A report with emphasis on immunohistochemistry. J Clin Diagn Res.
11:ZD16–ZD18. 2017.PubMed/NCBI
|
|
6
|
Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM,
Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC: Resveratrol-induced
autophagy and apoptosis in cisplatin-resistant human oral cancer
CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol.
50:873–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan CH, Horng CT, Lee CF, Chiang NN, Tsai
FJ, Lu CC, Chiang JH, Hsu YM, Yang JS and Chen FA: Epigallocatechin
gallate sensitizes cisplatin-resistant oral cancer CAR cell
apoptosis and autophagy through stimulating AKT/STAT3 pathway and
suppressing multidrug resistance 1 signaling. Environ Toxicol.
32:845–855. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang PY, Peng SF, Lee CY, Lu CC, Tsai SC,
Shieh TM, Wu TS, Tu MG, Chen MY and Yang JS: Curcumin-loaded
nanoparticles induce apoptotic cell death through regulation of the
function of MDR1 and reactive oxygen species in cisplatin-resistant
CAR human oral cancer cells. Int J Oncol. 43:1141–1150. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Zhang B, Jiang L, Zeng X, Chen Y,
Feng X, Guo Y and Chen Q: RACK1, an excellent predictor for poor
clinical outcome in oral squamous carcinoma, similar to Ki67. Eur J
Cancer. 45:490–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schoop RA, Noteborn MH and Baatenburg de
Jong RJ: A mouse model for oral squamous cell carcinoma. J Mol
Histol. 40:177–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen SC, Chang PM and Yang MH:
Cisplatin/tegafur/uracil/irinotecan triple combination therapy for
recurrent/metastatic head and neck squamous cell carcinoma: A phase
I/II clinical study. Oncologist. 21:537–538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Štulhofer Buzina D, Martinac I, Ledić
Drvar D, Čeović R, Bilić I and Marinović B: Adverse reaction to
cetuximab, an epidermal growth factor receptor inhibitor. Acta
Dermatovenerol Croat. 24:70–72. 2016.PubMed/NCBI
|
|
13
|
El-Deftar MF, El Gerzawi SM, Abdel-Azim AA
and Tohamy SM: Prognostic significance of ploidy and S-phase
fraction in primary intraoral squamous cell carcinoma and their
corresponding metastatic lymph nodes. J Egypt Natl Canc Inst.
24:7–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Naaj IA, Leiser Y, Shveis M, Sabo E and
Peled M: Incidence of oral cancer occult metastasis and survival of
T1-T2N0 oral cancer patients. J Oral Maxillofac Surg. 69:2674–2679.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SY, Kim YJ, Chung SO and Park SU:
Recent studies on ursolic acid and its biological and
pharmacological activity. EXCLI J. 15:221–228. 2016.PubMed/NCBI
|
|
16
|
Kashyap D, Tuli HS and Sharma AK: Ursolic
acid (UA): A metabolite with promising therapeutic potential. Life
Sci. 146:201–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katashima CK, Silva VR, Gomes TL, Pichard
C and Pimentel GD: Ursolic acid and mechanisms of actions on
adipose and muscle tissue: A systematic review. Obes Rev.
18:700–711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woźniak Ł, Skapska S and Marszałek K:
Ursolic acid-a pentacyclic triterpenoid with a wide spectrum of
pharmacological activities. Molecules. 20:20614–20641. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Zhang Y, Dong L, Gao Q, Yin L,
Quan H, Chen R, Fu X and Lin D: Ethnopharmacology, phytochemistry,
and pharmacology of Cornus officinalis Sieb. et Zucc. J
Ethnopharmacol. 213:280–301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Wei K, Xu J, Yang D, Zhang C,
Wang Z and Li M: Belamcanda chinensis (L.) DC-An
ethnopharmacological, phytochemical and pharmacological review. J
Ethnopharmacol. 186:1–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie C, Xie Z, Xu X and Yang D: Persimmon
(Diospyros kaki L.) leaves: A review on traditional uses,
phytochemistry and pharmacological properties. J Ethnopharmacol.
163:229–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Novotný L, Vachálková A and Biggs D:
Ursolic acid: An anti-tumorigenic and chemopreventive activity.
Minireview. Neoplasma. 48:241–246. 2001.PubMed/NCBI
|
|
23
|
Lu CC, Huang BR, Liao PJ and Yen GC:
Ursolic acid triggers nonprogrammed death (necrosis) in human
glioblastoma multiforme DBTRG-05MG cells through MPT pore opening
and ATP decline. Mol Nutr Food Res. 58:2146–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Ursolic acid in cancer prevention and
treatment: Molecular targets, pharmacokinetics and clinical
studies. Biochem Pharmacol. 85:1579–1587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuttan G, Pratheeshkumar P, Manu KA and
Kuttan R: Inhibition of tumor progression by naturally occurring
terpenoids. Pharm Biol. 49:995–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lernoux M, Schnekenburger M, Dicato M and
Diederich M: Anti-cancer effects of naturally derived compounds
targeting histone deacetylase 6-related pathways. Pharmacol Res.
129:337–356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gosepath EM, Eckstein N, Hamacher A,
Servan K, von Jonquieres G, Lage H, Györffy B, Royer HD and Kassack
MU: Acquired cisplatin resistance in the head-neck cancer cell line
Cal27 is associated with decreased DKK1 expression and can
partially be reversed by overexpression of DKK1. Int J Cancer.
123:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang LC, Hsieh MT, Yang JS, Lu CC, Tsai
FJ, Tsao JW, Chiu YJ, Kuo SC and Lee KH: Effect of
bis(hydroxymethyl) alkanoate curcuminoid derivative MTH-3 on cell
cycle arrest, apoptotic and autophagic pathway in triple-negative
breast adenocarcinoma MDA-MB-231 cells: An in vitro study. Int J
Oncol. 52:67–76. 2018.PubMed/NCBI
|
|
29
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840:2310–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang HP, Lu CC, Chiang JH, Tsai FJ, Juan
YN, Tsao JW, Chiu HY and Yang JS: Pterostilbene modulates the
suppression of multidrug resistance protein 1 and triggers
autophagic and apoptotic mechanisms in cisplatin-resistant human
oral cancer CAR cells via AKT signaling. Int J Oncol. Mar
2–2018.(Epub ahead of print). View Article : Google Scholar :
|
|
31
|
Gelles JD and Chipuk JE: Robust
high-throughput kinetic analysis of apoptosis with real-time
high-content live-cell imaging. Cell Death Dis. 7:e24932016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH,
Yang JS, Lai KC, Lin JP, Tang NY, Lin JG, et al: Antitumor effects
of emodin on LS1034 human colon cancer cells in vitro and in vivo:
Roles of apoptotic cell death and LS1034 tumor xenografts model.
Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS,
Wu SH and Chung JG: Benzyl isothiocyanate (BITC) inhibits migration
and invasion of human colon cancer HT29 cells by inhibiting matrix
metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC
and MAPK signaling pathway. J Agric Food Chem. 58:2935–2942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu SH, Hang LW, Yang JS, Chen HY, Lin HY,
Chiang JH, Lu CC, Yang JL, Lai TY, Ko YC and Chung JG: Curcumin
induces apoptosis in human non-small cell lung cancer NCI-H460
cells through ER stress and caspase cascade- and
mitochondria-dependent pathways. Anticancer Res. 30:2125–2133.
2010.PubMed/NCBI
|
|
36
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lin JJ, Huang WW, Tsuzuki M, Lee TH and Chung JG: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Glick JH, Zehngebot LM and Taylor SG IV:
Chemotherapy for squamous cell carcinoma of the head and neck: A
progress report. Am J Otolaryngol. 1:306–323. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao Y and Liu D: The roles of excision
repair cross-complementation group1 in objective response after
cisplatin-based concurrent chemoradiotherapy and survival in head
and neck cancers: A systematic review and meta-analysis. Oral
Oncol. 51:570–577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chiu YJ, Yang JS, Hsu HS, Tsai CH and Ma
H: Adipose-derived stem cell conditioned medium attenuates
cisplatin-triggered apoptosis in tongue squamous cell carcinoma.
Oncol Rep. 39:651–658. 2018.PubMed/NCBI
|
|
41
|
Hsieh MT, Chen HP, Lu CC, Chiang JH, Wu
TS, Kuo DH, Huang LJ, Kuo SC and Yang JS: The novel pterostilbene
derivative ANK-199 induces autophagic cell death through regulating
PI3 kinase class III/beclin 1/Atg-related proteins in
cisplatin-resistant CAR human oral cancer cells. Int J Oncol.
45:782–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu Z, Du S, Ding F, Guo S, Ying G and Yan
Z: Ursolic acid attenuates temozolomide resistance in glioblastoma
cells by downregulating O6-methylguanine-DNA methyltransferase
(MGMT) expression. Am J Transl Res. 8:3299–3308. 2016.PubMed/NCBI
|
|
43
|
Li J, Liang X and Yang X: Ursolic acid
inhibits growth and induces apoptosis in gemcitabine-resistant
human pancreatic cancer via the JNK and PI3K/Akt/NF-κB pathways.
Oncol Rep. 28:501–510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shan JZ, Xuan YY, Ruan SQ and Sun M:
Proliferation-inhibiting and apoptosis-inducing effects of ursolic
acid and oleanolic acid on multi-drug resistance cancer cells in
vitro. Chin J Integr Med. 17:607–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang L, Liu X, Lu Z, Yuet-Wa Chan J, Zhou
L, Fung KP, Wu P and Wu S: Ursolic acid induces
doxorubicin-resistant HepG2 cell death via the release of
apoptosis-inducing factor. Cancer Lett. 298:128–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang J, Liu L, Qiu H, Zhang X, Guo W, Chen
W, Tian Y, Fu L, Shi D, Cheng J, et al: Ursolic acid simultaneously
targets multiple signaling pathways to suppress proliferation and
induce apoptosis in colon cancer cells. PLoS One. 8:e638722013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao N, Cheng S, Budhraja A, Gao Z, Chen J,
Liu EH, Huang C, Chen D, Yang Z, Liu Q, et al: Ursolic acid induces
apoptosis in human leukaemia cells and exhibits anti-leukaemic
activity in nude mice through the PKB pathway. Br J Pharmacol.
165:1813–1826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang G, Yang T, Zhang W, Lu M, Ma X and
Xiang G: In vitro and in vivo antitumor effects of folate-targeted
ursolic acid stealth liposome. J Agric Food Chem. 62:2207–2215.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Subbaramaiah K, Michaluart P, Sporn MB and
Dannenberg AJ: Ursolic acid inhibits cyclooxygenase-2 transcription
in human mammary epithelial cells. Cancer Res. 60:2399–2404.
2000.PubMed/NCBI
|
|
50
|
Bui NL, Pandey V, Zhu T, Ma L and Lobie
PE: Bad phosphorylation as a target of inhibition in oncology.
Cancer Lett. 415:177–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cook SJ, Stuart K, Gilley R and Sale MJ:
Control of cell death and mitochondrial fission by ERK1/2 MAP
kinase signalling. FEBS J. 284:4177–4195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pareja F, Macleod D, Shu C, Crary JF,
Canoll PD, Ross AH and Siegelin MD: PI3K and Bcl-2 inhibition
primes glioblastoma cells to apoptosis through downregulation of
Mcl-1 and Phospho-BAD. Mol Cancer Res. 12:987–1001. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pereira JK, Machado-Neto JA, Lopes MR,
Morini BC, Traina F, Costa FF, Saad ST and Favaro P: Molecular
effects of the phosphatidylinositol-3-kinase inhibitor NVP-BKM120
on T and B-cell acute lymphoblastic leukaemia. Eur J Cancer.
51:2076–2085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin Z, Jiang J and Liu XS: Ursolic
acid-mediated apoptosis of K562 cells involves Stat5/Akt pathway
inhibition through the induction of Gfi-1. Sci Rep. 6:333582016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu B, Wang X, Chi ZF, Hu R, Zhang R, Yang
W and Liu ZG: Ursolic acid-induced apoptosis in K562 cells
involving upregulation of PTEN gene expression and inactivation of
the PI3K/Akt pathway. Arch Pharm Res. 35:543–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lin J, Chen Y, Wei L, Shen A, Sferra TJ,
Hong Z and Peng J: Ursolic acid promotes colorectal cancer cell
apoptosis and inhibits cell proliferation via modulation of
multiple signaling pathways. Int J Oncol. 43:1235–1243. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gai L, Cai N, Wang L, Xu X and Kong X:
Ursolic acid induces apoptosis via Akt/NF-κB signaling suppression
in T24 human bladder cancer cells. Mol Med Rep. 7:1673–1677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang Y, Kong C, Zeng Y, Wang L, Li Z,
Wang H, Xu C and Sun Y: Ursolic acid induces PC-3 cell apoptosis
via activation of JNK and inhibition of Akt pathways in vitro. Mol
Carcinog. 49:374–385. 2010.PubMed/NCBI
|
|
60
|
Meng Y, Lin ZM, Ge N, Zhang DL, Huang J
and Kong F: Ursolic acid induces apoptosis of prostate cancer cells
via the PI3K/Akt/mTOR pathway. Am J Chin Med. 43:1471–1486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chuang WL, Lin PY, Lin HC and Chen YL: The
apoptotic effect of ursolic acid on SK-Hep-1 cells is regulated by
the PI3K/Akt, p38 and JNK MAPK signaling pathways. Molecules.
21:4602016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Son HS, Kwon HY, Sohn EJ, Lee JH, Woo HJ,
Yun M, Kim SH and Kim YC: Activation of AMP-activated protein
kinase and phosphorylation of glycogen synthase kinase3 β mediate
ursolic acid induced apoptosis in HepG2 liver cancer cells.
Phytother Res. 27:1714–1722. 2013. View Article : Google Scholar : PubMed/NCBI
|