Introduction
Gastric cancer (GC), one of the most common cancer
types diagnosed in humans worldwide, is associated with poor
prognosis and metastasis (1,2). While
significant progress has been achieved, treatments for GC are not
yet satisfactory. The average 5-year survival rate of
advanced-stage patients is only ~5–20% (3), which is accompanied by tumor
metastasis and drug resistance.
Human epidermal growth factor receptor 2 (HER2) has
transmembrane tyrosine kinase activity that promotes cell
proliferation and migration (4).
HER2 is a proto-oncogene present on chromosome 17, and
overexpression or mutation of HER2 or abnormal production of HER2
ligands potentially leads to tumorigenesis in many tissues
(5). These factors even play vital
roles in the development and drug resistance of GC (6). Approximately 7–34% of GCs are
characterized by poor prognosis associated with amplification of
the HER2 gene (7,8). Chemotherapy in combination with
trastuzumab, a humanized monoclonal antibody against HER2, carries
a significant survival advantage; however, 12% of all HER2-positive
GC cases still exhibit cancer progression, cancer recurrence or
subsequent drug resistance (9).
Thus, we herein aimed to further study the molecular mechanisms
underlying HER2 in GC to develop more effective treatment for
patients.
Abnormal cellular glycosylation plays a key role in
cancer progression and malignancy (10–12).
Glycosylation is regulated by various glycosyltransferases, such as
fucosyl-, sialyl- and galactosyltransferases, which catalyze the
transfer of monosaccharide residues from nucleotide sugar donors to
specific acceptor substrates, forming glycosidic bonds. In
addition, sialyltransferases are the key enzymes that transfer
sialic acid from CMP-NeuAc2 to glycoproteins or glycolipids in the
biosynthesis of sialic acid-containing glycoproteins and
glycolipids, which are altered in carcinoma cells of different
origins (13) and specifically
correlated with carcinoma differentiation metastatic phenotypes
(14). One important
glycosyltransferase dysregulated in cancer cells is human
β-galactoside α2,6-sialyltransferase (ST6Gal-I) (15,16),
whose activity is low or absent in only normal cells and high in
metastatic tumor cells, such as breast, liver and colon cancer
cells (12,17–19).
ST6Gal-I is hypothesized to improve the progression and metastasis
of cancer cells via the sialylation-dependent modulation of cell
surface receptors. For example, ST6Gal-I overexpression was
revealed to increase the α2,6-sialylation levels on the Fas
receptor, certain integrins, and TNFR1 impeded apoptosis and
blocked TNF-stimulated cell death (20–22).
Furthermore, ST6Gal-I was revealed to induce the sialylation of
EGFR, resulting in resistance to EGFR-targeted chemotherapy
(23). However, the molecular
mechanisms by which ST6Gal-I mediates HER2 in GC cells have not
been well-characterized.
In addition, determining whether HER2
α2,6-sialylation plays a vital role in the malignancy of GCs is
necessary. To identify whether ST6Gal-I is responsible for HER2
sialylation, we prepared and characterized ST6Gal-I knockdown and
ST6Gal-I overexpression SGC7901 gastric carcinoma cells. Given that
HER2 activity and downstream signaling are highly correlated with
cell proliferation, we investigated the effects of HER2 sialylation
on the PI3K/Akt pathway and on trastuzumab sensitivity in SGC7901
cancer cells. Our results revealed that the HER2 sialylation level
along with HER2 mutations may be a reliable biomarker for anti-HER2
therapy in GC.
Materials and methods
Cell culture and transfection
SGC7901 GC cells, purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA), were maintained in
RPMI-1640 medium with 10% FBS and 1% antibiotic solution containing
penicillin/streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). All cells were cultured in a humidified
incubator at 37°C with 5% CO2. The stable ST6Gal-I
overexpression, knockdown and empty vector cell lines were
established as previously described (24). In brief, the pcDNA3.1(−)/ST6Gal-I,
sh-ST6Gal-I and empty vectors were transfected into SGC7901 cancer
cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Limiting dilution was applied to obtain sub-cell
line clones after 24 h of transfection. Blasticidin S HCl (1.5
µg/ml) was used to select the low-expressing ST6Gal-I clone and
G418 (350 µg/ml) was utilized to select the ST6Gal-I overexpression
clone. ST6Gal-I overexpression or knockdown was verified by
ST6Gal-I mRNA expression and protein expression analyses.
Quantitative real-time PCR
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was
synthesized using the PrimerScript® RT Master Mix kit
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol. qRT-PCR was performed on a Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PCR condition
was as following: 95°C for 5 min, followed by 40 cycles of 95°C for
15 sec, 50°C for 30 sec and 72°C for 30 sec. The sequences of
primers used for the real-time PCR assays were as follows:
ST6Gal-I: forward, 5′-CCTCTGGGATGCTTGGTATC-3′ and reverse,
5′-GTGCAGGCACTATCGAAGAA-3′; GAPDH: forward,
5′-AGCCTCAAGATCATCAGC-3′ and reverse, 5′-GAGTCCTTCCACGATACC-3′.
ST6Gal-I activity assay
We conducted lectin staining to assess ST6Gal-I
activity. Briefly, the cells were stained with FITC-conjugated SNA
lectin (EY Laboratories, San Mateo, CA, USA), which is specific for
2,6-sialic acids, according to the manufacturer's instructions.
Cells were stained for 40 min at 4°C with SNA-FITC at a 1:200
dilution and analyzed by fluorescence-activated cell sorting (FACS;
BD Biosciences, Franklin Lakes, NJ, USA). In addition, cells were
stained with SNA-FITC at a 1:100 dilution for 4 h for the cell
immunofluorescence assay.
Cell Counting Kit-8 (CCK-8) assay
To assess the proliferation of the transfected
cloned cell lines, a CCK-8 detection kit (Dojindo Laboratories,
Kumamoto, Japan) was used according to the manufacturer's
instructions. Approximately 3,000 cells were seeded onto a 96-well
plate in quintuplicate for 6 h, and complete medium was then
replaced with RPMI-1640 medium without fetal bovine serum (FBS) for
0, 24, 48, 72, 96, 120 or 144 h. Next, 10 µl of CCK-8 reagent was
added to each well, and the absorbance was measured at 450 nm using
a Multiskan Spectrum spectrophotometer (BioTek Instruments, Inc.,
Winooski, VT, USA). To assess drug resistance, cells were seeded
and incubated with different doses of trastuzumab (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 24 h and CCK-8 assays were then
performed.
Cell cycle and apoptosis analysis by
flow cytometry
To assess the cell cycle, ST6Gal-I overexpression or
ST6Gal-I knockdown cells were seeded onto 6-well plates and
cultured in medium containing no FBS for 24 h. The cells were
harvested, fixed with 70% cold ethanol at 4°C for 2 h, washed with
ice cold phosphate-buffered saline (PBS) 3 times and incubated with
staining solution (20 mg/ml propidium iodide and 200 µg/ml
DNase-free RNase-A in PBS) at room temperature for 30 min at 37°C.
The labeled cell cycle populations were quantified by FACSCalibur
flow cytometry (BD Biosciences) and cell cycle distributions were
analyzed by FlowJo software (Tree Star, Inc., Ashland, OR, USA). In
addition to the cell apoptosis assays, cells were incubated with
trastuzumab for 24 h, collected for staining, and stained with an
FITC-labeled Annexin V and propidium iodide staining kit (BD
Biosciences, San Jose, CA, USA) for 60 min at 37°C. Staining
controls were prepared with fixed cells single stained and
unstained; a positive apoptotic control was obtained by incubating
the cells with 1 mM hydrogen peroxide for 15 h (Sigma-Aldrich;
Merck KGaA). Cell populations stained by the 2 different reagents
were recorded by FACS.
Transwell invasion assay
In vitro invasion assays were performed using
Transwell-24 plates (Corning Inc., Corning, NY, USA);
~5×104 transfected cells in 150 µl of RPMI-1640 medium
without FBS were added to the upper compartment of the Transwell
chamber coated with 40 µl of Matrigel (pore size, 8.0 µm; diameter,
6.5 mm), and 350 µl of RPMI-1640 medium supplemented with 20% FBS
was added to the lower compartment as a chemoattractant. After
incubation for 24 h at 37°C and 5% CO2, the upper
chamber was washed 3 times with PBS, and the cells that did not
invade through the pores of the upper surface of the membrane were
removed by cotton swabs. The invaded cells were subsequently fixed
and stained with 0.1% crystal violet. After being washed with PBS
and dried, the cells were visually counted in 4 randomly selected
fields with an inverted microscope (Olympus IX71; Olympus Corp.,
Tokyo, Japan) at ×200 magnification.
Western blot analysis
Cells were incubated with trastuzumab for 24 h and
collected for western blot analysis. The cells were lysed using
cell lysis buffer, and protein concentrations were assessed with a
BCA assay kit (both from Beyotime Institute of Biotechnology,
Shanghai, China). Equal amounts of denatured proteins were
separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride (PVDF) membranes. The membranes were blocked with 5%
non-fat milk and then incubated overnight with primary antibodies
directed against ST6Gal-I (dilution at 1:500; cat. no. sc-6263),
ERK (dilution at 1:1,000; cat. no. sc-514302), p-ERK (dilution at
1:500; cat. no. sc-81492), AKT (dilution at 1:1000; cat. no.
sc-5298), p-AKT (dilution at 1:500; cat. no. sc-135650), HER2
(dilution at 1:500; cat. no. sc-7301), p-HER2 (dilution at 1:500;
cat. no. sc-81507), caspase-3 (dilution at 1:400; cat. no. sc-7272;
all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
GAPDH (dilution at 1:2,000; cat. no. ab9484; Abcam, Cambridge, MA,
USA), after washed with TBS-Tween (0.1%) three times, all membranes
were then incubated with mouse-IgGκ BP-HRP secondary antibody
(dilution at 1:2,000; cat. no. sc-516102; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. Proteins were visualized using
an enhanced chemiluminescence (ECL) kit (Amersham Biosciences,
Little Chalfont, UK) according to the manufacturer's instructions.
The relative amounts of proteins were determined by densitometry
using ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Immunoprecipitation assays
Cells were lysed, and 500 µg of lysed protein was
incubated overnight at 4°C with 50 µl of SNA-conjugated agarose (EY
Laboratories). α2,6-Sialylated proteins bound to SNA-agarose beads
were precipitated by centrifugation and washed extensively with
lysis buffer. Sialylated proteins were released from complexes by
boiling in SDS-PAGE sample buffer and immunoblotted for HER2 (Santa
Cruz Biotechnology, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The data were presented as
the mean value ± standard deviation (SD). Statistical comparisons
between different groups were performed by one-way analysis of
variance (ANOVA) with the Least Significant Difference (LSD) post
hoc test and statistical comparisons between two groups were
analyzed by t-test comparison. Differences were considered
statistically significant when P-values were <0.05.
Results
Establishment of ST6Gal-I
overexpression and ST6Gal-I knockdown clones of SGC7901 human GC
cells
ST6Gal-I and HER2 are biomarkers associated with the
malignant progression and poor prognosis of GC (25–27).
We confirmed that HER2, p-HER2 and SNA-HER2 were expressed in
SGC7901 GC cell lines, MCF7 breast cancer cell lines, and A549 and
H460 lung cancer cell lines (Fig.
1A). To evaluate the role of ST6Gal-I-induced sialylation in
regulating GC development, we constructed and transfected ST6Gal-I
overexpression or ST6Gal-I knockdown plasmids into SGC7901 cells.
After limiting dilution and persistent culture, we obtained several
different sub-cell line clones with stable expression. The vector 2
cell line was selected as the control group, the X61-4 cell line
was selected as the knockdown group, and the PS7 cell line was
selected as the overexpression group. ST6Gal-I expression was
further confirmed by PCR and western blotting. Endogenous ST6Gal-I
gene and protein expression were stably knocked down in the X61-4
cell line, while the PS7 cell line expressed the ST6Gal-I gene and
protein at high levels compared to that in the vector cell line
(Fig. 1B-E). Collectively, these
data strongly demonstrated that ST6Gal-I was stably overexpressed
or knocked down in SGC7901 cells.
 | Figure 1.Establishment of ST6Gal-I
overexpression and ST6Gal-I knockdown clones of SGC7901 human
gastric cancer cells. (A) The expression levels of HER2, p-HER2 and
SNA-HER2 in SGC7901, MCF7 and A549 cancer cell lines as determined
by western blotting. (B-E) The vector, X61-4 and PS7 cell lines
were chosen from stable cell clones of the empty vector group, the
ST6Gal-I knockdown group and the ST6Gal-I overexpression group,
respectively, and the mRNA and protein levels of ST6Gal-I in the
three groups were assessed by PCR and western blotting. *P<0.05,
**P<0.01. ST6Gal-I, β-galactoside α2,6-sialyltransferase; HER2,
human epidermal growth factor receptor 2. |
Overexpression of ST6Gal-I impacts the
α2,6-linked sialic acids levels on HER2
To assess the functional consequence of ST6Gal-I
upregulation or downregulation in tumor cells, we measured the
levels of α2,6-sialylation on the HER2 receptor. FITC-conjugated
SNA lectin was used to recognize α2,6-linked sialic acids using
flow cytometry and immunofluorescence microscopy. The FACS results
indicated that the SNA fluorescence in PS7 cells was stronger than
that in the X61-4 and vector groups (Fig. 2A and B). The immunofluorescence
results also revealed that PS7 cells expressed significantly higher
levels of α2,6-linked sialic acids than the vector cells, while
X61-4 cells expressed lower levels of α2,6-linked sialic acids than
the vector control cells (Fig. 2C and
D). Moreover, X61-4 cells and PS7 cells were incubated with
agarose-conjugated SNA lectin. The α2,6-sialylated proteins bound
by SNA-agarose were isolated by SDS-PAGE and immunoblotted for
HER2. The SNA-HER2 expression in PS7 cells was much higher than
that in the vector group, and SNA-HER2 expression in X61-4 cells
was lower than that in the vector group (Fig. 2E). Thus, overexpressing ST6Gal-I
hypersialylated HER2, while knocking down ST6Gal-I hyposialylated
HER2.
 | Figure 2.Overexpressing ST6Gal-I impacts the
levels of α2,6-linked sialic acids on HER2. (A) The level of
α2,6-linked sialic acids was recognized by FITC-conjugated SNA
lectin using flow cytometry. (B) The graph depicts the relative
fluorescence intensities of the three groups, each of which was
analyzed in triplicate. **P<0.01. (C) Cells were stained with
FITC-SNA and then assessed for α2,6 sialylation on the cell surface
by fluorescence microscopy. Scale bar, 50 µm. (D) The graph depicts
the relative fluorescence intensities of the three groups, each of
which was analyzed in triplicate. *P<0.05, **P<0.01. (E)
Sialylated HER2 was harvested and lysed for immunoblotting
analysis. **P<0.01. ST6Gal-I, β-galactoside
α2,6-sialyltransferase; HER2, human epidermal growth factor
receptor 2. |
Overexpression of ST6Gal-I impacts the
tumor cell cycle and invasion ability
To investigate whether ST6Gal-I protected GC cells
from serum starvation by regulating cell cycle progression, FACS
was used to analyze the cell cycles of PS7 cells, X61-4 cells and
vector cells. Representative FACS results for the three group cells
are shown in Fig. 3A, and FACS data
are summarized in Fig. 3B. Among
the PS7 cells, 35.07% were in the S phase, which was much higher
than that in X61-4 cells (26.6%) and vector control cells (30.71%).
Accordingly, the proportion of PS7 cells remaining in the G2/M
phase was 11.66%, compared to 6.74% for X61-4 cells and 8% for
vector cells (P<0.01). Serum-starved cells highly expressing
ST6Gal-I maintained more cells in the S phase than low
ST6Gal-I-expressing cells, suggesting that ST6Gal-I expression
promoted GC cells from the G0/G1 phase into the S phase, thus
enhancing proliferation. Thus, disturbances in ST6Gal-I-mediated
sialylation altered cell proliferation.
To further assess whether ST6Gal-I expression
promoted the proliferation of GC cells, CCK-8 assays were
conducted. The growth rates of PS7 and X61-4 cloned cells were
increased and markedly decreased, respectively, compared to those
of vector cells under serum starvation (Fig. 3C). Matrigel-coated Transwell assays
were applied to detect the invasion abilities of the cloned cell
lines. As shown in Fig. 3D and E,
more PS7 clone cells penetrated the Matrigel-coated membrane than
vector cells, and fewer ST6Gal-I knockdown cells penetrated the
Matrigel-coated membrane than vector cells, indicating that
ST6Gal-I-overexpressing cells exhibited a much higher invasion
ability than vector cells (P<0.05). Collectively, these results
indicated that ST6Gal-I may be an essential factor promoting GC
cell proliferation and invasion.
Elevated HER2 α2,6-sialylation
increases trastuzumab drug resistance
For future therapeutic applications, we investigated
whether HER2 sialylation decreases apoptosis and enhances
resistance to trastuzumab treatment. FACS analysis and a CCK-8
assay were performed to analyze trastuzumab inhibition in GC cells.
Trastuzumab inhibition was dose-dependent in each group, and the
inhibition effect of trastuzumab on PS7 cells was lower than that
on vector cells; however, X61-4 cells were inhibited at a much
higher rate than vector cells. When the drug concentration reached
10 µM, each group exhibited evident cell death. Moreover,
FITC-labeled Annexin V and propidium iodide staining were recorded
by FACS to indicate cell apoptosis. As shown in Fig. 4B and C, a small population of PS7
cells were apoptotic (including 2.08% early-stage apoptosis and
9.10% late-stage necrosis), whereas a large population of X61-4
cells were apoptotic (76.3% early-stage apoptosis and 0.52%
late-stage necrosis) under serum starvation. After incubation with
trastuzumab, the number of apoptotic PS7 cells was not
significantly increased (11.90% with treatment vs. 11.18% without),
but the number of apoptotic X61-4 cells was distinctly increased
(88.04% with treatment vs. 76.82% without). In addition, caspase-3
was reportedly the executioner of apoptosis (28). Our western blot results revealed
much higher caspase-3 expression in X61-4 cells compared to that in
PS7 cells, and trastuzumab treatment increased caspase-3 expression
in all three groups compared to that in cells not treated with
trastuzumab (Fig. 4D).
Collectively, elevated HER2 α2,6 sialylation reduced cell apoptosis
and increased resistance to trastuzumab treatment. In addition,
HER2 α2,6 sialylation promoted GC cell survival against many
antitumor treatments.
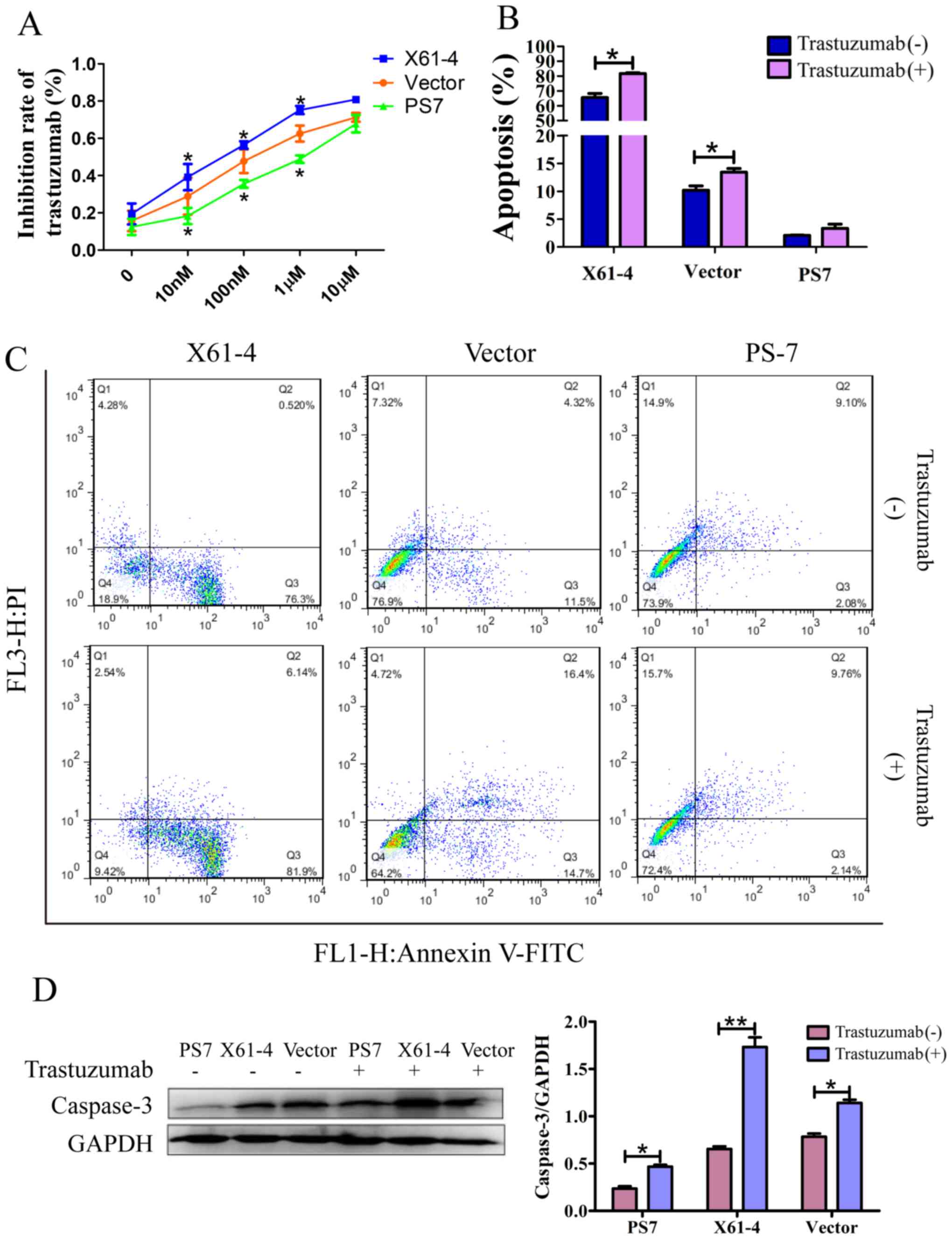 | Figure 4.Elevated HER2 α2,6-sialylation
increases trastuzumab drug resistance. (A) The trastuzumab
inhibition rate in SGC7901 cells in which ST6Gal-I was
overexpressed or knocked down was analyzed using a CCK-8 assay. The
graph depicts the average of three separate experiments, each of
which was performed in triplicate. *P<0.05 vs. the vector. (B)
Graph depicting the quantitative analysis of apoptosis populations
in SGC7901 cells in which ST6Gal-I was overexpressed or knocked
down that were treated with or without trastuzumab; each experiment
was performed in triplicate. *P<0.05. (C) Representative
apoptosis populations were stained with FITC Annexin V and
propidium iodide (PI) in cells in which ST6Gal-I was overexpressed
or knocked down that were treated with or without trastuzumab.
Lower right quadrant, Annexin V-positive; upper right quadrant,
Annexin V- and PI-positive. (D) Caspase-3 levels were detected in
cells in which ST6Gal-I was overexpressed or knocked down that were
treated with or without trastuzumab by western blotting, and a
quantitative analysis of caspase-3 expression is shown, *P<0.05,
**P<0.01. HER2, human epidermal growth factor receptor 2;
ST6Gal-I, β-galactoside α2,6-sialyltransferase; CCK-8, Cell
Counting Kit-8. |
HER2 α2,6 sialylation impacted the Akt
and ERK signaling pathways
To investigate how HER2 sialylation affects the
downstream signaling pathways exerting drug resistance, we examined
the protein levels of HER2, Akt and ERK. HER2 was sialylated by
ST6Gal-I in cancer cells, and the SNA-HER2 level in PS7 cells was
distinctly higher than that in X61-4 cells. HER2 sialylation was
not attenuated with 10 µM trastuzumab treatment for 24 h, while
HER2 sialylation in X61-4 cells was much lower after trastuzumab
treatment (Fig. 5A and B).
Phosphorylated HER2 expression was significantly higher in X61-4
cells than in PS7 cells, and the phosphorylation levels in the
three groups were substantially decreased after trastuzumab
treatment. The HER2 protein expression levels in the three groups
were similar (Fig. 5A and C). It
has been revealed that HER2 activation cοuld initiate the Akt and
ERK signaling pathways by increasing Akt and ERK phosphorylation
(29). Western blot analyses
revealed that the Akt and ERK phosphorylation levels were
significantly higher in PS7 cells than in X61-4 cells and vector
cells, and trastuzumab treatment did not alter the phosphorylation
levels in any of the groups. Furthermore, the total Akt and Erk
repression levels were consistent among all the groups (Fig. 5A, D and E). Collectively, our
results indicated that overexpressing ST6Gal-I in cancer cells
elevated HER2 α2,6 sialylation, activating the downstream cascade,
increasing the phosphorylation of Akt and ERK, and inhibiting the
effect of trastuzumab treatment.
 | Figure 5.HER2 α2,6-sialylation impacts the Akt
and ERK signaling pathways. (A) Cells in which ST6Gal-I was
overexpressed or knocked down were treated with or without
trastuzumab for 24 h, and the protein levels of SNA-HER2, p-HER2,
HER2, p-Akt, Akt, p-ERK, ERK and GAPDH were then measured by
western blotting. (B-E) The relative protein intensities of these
proteins were detected by ImageJ software. *P<0.05, **P<0.01.
HER2, human epidermal growth factor receptor 2; ST6Gal-I,
β-galactoside α2,6-sialyltransferase. |
Discussion
HER2 has transmembrane tyrosine kinase activity that
promotes cell proliferation and suppresses apoptosis, and HER2 is
thereby involved in the pathogenesis and poor outcomes of advanced
GCs (5). Overexpressed or aberrant
HER2 expression is proposed to promote gastric epithelial cancer
cell resistance to HER2-target therapeutics, such as trastuzumab
and lapatinib (30,31). In addition, cardiotoxicity is a
well-known toxicity caused by high doses of trastuzumab (32). Thus, substantial researchexploring
the mechanism of HER2 drug resistance and effective trastuzumab
usage in GCs must be performed.
Evidence has indicated that dysregulated
α2,6-sialylation reduces cell apoptosis and promotes drug
resistance in cancers (22,23). ST6Gal-I is the key enzyme regulating
sialic acid production, and sialic acid is the only sugar in
glycoproteins that bears a net negative charge at physiological pH.
Due to their terminal location and negative charge, sialic acids
can inhibit cell-cell adhesion and enhance adhesion to the
extracellular matrix (33). The
activity of ST6Gal-I is low or absent in only normal mucosa cells,
and its activity is particularly high in metastasizing carcinomas
(17,34). We verified that ST6Gal-I was highly
expressed in GC, breast cancer and lung cancer cells. ST6Gal-I mRNA
and protein expression were assessed after cancer cells were
transfected with ST6Gal-I overexpression or ST6Gal-I knockdown
plasmids (Fig. 1).
ST6Gal-I adds α2,6-linked sialic acids to select
receptors, such as the β1 integrin receptor (35), the Fas receptor and TNFR1 death
receptors (20,22) and multiple other membrane proteins.
In the present study, we made significant progress toward defining
the role of ST6Gal-I in GC drug resistance by revealing that HER2
is sialylated by ST6Gal-I (Fig. 2).
High HER2 sialylation may impact the cycle of GC cells by enriching
the proportion of cells in the S phase and promoting the invasive
ability of cancer cells under serum deprivation (Fig. 3). ST6Gal-I activation has been
revealed to promote the expression of cyclin D2 and increase the
phosphorylation of pRb, accelerating the transition between the G1
and S phases (24). In addition,
HER2 overexpression was demonstrated to activate the HER2-PI3K/Akt
pathways and induce G2-M arrest in breast cancer (36,37).
The results thus far prompted us to further study how sialylated
HER2 regulates intracellular cell cycle molecules in GC. Consistent
with these results, the caspase-3 expression and incidence of
apoptosis in ST6Gal-I-overexpressing cells were low, and the cells
exhibited strong resistance to trastuzumab.
The PI3K/Akt pathway provides critical cell
mitogenic and survival signals required for tumor progression, and
ERK and Akt activation may represent biologically relevant targets
for anticancer therapy (38,39).
Recent research illustrated that the amplification and/or
overexpression of HER2 and phosphorylation of HER2 induced
downstream signaling transduction, including activation of the
MEK/ERK and PI3K/Akt pathways, promoting trastuzumab resistance in
cancer cells (40,41). Results revealed that overexpression
of ST6Gal-I induced high HER2 α2,6-sialylation levels and led to
low HER2 phosphorylation, while the phosphorylation levels of Akt
and ERK were not attenuated. Consequently, ST6Gal-I overexpression
increased the proliferation and migration of GC cells. ST6Gal-I
overexpression inhibits the phosphorylation of targeted protein
sites but may initiate other effector molecules activating the Akt
and ERK proteins (42). Thus,
multiple mechanisms of resistance may coexist in
trastuzumab-resistant cells (43).
In conclusion, our data indicated that amplified or
aberrant HER2 expression may not be the only factor underlying
trastuzumab resistance in GC. The overexpression level of ST6Gal-I
may induce high HER2 α2,6-sialylation, affecting the proliferation,
adhesion and invasion of cancer cells and consequently inhibiting
cell apoptosis via the PI3K/Akt and ERK pathways. In addition,
inhibiting the α2,6-sialylation of HER2 may be one of numerous
therapeutic strategies to overcome or avoid trastuzumab resistance.
Candidate strategies in combination with HER2-targeted therapies
promoting non-cross resistance and non-overlapping toxicity may be
logically ideal.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (no. 81071751), the Natural
Science Foundation of Guangdong Province (no. 2018A030313639), the
Guangzhou Science and Technology Project (no. 201704030059) and the
Opening Project of Zhejiang Provincial Top Key Discipline of
Pharmaceutical Sciences (no. 2016009).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SL and XW conceived and designed the study. MZ
mainly contributed to the flow cytometry-related experiments,
western blot analysis, immunoprecipitation assays and Transwell
invasion assay and prepared the first manuscript. YiL, YS and GT
mainly contributed to the cell culture. XG, JL and YaL mainly
contributed to the RNA isolation and real-time PCR experiments. JW
performed the cell transfection. JW and LJ mainly contributed to
construct the stable overexpression cell line, JW and XH mainly
contributed to construct the stable knockdown cell line. ZD and XC
mainly contributed to the CCK-8 assay. NL provided guidance and
solutions for all the experiments and contributed to data
statistical analysis. SL, XW and NL reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ST6Gal-I
|
β-galactoside
α2,6-sialyltransferase
|
|
HER2
|
human epidermal growth factor receptor
2
|
References
|
1
|
Power DG, Kelsen DP and Shah MA: Advanced
gastric cancer-slow but steady progress. Cancer Treat Rev.
36:384–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HJ, Eun JY, Jeon YW, Yun J, Kim KH,
Kim SH, Kim HJ, Lee SC, Bae SB, Kim CK, et al: Efficacy and safety
of oxaliplatin, 5-Fluorouracil, and folinic Acid combination
chemotherapy as first-line treatment in metastatic or recurrent
gastric cancer. Cancer Res Treat. 43:154–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah MA, Janjigian YY, Stoller R, Shibata
S, Kemeny M, Krishnamurthi S, Su YB, Ocean A, Capanu M, Mehrotra B,
et al: Randomized multicenter phase II study of modified docetaxel,
cisplatin, and fluorouracil (DCF) versus DCF plus growth factor
support in patients with metastatic gastric adenocarcinoma: A study
of the US gastric cancer consortium. J Clin Oncol. 33:3874–3879.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jorissen RN, Walker F, Pouliot N, Garrett
TP, Ward CW and Burgess AW: Epidermal growth factor receptor:
Mechanisms of activation and signalling. Exp Cell Res. 284:31–53.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boku N: HER2-positive gastric cancer.
Gastric Cancer. 17:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sui M, Jiao A, Zhai H, Wang Y, Wang Y, Sun
D and Li P: Upregulation of miR-125b is associated with poor
prognosis and trastuzumab resistance in HER2-positive gastric
cancer. Exp Ther Med. 14:657–663. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanner M, Hollmén M, Junttila TT, Kapanen
AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al:
Amplification of HER-2 in gastric carcinoma: Association with
Topoisomerase II alpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taniguchi N and Korekane H: Branched
N-glycans and their implications for cell adhesion, signaling and
clinical applications for cancer biomarkers and in therapeutics.
BMB Rep. 44:772–781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dall'Olio F, Malagolini N, Trinchera M and
Chiricolo M: Mechanisms of cancer-associated glycosylation changes.
Front Biosci. 17:670–699. 2012. View
Article : Google Scholar
|
|
12
|
Schultz MJ, Swindall AF and Bellis SL:
Regulation of the metastatic cell phenotype by sialylated glycans.
Cancer Metastasis Rev. 31:501–518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y and Chen X: Sialic acid metabolism
and sialyltransferases: Natural functions and applications. Appl
Microbiol Biotechnol. 94:887–905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shaikh FM, Seales EC, Clem WC, Hennessy
KM, Zhuo Y and Bellis SL: Tumor cell migration and invasion are
regulated by expression of variant integrin glycoforms. Exp Cell
Res. 314:2941–2950. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J and Gu J: Significance of
β-galactoside α2,6 sialyltranferase 1 in Cancers. Molecules.
20:7509–7527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Büll C, Stoel MA, den Brok MH and Adema
GJ: Sialic acids sweeten a tumor's life. Cancer Res. 74:3199–3204.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Swindall AF, Londoño-Joshi AI, Schultz MJ,
Fineberg N, Buchsbaum DJ and Bellis SL: ST6Gal-I protein expression
is upregulated in human epithelial tumors and correlates with stem
cell markers in normal tissues and colon cancer cell lines. Cancer
Res. 73:2368–2378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dall'Olio F, Chiricolo M, D'Errico A,
Gruppioni E, Altimari A, Fiorentino M and Grigioni WF: Expression
of β-galactoside α2,6 sialyltransferase and of α2,6-sialylated
glycoconjugates in normal human liver, hepatocarcinoma, and
cirrhosis. Glycobiology. 14:39–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin S, Kemmner W, Grigull S and Schlag PM:
Cell surface α2,6 sialylation affects adhesion of breast carcinoma
cells. Exp Cell Res. 276:101–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swindall AF and Bellis SL: Sialylation of
the Fas death receptor by ST6Gal-I provides protection against
Fas-mediated apoptosis in colon carcinoma cells. J Biol Chem.
286:22982–22990. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhuo Y, Chammas R and Bellis SL:
Sialylation of beta1 integrins blocks cell adhesion to galectin-3
and protects cells against galectin-3-induced apoptosis. J Biol
Chem. 283:22177–22185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Swindall AF, Kesterson RA, Schoeb
TR, Bullard DC and Bellis SL: ST6Gal-I regulates macrophage
apoptosis via α2-6 sialylation of the TNFR1 death receptor. J Biol
Chem. 286:39654–39662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JJ, Yi JY, Jin YB, Lee YJ, Lee JS,
Lee YS, Ko YG and Lee M: Sialylation of epidermal growth factor
receptor regulates receptor activity and chemosensitivity to
gefitinib in colon cancer cells. Biochem Pharmacol. 83:849–857.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Britain CM, Dorsett KA and Bellis SL: The
glycosyltransferase ST6Gal-I protects tumor cells against serum
growth factor withdrawal by enhancing survival signaling and
proliferative potential. J Biol Chem. 292:4663–4673. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baek DW, Kang BW, Hwang S, Kim JG, Seo AN,
Bae HI, Kwon OK, Lee SS, Chung HY and Yu W: Clinical significance
of p53 protein expression, beta-catenin expression and HER2
expression for Epstein-Barr virus-associated gastric cancer.
Chonnam Med J. 53:140–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahmed S, Sami A and Xiang J: HER2-directed
therapy: Current treatment options for HER2-positive breast cancer.
Breast Cancer. 22:101–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oh DY, Jung K, Song JY, Kim S, Shin S,
Kwon YJ, Oh E, Park WY, Song SY and Choi YL: Precision medicine
approaches to lung adenocarcinoma with concomitant MET and HER2
amplification. BMC Cancer. 17:5352017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walsh JG, Cullen SP, Sheridan C, Lüthi AU,
Gerner C and Martin SJ: Executioner caspase-3 and caspase-7 are
functionally distinct proteases. Proc Natl Acad Sci USA.
105:12815–12819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Joslin EJ, Opresko LK, Wells A, Wiley HS
and Lauffenburger DA: EGF-receptor-mediated mammary epithelial cell
migration is driven by sustained ERK signaling from autocrine
stimulation. J Cell Sci. 120:3688–3699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yk W, Cf G, T Y, Z C, Xw Z, Xx L, Nl M and
Wz Z: Assessment of ERBB2 and EGFR gene amplification
and protein expression in gastric carcinoma by immunohistochemistry
and fluorescence in situ hybridization. Mol Cytogenet. 4:142011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Apicella M, Corso S and Giordano S:
Targeted therapies for gastric cancer: Failures and hopes from
clinical trials. Oncotarget. 8:57654–57669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nemeth BT, Varga ZV, Wu WJ and Pacher P:
Trastuzumab cardiotoxicity: From clinical trials to experimental
studies. Br J Pharmacol. 174:3727–3748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan Y, Wu L, Shen S, Wu S and Burdick MM:
Effect of α2,6 sialylation on integrin-mediated adhesion of breast
cancer cells to fibronectin and collagen IV. Life Sci. 149:138–145.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hedlund M, Ng E, Varki A and Varki NM:
α2-6-Linked sialic acids on N-glycans modulate carcinoma
differentiation in vivo. Cancer Res. 68:388–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hou S, Hang Q, Isaji T, Lu J, Fukuda T and
Gu J: Importance of membrane-proximal N-glycosylation on
integrin β1 in its activation and complex formation. FASEB J.
30:4120–4131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma M, Ma Y, Zhang GJ, Liao R, Jiang XF,
Yan XX, Bie FJ, Li XB and Lv YH: Eugenol alleviated breast
precancerous lesions through HER2/PI3K-AKT pathway-induced cell
apoptosis and S-phase arrest. Oncotarget. 8:56296–56310.
2017.PubMed/NCBI
|
|
37
|
Jandial DD, Krill LS, Chen L, Wu C, Ke Y,
Xie J, Hoang BH and Zi X: Induction of G2M arrest by Flavokawain A,
a kava chalcone, increases the responsiveness of
HER2-overexpressing breast cancer cells to herceptin. Molecules.
22:pii E462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bahrami A, Hasanzadeh M, Hassanian SM,
ShahidSales S, Ghayour-Mobarhan M, Ferns GA and Avan A: The
potential value of the PI3K/Akt/mTOR signaling pathway for
assessing prognosis in cervical cancer and as a target for therapy.
J Cell Biochem. 118:4163–4169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Teplinsky E and Muggia F: Targeting HER2
in ovarian and uterine cancers: Challenges and future directions.
Gynecol Oncol. 135:364–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ikink GJ, Boer M, Bakker ER and Hilkens J:
IRS4 induces mammary tumorigenesis and confers resistance to
HER2-targeted therapy through constitutive PI3K/AKT-pathway
hyperactivation. Nat Commun. 7:135672016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yen HY, Liu YC, Chen NY, Tsai CF, Wang YT,
Chen YJ, Hsu TL, Yang PC and Wong CH: Effect of sialylation on EGFR
phosphorylation and resistance to tyrosine kinase inhibition. Proc
Natl Acad Sci USA. 112:6955–6960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Köninki K, Barok M, Tanner M, Staff S,
Pitkänen J, Hemmilä P, Ilvesaro J and Isola J: Multiple molecular
mechanisms underlying trastuzumab and lapatinib resistance in
JIMT-1 breast cancer cells. Cancer Lett. 294:211–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|



















