Introduction
With an estimation of 55,440 new cases (29,200 in
men, 26,240 in women), and a mortality rate of 44,330 (23,020 men,
21,310 women) in 2018, pancreatic cancer remains the fourth leading
cause of cancer-associated mortality in the USA, and is one of the
most life-threatening malignancies (1). As pancreatic cancer shows high level
of heterogeneity and often metastasizes early, management of
pancreatic cancer has always been challenging (2,3). The
majority of patients with pancreatic cancer (~53%) are diagnosed at
an advanced stage, for whom the 5-year-survival rate is only 2–5%,
which is among the lowest of all types and stages of malignancy
(4). Even in the 10% patients who
are diagnosed at early stages, the 5-year-survival rate is only
32%. Gemcitabine as the first line chemotherapy provides limited
benefit on the overall survival rate of patients with locally
advanced and metastatic pancreatic cancer (5,6).
Numerous efforts have been made to improve the treatment outcome.
The development of treatment regimens, including FOLFIRINOX
(7,8) or nab-paclitaxel plus gemcitabine
(9) have led to improvement in
survival and response rates, however, they significantly increase
toxic side effects (10,11). Novel treatment options are urgently
required for pancreatic cancer.
One of the reasons for the poor treatment outcomes
is that pancreatic cancer has an enriched cancer stem cell (CSC)
population (6). CSCs are
responsible for tumor generation (3), are resistant to current chemotherapy
and radiation therapies (12), and
are prone to metastasis (13). The
cells survive current treatments and eventually give rise to new
tumors either at the primary or metastatic sites (14–16).
Depending on the microenvironment, a CSC can be characteristically
quiescent, and the dormancy protects them from chemotherapeutic
agents that target actively dividing cells (17). Alternately, a CSC can divide and
generate daughter cells which give rise to all cell types found in
a particular bulk of tumor (18),
and/or generate daughter cells which do not differentiate but
maintain the full potential for differentiation as the parent stem
cell (self-renewal) (17). The
self-renewal ability maintains the number of CSCs within the tumor,
whereas its descendent progeny constitute the bulk of the tumor.
CSCs also exhibit unique features, including drug resistance and
metastatic ability. If a treatment does not eliminate CSCs, the
CSCs eventually promote tumor recurrence. Therefore, therapies that
inhibit CSCs offers promise in eliminating the whole cancer cell
population.
Herbal preparations of Rauwolfia vomitoria
(Rau), a tropical shrub in the family Apocynaceae, is a traditional
folk medicine in Africa used to treat a variety of conditions,
including hypertension (19,20),
fever (21,22), gastrointestinal diseases (23), liver diseases (24) and cancer (25). The extract as a whole mixture is
widely used as a health supplement. Extracts from the root bark of
this plant are enriched with β-carboline alkaloids and indole
alkaloids (26). β-carboline
alkaloids have been reported to have several bioactivities,
including antitumor effects (27,28).
In our previous study, it was reported that an extract of Rau, with
its hypotensive component reserpine removed, induced pancreatic
cancer cell apoptosis, and inhibited pancreatic tumor growth in
mice (29). The combination of Rau
and gemcitabine showed synergistic antitumor effects (29). In the present study, the activities
of the same extract on inhibiting pancreatic CSCs in vitro
and in vivo were investigated.
Materials and methods
Cell lines and reagents
The PANC-1, AsPC-1, HPAF-II, BxPC-3 and MiA PaCa-2
human pancreatic cancer cell lines were obtained from the American
Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in
the laboratory. The MRC-5 immortalized human lung epithelial cell
line was provided by Dr Sitta Sittampalam at the National Center
for Advancing Translational Sciences, NIH (Bethesda, MD, USA), and
was used as a comparison to the cancer cells. All cells were
cultured at 37°C in 5% CO2/95% air in recommended growth
media: PANC-1 and Mia PaCa-2 in DMEM (cat no. 10-013-CV; Corning,
Inc., Corning, NY, USA); AsPC-1 and BxPc-3 in RPMI-1640 (cat. no.
10-040-CV; Corning, Inc.) and HPAF-II in EMEM (cat. no. 10-010-CV;
Corning Inc.), containing 10% fetal bovine serum (FBS;
Sigma-Aldrich, St. Louis, MO, USA; cat. no. F0926) and 1%
antibiotics (cat. no. 30-001-C; Corning, Inc.). The Rau extract was
provided by Natural Source International, Ltd. (New York, NY, USA)
and was prepared in sterile phosphate-buffered saline (PBS) in 10
mg/ml stock solutions and stored at −20°C.
Cell viability assay
The cells were assessed for viability using a 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
assay at 48 h of treatment. Cells in the exponential growth phase
were exposed to serial dilutions of Rau for 48 h. The medium was
then replaced with fresh media containing MTT and cells were
incubated for 4 h at 37°C. The colorimetric MTT assay assesses
relative proliferation, based on the ability of living, but not
dead cells, to reduce MTT to formazan. The cells did not reach a
plateau phase during the incubation period. The 50% inhibitory
concentration (IC50) was defined as the concentration of
drug that inhibited cell growth by 50% relative to the untreated
control. Pilot experiments for each cell line were performed to
optimize cell density and assay duration, and to center drug
dilution series approximately on the IC50.
Tumor spheroid formation assay
For the PANC-1 cells, a single-cell suspension was
plated into 24-well ultra-low attachment plates (Corning Inc.) at a
density of 5,000 cells/well in stem cell media and incubated at
37°C in a humidified atmosphere of 95% air and 5% CO2.
For the MIA PaCa-2 cells, a single-cell suspension was plated into
96-well ultra-low attachment plates (Corning Inc.) at a density of
100 cells/well in stem cell media and incubated under the same
conditions. The stem cell media consisted of DMEM (Corning Inc.)
supplemented with 1X B27 Supplement, 20 ng/ml human basic
fibroblast growth factor, 20 ng/ml epidermal growth factor, 100
U/ml penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) and 4 µg/ml heparin calcium salt (Thermo Fisher Scientific,
Inc.). The PANC-1 spheroids were counted following 4 weeks of
culture and the MIA PaCa-2 spheroids were counted following 2 weeks
of culture under the microscope. Spheroid diameter was measured
using ImageJ software v1.48 (NIH, Bethesda, MD, USA).
Flow cytometry for the detection of
CSC surface markers
Rau has marked autofluorescence in two ranges of
emission wavelength, at 400–600 nm and 800–900 nm, overlapping the
emission wavelength of several fluorescent labeling molecules.
Therefore, PE-Cy7-conjugated CD24 and APC-conjugated EpCam
antibodies were used as indicative markers for pancreatic CSCs
(CD24+EpCam+) to avoid overlapping with Rau
autofluorescence. The cells were exposed to various concentrations
of Rau for 24 or 48 h. The cells were then washed with PBS three
times, and resuspended in binding buffer (PBS supplemented with
0.1% bovine serum albumin (BSA; Fisher BioReagents, Waltham, MA,
USA; cat. no. BP1605-100) for 15 min. PE-Cy-7-conjugated anti-CD24
antibody (dilution 1:100; cat. no. 311119; BioLegend, Inc., San
Diego, CA, USA) and APC-conjugated anti-EpCam antibody (dilution
1:100; cat. no. 324207; BioLegend, Inc.) were added into the cell
suspension and incubated for 15 min according to the manufacturer's
protocol. The cells were washed in PBS three times following
staining and then analyzed using a BD LSR II flow cytometer. The
data were normalized to cell death, as follows: Normalized CSC
population = original CSC population detected with flow cytometry ×
% cell viability detected with the MTT assay.
Flow cytometry for sorting of side
population from pancreatic cancer cells
Dye Cycle Violet (DCV; Invitrogen; Thermo Fisher
Scientific, Inc.) was used for staining of the non-CSC population.
Cells that efficiently exclude DVC from the cytoplasm are
considered CSC-like population (DCV− cells). The MIA
PaCa-2 cells were suspended at a density of 1×106
cells/ml in DEME supplemented with 10% FBS and 10 mM HEPES. DCV (10
µM) was added and incubated for 30 min at room temperature. The
cells were then washed twice with PBS, and resuspended in DMEM
supplemented with 10% FBS and 10 mM HEPES for 1 h. The cells were
transferred to ice-cold HBSS/2% FBS/10 mM HEPES buffer immediately
prior to flow cytometric sorting. The DCV− and
DCV+ cells were separately collected for further
analysis. Gate setting was performed using cells treated with a
pump inhibitor (verapamil; 200 µM) prior to DCV staining.
SDS PAGE and western blot
analysis
The cells were lysed with RIPA buffer containing
protease inhibitors and phosphatase inhibitors (Sigma-Aldrich; EMD
Millipore) followed by sonication for 10 sec. Either whole cell
lysate or supernatant was used for further experiments, depending
on the proteins of interest. The BCA method was used for protein
quantification (Pierce BCA protein assay kit; Thermo Fisher
Scientific, Inc.). SDS-PAGE and western blot analyses were
performed as routine: 10 µg total protein or 2 µg nuclear or
cytoplasmic fractions were loaded on a 10% SDS-PAGE gel, and
electrophoresis was performed at 60 V for 35 min followed by 90 V
for 90 min. Proteins were transferred to PVDF membrane (cat. no.
ISEQ00010; ED Millipore, Burlington, USA) overnight. Membrane was
then blocked with 5% blocking grade blocker (cat. no. 170-6404;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) in 1X TBS-T
(Tween-20, 0.1%) for 2 h at room temperature with constant shaking.
Primary and secondary antibodies were from Cell Signaling
Technology Inc. (Danvers, MA, USA): rabbit anti-β-catenin (dilution
1:1,000; cat. no. 9582), rabbit anti-vinculin (dilution 1:1,000;
cat. no. 4650), rabbit anti-Histone H3 (dilution 1:2,000; cat. no.
4499), rabbit anti-Nanog (dilution 1:2,000; cat. no. 4903), mouse
anti-β-actin (dilution 1:2,000; cat. no. 3700), and goat
anti-rabbit (dilution 1:5,000; cat. no. 7074) or anti-mouse
(dilution 1:5,000; cat. no. 7076) IgG. Primary antibodies were
incubated overnight at 4°C and secondary antibodies were incubated
for 2 h at room temperature. The blots were established using a
chemiluminescence detection kit (Pierce ECL; cat. no. 32106) or ECL
Plus Western Blotting Substrate (cat. no. 32132) which were both
from Thermo Fisher Scientific, Inc.
RNA isolation, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis
Total RNA was extracted from cells or tissue samples
using TRIzol reagent according to the protocol of the manufacturer
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA synthesis was
performed with 1 µg of total RNA using an Omniscript RT kit
according to the manufacturer's protocol (Qiagen, Inc., Valencia,
CA, USA). cDNA was diluted 1:5 in DEPC-treated nanopure water and
used for further analysis. RT-qPCR analysis was performed using a
Bio-Rad iQ iCycler detection system with iQ SYBR green supermix
(Bio-Rad Laboratories, Inc.). The reactions were performed in a
total volume of 10 µl, including 5 µl of 2X iQ SYBR Green supermix,
0.4 µl of primers at 20 pmol/µl and 0.4 µl of cDNA template. Primer
sequences are as follows: BCL2L2 (F, GCGGAGTTCACAGCTCTATAC and R,
AAAAGGCCCCTACAGTTACCA); Cox-2 (F, CTGGCGCTCAGCCATACAG and R,
CGCACTTATACTGGTCAAATCCC); MMP14 (F, GGCTACAGCAATATGGCTACC and R,
GATGGCCGCTGAGAGTGAC); MYC (F, TCCCTCCACTCGGAAGGAC and R,
CTGGTGCATTTTCGGTTGTTG); hBim (F, TACTCCAGTGCAGTCTCCTC and R,
TCCCATCTTTCCTAACACAG); hDppa4 (F, AAAAGCAAGAAGGGAGAGTGA and R,
CGGAGATTGCACTGAACTGA); hEsrrb (F, TCAGAGAGCAGCCCATACCT and R,
GCGTCACAAACTCCTCCTTC); hOct4 (F, GAGAATTTGTTCCTGCAGTGC and R,
GTTCCCAATTCCTTCCTTAGTG); hSox2 (F, ATGGGTTCGGTGGTCAAGTC and R,
GTGGATGGGATTGGTGTTCTC); hTbx3 (F, GAAGAAGAGGTGGAGGACGA and R,
ATTCAGTTTCGGGGAACAAG); hTcl1 (F, GATACCGATCCTCAGACTCCA and R,
GAGGGACAGAAGGGACAGAA); GAPDH (F, CCAGGTGGTCTCCTCTGACTTCAACA and R,
AGGGTCTCTCTCTTCCTCTTGTGCTC). All qPCR were run according to the
following thermocyclers: Initial denaturation and enzyme activation
at 95°C for 3 min, followed by 40 cycles of denaturing (95°C for 15
sec), annealing (55–60°C for 30 sec), and extension (72°C for 30
sec). Melt curve was carried out at 55-95oC (in 0.5oC increments)
for 30 sec.
All reactions were performed in four repeats for
every sample and with three independent experiments for each group.
GAPDH was used as housekeeping gene for normalization. Gene
expression was quantified using ∆∆CT method and 2−∆∆Ct
was used as the relative expression changes for each gene (30).
Pancreatic cancer xenograft mouse
model
All animal experiments followed a protocol approved
by the Institutional Animal Care and Use Committee of the
University of Kansas Medical Center (Kansas City, KS, USA). Single
treatment and repeated treatments were each used for the
measurement of tumorigenecity. In the single treatment model, the
PANC-1 pancreatic cancer cells at three densities were used for
tumor inoculation (2×104 cells per injection,
2×105 cells per injection, or 1×106 cells per
injection). The PANC-1 cells were suspended in PBS as single cell
suspension and then mixed with either 200 mg/ml Rau or PBS. At each
cell injection number, cells mixed with Rau were injected
subcutaneously into the left flank of the mouse, and cells mixed
with PBS were injected into the right flank of the same mouse. A
total of 10 mice (Athymic Ncr nu/nu, female, 4–6 weeks, 15–20 g)
were used for each cell density. The formation of tumors were
monitored daily, and longitudinal tumor growth was measured using
calipers. Mice were housed in 5 mice/cage in a sterile rodent room
with 12-h/12 h-light/dark cycle. Housing was handled by the
University of Kansas Medical Center Laboratory Animal Resources
following standard protocol.
In the repeated treatment model, a single cell
suspension of PANC-1 cells was mixed with 200 mg/ml Rau, and then
inoculated into 10 mice at 2×105 cells per injection, in
the left and right flanks. Treatment was started the following day
with oral gavage of 20 mg/kg Rau, five times per week for 3 weeks.
In the control group (10 mice), the mice were inoculated with the
same number of cells in PBS, and were then gavaged with an
equivalent volume of saline solution. Tumor formation was monitored
daily, and longitudinal tumor growth was measured using
calipers.
Statistical analysis
Statistical analysis was performed using IBM SPSS
statistical software, version 23 (IBM PSS, Armonk, NY, USA).
Student's t-test and a log-rank test were used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhibition of pancreatic cancer tumor
spheroid formation in vitro
Five human pancreatic cancer cell lines (PANC-1, MiA
PaCa-2, AsPC-1, HPAF-II and BxPC-3) and an immortalized epithelial
cell line (MRC-5) were treated with various concentrations of Rau,
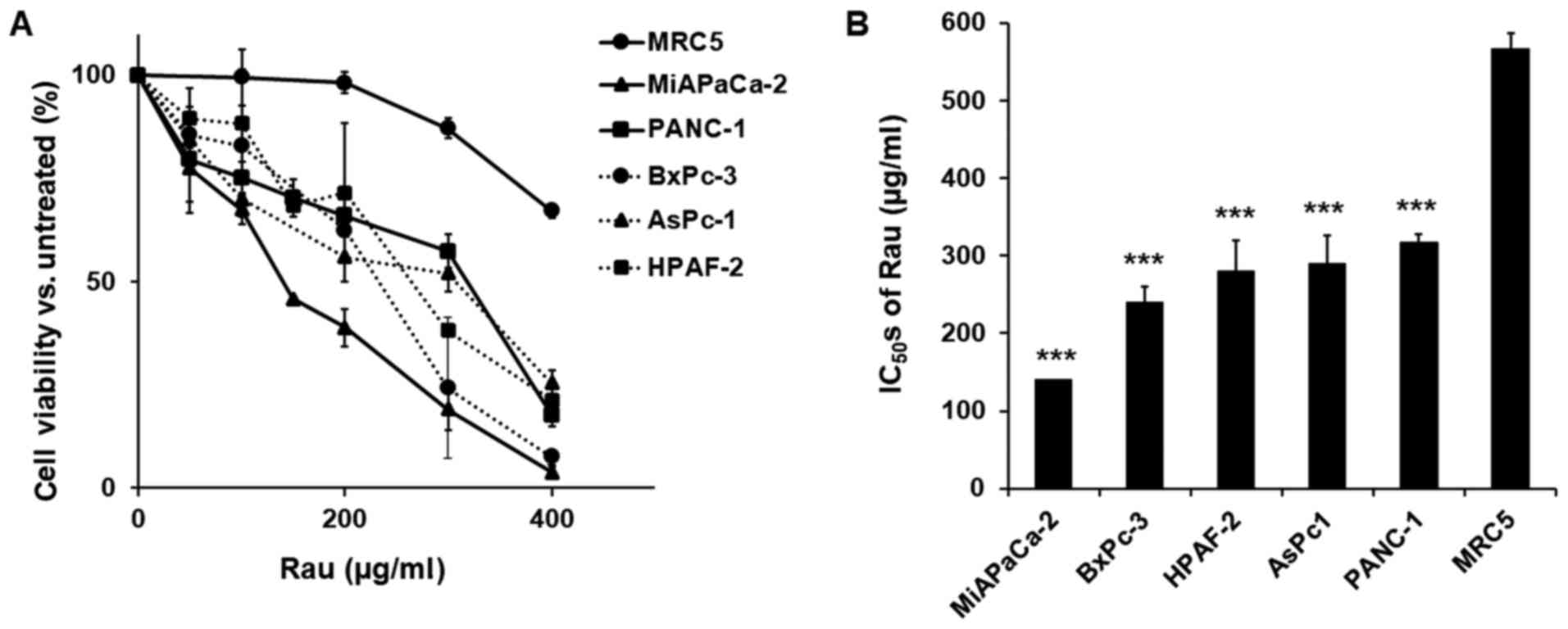
and cell viability was detected 48 h later. Rau inhibited the
proliferation of all five cancer cells (Fig. 1A), with IC50 values
ranging between 140 and 317 µg/ml. The non-cancerous MRC-5
epithelial cell line was less affected by Rau treatment, with a
higher IC50 value of 567 µg/ml (Fig. 1B). These results are consistent with
our previous findings that Rau inhibited the overall proliferation
of pancreatic cancer cells (29).
To investigate the inhibitory effect of Rau in CSCs,
a tumor spheroid formation assay was performed. The ability to form
tumor spheroids is an in vitro indication of the tumorigenic
capacity and self-renew ability of CSCs. When cancer cells are
cultured in non-adherent, serum-free conditions, non-CSC
populations die by anoikis, whereas CSCs overcome anoikis and go
through division leading to the formation of tumor spheroids
(32,33). Single cell suspensions were treated
with Rau and tumor spheroids were counted 4 weeks later. Data
showed that Rau significantly reduced the number of the PANC-1
tumor spheroids at the concentrations of 50 and 100 µg/ml, and
completely eliminated the tumor spheroids at 200 µg/ml (Fig. 2A and B). The estimated
IC50 value for tumor spheroids inhibition is 39.44
µg/ml. By contrast, the IC50 value of Rau to the bulk of
PANC-1 cells was 317 µg/ml (Fig.
1B). The MIA PaCa-2 pancreatic cancer cells were also treated
by Rau for the detection of tumor spheroids. Similar results were
obtained. Rau reduced the number of the MIA PaCa-2 spheroids at 50
µg/ml, and completely inhibited spheroid formation at ≥100 µg/ml
(Fig. 2C and D). The estimated
IC50 value of 34 µg/ml (Fig.
2D) was lower than the IC50 value for the bulk of
the MIA PaCa-2 cells (Fig. 1A).
Cells with stemness features are reported to exclude
dyes as side populations (34,35).
In order to separate the CSC-like population, MIA PaCa-2 cells were
sorted using flow cytometry with DCV staining. The DCV−
cells (CSC-like) and DCV+ (non CSC-like) cells were
collected and treated with Rau. Rau inhibited the viability in all
unsorted, DCV+ and DCV− cells, preferentially
inhibiting DCV− cells (Fig.
3A). The estimated IC50 values were 162 µg/ml in
unsorted cells, 177 µg/ml in DCV+ cells and 122 µg/ml in
DCV− cells. This result suggested that Rau
preferentially inhibited CSC-like cells.
Tumor spheroid formation was detected. Although cell
spheroids were also formed in the DCV+ cell culture,
they were significantly smaller (Fig.
3B). By contrast, DCV− cells formed large spheroids,
as expected. As there is currently no exclusive way to pin-point
pancreatic CSCs, the formation of spheroids in DCV+
cells may due to the remaining CSC-like cells in the
DCV+ population. However, the DCV staining and sorting
provided a side population enriched with ‘stemness’. Rau at 50
µg/ml inhibited spheroids in the DCV− and
DCV+ populations (Fig.
3B), a result consistent with those in unsorted cells.
Reduction of the CSC marker-positive
cell population
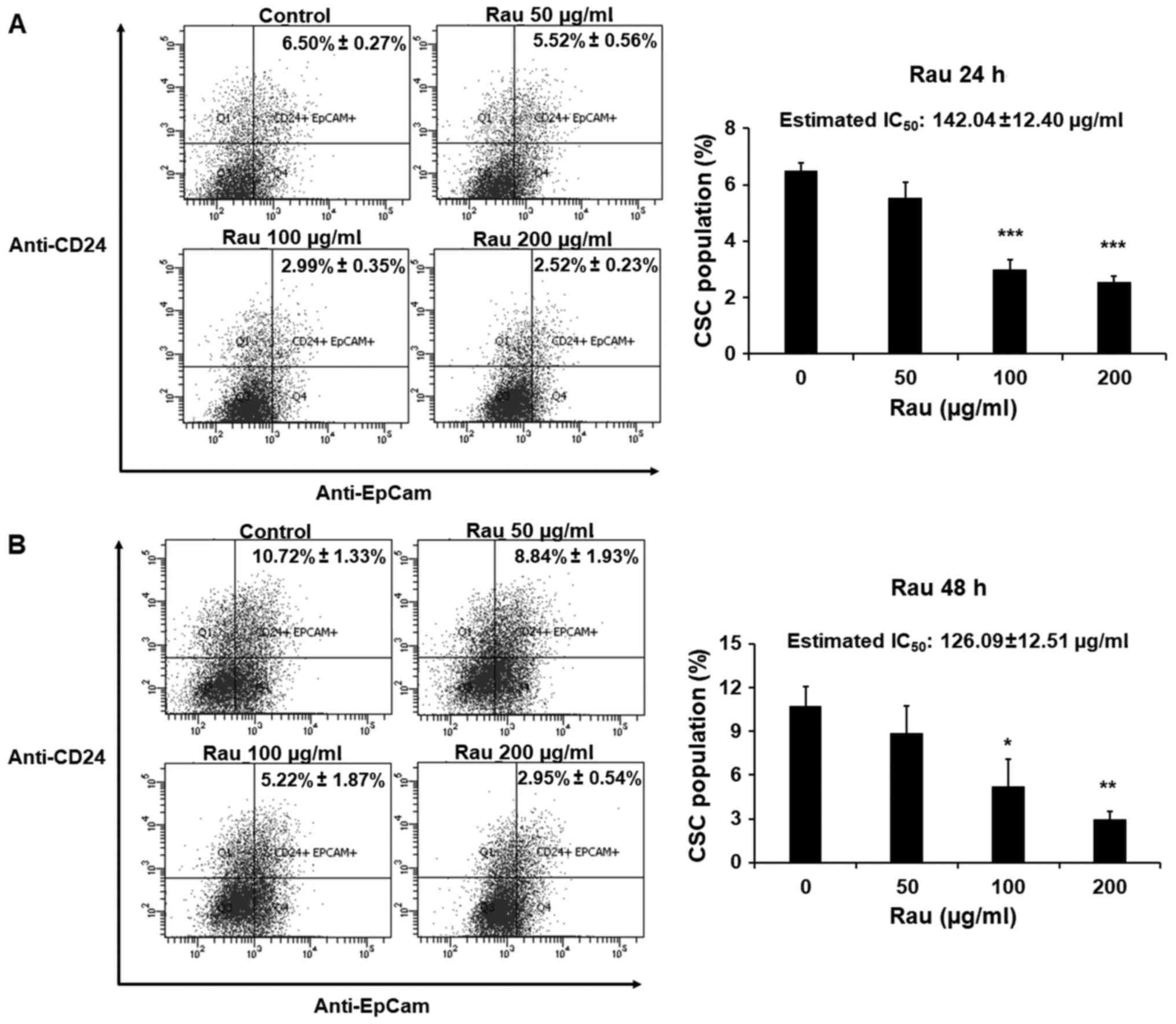
The effects of Rau on CSCs in a shorter time period
were also examined. The PANC-1 cells were treated with Rau for 24
or 48 h at concentrations of 50, 100 or 200 µg/ml. The pancreatic
CSC markers CD24 and EpCAM were examined by immune staining
followed by flow cytometric analysis. Rau reduced the
CD24+EpCam+ cell population following 24 and
48 h treatment (Fig. 4A and B). In
the control groups, CD24+EpCam+ cells
consisted of 6.50–10.72% of the whole population. At the
concentration of 200 µg/ml, Rau significantly reduced
CD24+EpCam+ cells to 2.52–2.95% following 24
and 48 h of treatment (Fig. 4A and
B). At a lower concentration of 100 µg/ml, Rau also
significantly reduced the CD24+EpCam+ cells
to 2.99–5.22% following 24 and 48 h of treatment (Fig. 4B). It was estimated that the
IC50 value at 24-h treatment was 142.04±12.40 µg/ml, and
at 48 h treatment was 126.09±12.51 µg/ml (Fig. 4A and B), and these values were lower
than the IC50 values for the bulk tumor cells. These
data are consistent with the results above showing that Rau
preferentially inhibited pancreatic CSCs.
One of the essential pathways in maintaining the
self-renewal and spheroid formation capacities of CSCs is
activation of the canonical Wnt/β-catenin signaling pathway
(18,36). When there is active Wnt signaling,
the β-catenin degradation complex in the cytosol dissociates, and
β-catenin accumulates in the nucleus and functions as a
transcriptional factor to upregulate genes that promote CSC
stemness, including Nanog (37). In
the present study, the cytoplasmic and nuclear fractions of the
PANC-1 cells were each examined for β-catenin levels with or
without Rau treatment. Treatment with Rau (100 µg/ml) for 24 and 48
h reduced the levels of β-catenin in the nucleus (Fig. 5A), whereas the cytoplasmic β-catenin
levels were not changed (Fig. 5A).
A panel of β-catenin downstream target genes, including B-cell
lymphoma 2-like 2 (BCL2L2), cyclooxygenase-2 (COX-2), matrix
metalloproteinase (MMP)14 and MYC, were examined by RT-qPCR
analysis (Fig. 5B). Following
treatment for 48 h, the expression of MYC was significantly
decreased by Rau treatment, which is consistent with Wnt/β-catenin
signaling pathway inhibition. Studies have shown that the stem
cell-related gene Nanog has the ability to induce β-catenin
phosphorylation and enhances its degradation (38). Therefore, the present study examined
the expression of Nanog by western blot analysis. Nanog was
increased following 24 h of Rau treatment, and was then decreased
following 48 h of Rau treatment (Fig.
5C). It was hypothesized that the increase in Nanog at the
earlier time point enhanced β-catenin degradation and therefore
suppressed nuclear levels of β-catenin. The suppressed β-catenin
levels subsequently resulted in inhibition of the expression of
Nanog at a later time-point (39,40).
As a result, the Nanog and Wnt signaling pathway was suppressed by
Rau.
 | Figure 5.Decrease of nuclear β-catenin by Rau.
PANC-1 cells were treated with Rau at 100 µg/ml for 24 and 48 h.
(A) Expression of β-catenin was detected by western blot analysis
in cytoplasmic and nuclear fractions. Vinculin was a loading
control for cytoplasmic proteins, and histone H3 was a loading
control indicative for the nuclear fraction. (B) Expression of
β-catenin downstream target genes at 48 h of Rau treatment,
detected by RT-qPCR analysis. (C) Expression of Nanog was detected
by western blot analysis. (D) Expression of CSC-related genes
following 48 h of Rau treatment, detected by RT-qPCR analysis. (E)
Suggested mechanism of Rau inhibiting Nanog and nuclear β-catenin.
Rau treatment has an early effect in increasing the expression of
Nanog, which leads to the phosphorylation and degradation of
β-catenin, which represses nuclear β-catenin. The decreasing level
of nuclear β-catenin negatively influenced the expression of Nanog.
Rau treatment also appeared to directly inhibit β-catenin nuclear
accumulation. Both can result in overall suppression of levels of
Nanog and nuclear β-catenin. Rau also inhibited the RNA level of
CSC-related genes, including Dppa4 and Esrrb. *P<0.05;
**P<0.01; ***P<0.001, compared with the untreated control
group. Rau, Rauwolfia vomitoria; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; BCL2L2,
B-cell lymphoma 2-like 2, COX-2, cyclooxygenase-2, MMP14, matrix
metalloproteinase 14; Dppa4, developmental pluripotency associated
4, Esrrb, estrogen related receptor β; Oct4, octamer-binding
transcription factor 4; Tbx3, T-box 3; Tcl1, T cell leukemia 1. |
A panel of other CSC-related genes were also
examined by RT-qPCR analysis (31).
Data showed that the expression levels of developmental
pluripotency associated 4 (Dppa4), estrogen related receptor β
(Esrrb) and SRY-box 2 (Sox2) were inhibited following 48 h of Rau
treatment (Fig. 5D).
Taken together, Rau treatment had an early effect in
increasing the expression of Nanog, which led to the
phosphorylation and degradation of β-catenin, and repressed the
nuclear level of β-catenin. The decreasing level of nuclear
β-catenin negatively affected the expression of Nanog. Rau
treatment also appeared to directly inhibit the nuclear
accumulation of β-catenin and other CSC-related genes, including
MYC, resulting in the overall suppression of Nanog and nuclear
β-catenin (Fig. 5E). The full
mechanism of Rau-induced CSC inhibition warrants further
investigation.
Inhibition of pancreatic cancer stem
cells in vivo
The inhibitory effects of Rau against pancreatic
CSCs were examined in vivo by tumorigenicity in
immunocompromised mice. Single treatment was performed first using
inoculation of different numbers of PANC-1 cells at limited
dilutions. The cells (2×104, 2×105 and
1×106) were mixed with 200 mg/ml Rau and injected
subcutaneously into the left flanks of nude mice (n=10),
respectively. For the control, the same number of cells were mixed
with PBS and inoculated into the right flanks of the same mouse.
The results are shown in Fig. 6A-F.
At the lowest inoculation number (2×104 cells), the
tumor formation rate was low and no difference was observed between
the treated and untreated groups. At the highest inoculation number
(1×106 cells per injection), the untreated group reached
a maximum of 90% tumor formation and the treated group reached 80%,
with no significant difference between the two. There was also no
difference in the growth of the formed tumors between the treated
and untreated groups. At 2×105 cells per injection, the
single Rau treatment significantly inhibited the tumor formation
rate. The growth of the formed tumors was also inhibited.
As single Rau treatment showed limited effect on the
inhibition of tumor formation rate and tumor size, repeated
treatment was performed with oral administration of Rau. The
optimal cell number for injection was selected as 2×105
per injection. The mice (n=10) were injected subcutaneously in the
left and right flanks with PANC-1 cells mixed with 200 mg/ml of
Rau. Treatment started the following day and lasted for 3 weeks
with oral gavage of 20 mg/kg Rau, five times per week. The control
mice (n=10) were inoculated with the same number of cells mixed
with PBS, and were administered with equivalent volumes of
saline.
The rate of tumor formation and time of tumor
formation were significantly different between the control and
treated groups (Fig. 7A). At day 6,
the tumor formation rate in the control group reached 80%, whereas
that in the Rau-treated group was only 35%. At day 20 when the
treatment had stopped, all mice in the control group were bearing
tumors on both flanks (100% tumor formation), whereas the
Rau-treated group only had 65% tumor formation. All mice were kept
for 2 months following the end of treatment. At the end of the
experiment, the Rau-treated group had a maximum of 85% tumor
formation, compared with 100% tumor formation in the control group.
These data indicated that Rau administration at 20 mg/kg orally
eliminated CSCs in 15% of the injection sites.
The growth of the formed tumors was not
significantly inhibited by Rau treatment compared with the control
group, which indicated the lack of a long-term inhibitory effect on
tumor growth following the end of treatment (Fig. 7B). No adverse effects were observed
in either group during the treatment (Fig. 7C).
Discussion
Targeting CSCs has been an attractive strategy for
developing novel treatments with the aim of eliminating the entire
cancer cell population. However, targeting CSCs has been
challenging. First, as CSCs are only a small population in the bulk
of cancer cells, anticancer agents that have cytotoxicity to the
bulk of cancer cells do not necessarily inhibit CSCs (5,6). CSCs
possess self-renewal ability and are able to give rise to new
tumors (17). CSCs are also found
to be drug resistant (12,41). The mechanism by which CSCs become
drug resistant remains to be fully elucidated. A partial reason is
the quiescent status of CSCs in a growing tumor. Other potential
mechanism are the upregulated expression of the ABCG2 transporter,
which facilitates the efflux of chemotherapeutic drugs from the
cytosol (41), overexpression of
detoxifying enzymes, enhanced DNA repair ability, and
overexpression of anti-apoptotic proteins (12). Given the roles of CSC in tumor
generation, metastasis and drug resistance, the identification and
development of novel drugs that can inhibit CSCs may lead to a
promising outcome in the comprehensive inhibition of tumor growth,
metastasis and recurrence, and overcoming drug resistance. In the
present study, it was demonstrated that the Rau extract inhibited
pancreatic CSCs in vitro and in vivo. Previously, it
was reported that the same Rau extract induced the apoptosis of
pancreatic cancer cells and sensitized pancreatic cancer cells to
gemcitabine treatment (29). The
inhibition of CSCs may be another factor contributing to
Rau-induced gemcitabine sensitivity in addition to its
apoptosis-inducing activity. The data suggested that Rau had
preferential inhibitory effects towards pancreatic CSCs, and also
inhibited the bulk of cancer cells. This may be advantageous in
cancer therapy as one treatment inhibits CSC and non-CSCs. The
overall inhibitory effect in non-CSC and CSCs results in the
inhibition of tumor growth, whereas the inhibition in CSCs is
likely also to reduce metastasis and chemotherapy resistance. Given
the lack of treatment options for pancreatic cancer, the benefits
of Rau in pancreatic cancer treatment warrant further
investigation, particularly in combination with current
chemotherapies.
Dye exclusion and CSC surface markers are used in
CSC isolation and provide consistent data for the enrichment of
pancreatic CSCs. However, there has not been an efficient method to
definitively identify and isolated a pure pancreatic CSCs and
maintain/amplify them for drug development purposes (42). Functional assays, including tumor
spheroid assays and tumorigenicity in mice are commonly used
(43). Due to the difficulties in
obtaining and maintaining a pure CSC population (44), the isolated CSCs may lose their
natural environment in the bulk population (17). In the present study, the bulk of
pancreatic cancer cells were treated, in addition to a
dye-excluding side population, and the CSC-specific outcomes were
examined. The inhibition of CSCs was shown, and was not likely due
to the general cytotoxicity of Rau to the bulk of cancer cells. The
data showed that Rau had an IC50 value of 317 µg/ml over
48 h of treatment towards the bulk of PANC-1 cells, and had
markedly lower IC50 values of 126.9–142.04 µg/ml for the
reduction of CD24+EpCam+ cells at a shorter
treatment time of 24–48 h. Furthermore, in the tumor spheroid
formation assay, Rau had an IC50 value of 39.44 µg/ml in
inhibiting the number of spheroids. These data suggested that Rau
had a preferential inhibitory activity towards pancreatic CSCs.
The data obtained in the present study showed that
Rau reduced protein levels of nuclear β-catenin and Nanog in PANC-1
cells, which are important in stem cell initiation and maintenance.
Rau also reduced the mRNA levels of several CSC-related genes,
namely Dppa4, Esrrb and Sox2. The in-depth mechanism underlying how
Rau interacts with Nanog and/or the β-catenin signaling pathway
requires further investigation. In addition, as plant extracts
contain a complex mixture of natural compounds, it is possible that
Rau also affects other molecular targets and pathways that lead to
its CSC inhibitory effect.
Previous studies on extracts of Rau showed
inhibitory effects on the proliferation on pancreatic, ovarian and
prostate cancer (25,29,45).
The data from animal experiments in the present study revealed the
promising effects of Rau in inhibiting tumorigenicity, at a dose
and administration route that can be easily translated into
clinical use. No toxic side-effects were observed in mice at this
dosage. The inhibition of tumorigenicity indicated the possible
role of Rau in the prevention of cancer, in addition to data
indicating a treatment role. As extracts of Rauwolfia
vomitoria are consumed by the American public as a health
supplement, the safety, toxicity and effects of Rau as an
anticancer agent require further investigation clinically.
Acknowledgements
The authors would like to thank Dr Sitta Sittampalam
at the National Center for Advancing Translational Sciences, NIH
for providing MRC-5 cells. We also thank our previous Research
Assistant Iman Joker at KUMC and previous Postdoctoral Fellow Jun
Yu at KUMC for their exploratory work on this study.
Funding
The present study was supported by a research grant
provided by the Beljanski Foundation. The Beljanski Foundation had
no influence on the design, performance, data collection, data
analysis or manuscript preparation of the study.
Availability of data and materials
All the original data concerning this publication is
available upon request.
Authors' contributions
QC conceptualized, designed and oversaw the studies.
RD participated in the study design and performed the majority of
the experiments. PC performed part of the cell experiments. RD and
PC collected the data. RD, PC and QC analyzed the data. All authors
participated in manuscript preparation and all approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All animal experiments followed a protocol approved
by the Institutional Animal Care and Use Committee of the
University of Kansas Medical Center.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fingerhut A, Vassiliu P, Dervenis C,
Alexakis N and Leandros E: What is in a word: Pancreatoduodenectomy
or pancreaticoduodenectomy? Surgery. 142:428–429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu Y, Ramena G and Elble RC: The role of
cancer stem cells in relapse of solid tumors. Front Biosci (Elite
Ed). 4:1528–1541. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oettle H and Neuhaus P: Adjuvant therapy
in pancreatic cancer: A critical appraisal. Drugs. 67:2293–2310.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Renouf D and Moore M: Evolution of
systemic therapy for advanced pancreatic cancer. Expert Rev
Anticancer Ther. 10:529–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Conroy T, Gavoille C, Samalin E, Ychou M
and Ducreux M: The role of the FOLFIRINOX regimen for advanced
pancreatic cancer. Curr Oncol Rep. 15:182–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faris JE, Blaszkowsky LS, McDermott S,
Guimaraes AR, Szymonifka J, Huynh MA, Ferrone CR, Wargo JA, Allen
JN, Dias LE, et al: FOLFIRINOX in locally advanced pancreatic
cancer: The Massachusetts General Hospital Cancer Center
experience. Oncologist. 18:543–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vishnu P and Roy V: Safety and efficacy of
nab-paclitaxel in the treatment of patients with breast cancer.
Breast Cancer (Auckl). 5:53–65. 2011.PubMed/NCBI
|
|
11
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: Groupe Tumeurs Digestives of Unicancer;
PRODIGE Intergroup: FOLFIRINOX versus gemcitabine for metastatic
pancreatic cancer. N Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vinogradov S and Wei X: Cancer stem cells
and drug resistance: The potential of nanomedicine. Nanomedicine
(Lond). 7:597–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiozawa Y, Nie B, Pienta KJ, Morgan TM
and Taichman RS: Cancer stem cells and their role in metastasis.
Pharmacol Ther. 138:285–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmied BM, Ulrich A, Matsuzaki H, Li CH
and Pour PM: In vitro pancreatic carcinogenesis. Ann Oncol. 10
Suppl 4:41–45. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du Z, Qin R, Wei C, Wang M, Shi C, Tian R
and Peng C: Pancreatic cancer cells resistant to chemoradiotherapy
rich in ‘stem-cell-like’ tumor cells. Dig Dis Sci. 56:741–750.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lonardo E, Cioffi M, Sancho P, Crusz S and
Heeschen C: Studying pancreatic cancer stem cell characteristics
for developing new treatment strategies. J Vis Exp.
100:e528012015.
|
|
17
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ajani JA, Song S, Hochster HS and
Steinberg IB: Cancer stem cells: The promise and the potential.
Semin Oncol. 42 Suppl 1:S3–S17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
La Barre J: Hypotensive effects of the
completely dereserpinised extract of Rauwolfia vomitoria.
Arzneimittelforschung. 23:600–605. 1973.PubMed/NCBI
|
|
20
|
Gbolade A: Ethnobotanical study of plants
used in treating hypertension in Edo State of Nigeria. J
Ethnopharmacol. 144:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pesewu GA, Cutler RR and Humber DP:
Antibacterial activity of plants used in traditional medicines of
Ghana with particular reference to MRSA. J Ethnopharmacol.
116:102–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kweifio-Okai G: Antiinflammatory activity
of a Ghanaian antiarthritic herbal preparation: I. J
Ethnopharmacol. 33:263–267. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
La Barre J and Castiau J: Effect of
reserpine-free Rauwolfia vomitoria extract on gastric motility in
dogs. C R Seances Soc Biol Fil. 151:2222–2224. 1957.(In French).
PubMed/NCBI
|
|
24
|
Isaiah AM, Olawale O, Effiong EE,
Idongesit NJ, Fidelis UA and Friday UU: Vitamin e supplementation
with Rauwolfia vomitoria root bark extract improves hematological
indices. N Am J Med Sci. 4:86–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bemis DL, Capodice JL, Gorroochurn P, Katz
AE and Buttyan R: Anti-prostate cancer activity of a beta-carboline
alkaloid enriched extract from Rauwolfia vomitoria. Int J Oncol.
29:1065–1073. 2006.PubMed/NCBI
|
|
26
|
Iwu MM and Court WE: Root alkaloids of
Rauwolfia vomitoria afz. Planta Med. 32:88–99. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beljanski M and Beljanski MS: Three
alkaloids as selective destroyers of cancer cells in mice. Synergy
with classic anticancer drugs. Oncology. 43:198–203. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Q, Chao R, Chen H, Hou X, Yan H, Zhou
S, Peng W and Xu A: Antitumor and neurotoxic effects of novel
harmine derivatives and structure-activity relationship analysis.
Int J Cancer. 114:675–682. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu J and Chen Q: Antitumor activities of
Rauwolfia vomitoria extract and potentiation of gemcitabine effects
against pancreatic cancer. Integr Cancer Ther. 13:217–225. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amini S, Fathi F, Mobalegi J,
Sofimajidpour H and Ghadimi T: The expressions of stem cell
markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3,
Dppa4, and Esrrb in bladder, colon, and prostate cancer, and
certain cancer cell lines. Anat Cell Biol. 47:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vermeulen L, Todaro M, de Sousa Mello F,
Sprick MR, Kemper K, Alea Perez M, Richel DJ, Stassi G and Medema
JP: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:13427–13432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SY, Hong SH, Basse PH, Wu C, Bartlett
DL, Kwon YT and Lee YJ: Cancer stem cells protect non-stem cells
from anoikis: Bystander effects. J Cell Biochem. 117:2289–2301.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goodell MA, McKinney-Freeman S and Camargo
FD: Isolation and characterization of side population cells.
Methods Mol Biol. 290:343–352. 2005.PubMed/NCBI
|
|
35
|
Richard V, Nair MG, Kumar Santhosh TR and
Pillai MR: Side population cells as prototype of chemoresistant,
tumor-initiating cells. BioMed Res Int. 2013:5172372013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng P, Sun X, Yin D, Xu F, Yang K, Qin
L, Dong Y, Guo F, Chen A, Zhang W, et al: Nanog down-regulates the
Wnt signaling pathway via β-catenin phosphorylation during
epidermal stem cell proliferation and differentiation. Cell Biosci.
5:52015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo
G, Hu CJ, Dong H and Yang SM: Helicobacter pylori upregulates Nanog
and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem
cell-like properties in human gastric cancer. Cancer Lett.
374:292–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takao Y, Yokota T and Koide H:
Beta-catenin up-regulates Nanog expression through interaction with
Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun.
353:699–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
An Y and Ongkeko WM: ABCG2: The key to
chemoresistance in cancer stem cells? Expert Opin Drug Metab
Toxicol. 5:1529–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li C, Lee CJ and Simeone DM:
Identification of human pancreatic cancer stem cells. Methods Mol
Biol. 568:161–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salmaggi A, Boiardi A, Gelati M, Russo A,
Calatozzolo C, Ciusani E, Sciacca FL, Ottolina A, Parati EA, La
Porta C, et al: Glioblastoma-derived tumorospheres identify a
population of tumor stem-like cells with angiogenic potential and
enhanced multidrug resistance phenotype. Glia. 54:850–860. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nagare RP, Sneha S, Priya SK and Ganesan
TS: Cancer stem cells - Are surface markers alone sufficient? Curr
Stem Cell Res Ther. 12:37–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu J, Ma Y, Drisko J and Chen Q: Antitumor
activities of Rauwolfia vomitoria extract and potentiation of
carboplatin effects against ovarian cancer. Curr Ther Res Clin Exp.
75:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|