Introduction
Acute lymphoblastic leukemia (ALL) is the most
common malignancy in childhood and accounts for ~25% of cases
diagnosed in children <15 years old (1). ALL is a heterogeneous disease that
results from the malignant transformation of lymphoid progenitor
cells in the bone marrow, blood and extramedullary sites (2). In children with ALL, T-cell ALL
(T-ALL) accounts for 12–15%, and compared with B-cell ALL, the
prognosis of T-ALL is poorer due to increased relapse risk and
reduced response to salvage therapy (3). In recent years, the outcome of
children with T-ALL has improved with contemporary risk and
response-based treatment protocols; additionally, the 5-year
event-free survival rate reported by a number of foreign research
centers reached 85%, but the majority of the research centers
remain at ~60% (4–6). Currently, the primary focus for
first-line therapy is on reducing the burden of long-term toxicity,
decreasing the chemotherapy intensity for specific low-risk
patients and strengthening treatment for high-risk groups (7). Since antineoplastic agents derived
from natural products exhibit notable advantages, including low
toxicity, insignificant or no side-effects, targeting multiple
targets and a reduced chance to generate drug resistance (8), researchers focus on searching for
novel antineoplastic drugs from natural products and Chinese
traditional herbs.
Timosaponin A-III (TAIII), a steroidal saponin
isolated from the rhizomes of anemarrhena asphodeloides, has been
credited with a wide spectrum of bioactivities, including
controlling hyperglycemia (9),
improving learning and memory (10), inhibiting inflammation (11), suppressing allergic reaction
(12) and restraining platelet
aggregator (13). In recent years,
more importance has been attached to its antitumor effect;
additionally, accumulating evidence indicate that TAIII serves
important roles in suppressing proliferation, inducing apoptosis
(14), activating autophagy
(15,16) and reversing multidrug resistant
(17) of tumor cells. TAIII was
observed to exhibit cytotoxicity towards a panel of carcinoma cell
lines, including human breast carcinoma cells (MDA-MB-231 and
MCF-7), hepatocellular carcinoma cells (Hep3b and HepG2), human
lung cancer cells (A549), human colorectal cancer cells (HCT-15,
HCT-116, HT-29, SW-480 and SW-620), human cervical epithelioid
carcinoma cells (HeLa), human nasopharyngeal carcinoma cells
(SUNE-1) and human chronic myeloid leukemia cell line (K562/ADM),
but had a reduced effect on the viability of normal cells (16–19).
However, to the best of our knowledge, limited research has been
conducted to investigate the function of TAIII in T-ALL Jurkat
cells.
In the present study, the aim was to investigate the
effect of TAIII on cell apoptosis and autophagy in T-ALL Jurkat
cells. It was determined that TAIII induced cell apoptosis and
autophagy in a dose-dependent manner. Further study revealed that
TAIII promoted autophagy via inhibiting the phosphoinositide
3-kinase (PI3K)/Akt/mechanistic target of rapamycin kinase (mTOR)
pathway. The present study revealed the possible antitumor
mechanisms of TAIII in T-ALL, providing novel strategies and
targets for T-ALL treatment.
Materials and methods
Cell line and cell culture
The Human T-ALL Jurkat cell line was purchased from
the Department of Pharmacology, The Institute of Hematology of
Chinese Academy of Medical Sciences (Tianjin, China). The cells
were maintained in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS; both from HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), 100 U/ml penicillin and 0.1 mg/ml streptomycin at
37°C in a humidified atmosphere containing 5% CO2. The
cells cultured to 70–80% confluence were used in experiments.
Chemicals and reagents
TAIII (purity>98%; Shanghai Yuanye Bio-Technology
Co., Ltd., Shanghai, China) and monodansylcadaverine (MDC;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were dissolved in
dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) at the final
concentration of 20 mM and 50 µM, respectively, stored at
−20°C in the dark, and diluted in RPMI-1640 medium to the desired
concentration prior to each experiment. The final DMSO
concentration did not exceed 0.1% throughout the study. Rabbit
polyclonal antibodies, including B-cell lymphoma 2 (Bcl-2; cat. no.
bs-0032R), Bcl-2-associated X (Bax; cat. no. bs-20386R), Beclin 1
(cat. no. bs-1353R), LC3-I/LC3-II (cat. no. bs-2912R), Akt (cat.
no. bs-0115R), phospho(p)-Akt (cat. no. bs-0876R), mTOR (cat. no.
bs-1992R), p-mTOR (cat. no. bs-3495R), PI3K (cat. no. bs-6423R) and
p-PI3K (cat. no. bs-4160R), were purchased from Beijing
Biosynthesis Biotechnology Co., Ltd. (Beijing, China). Rabbit
polyclonal antibody against GAPDH (cat. no. AB-P-R001) was a
product of Hangzhou Goodhere Biotechnology Co., Ltd. (Hangzhou,
China).
Cell Counting Kit-8 (CCK-8) assay for
cell proliferation
CCK-8 (Dojindo Molecular Technologies, Inc.,
Shanghai, China) was used to determine the survival rate of cells
incubated with TAIII. Jurkat cells were seeded into a 96-well plate
at a density of 1×104 cells/well in RPMI-1640 containing
10% FBS. Subsequently, various concentrations of TAIII (2, 4, 8, 16
and 32 µM) were added. After the cells were incubated at 37°C in an
atmosphere containing 5% CO2 for 24, 48 or 72 h, 10 µl
CCK-8 solution was added to each well and incubated for an
additional 4 h at 37°C. The absorbance was measured at 580 nm with
Model 680 microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). A blank well containing only RPMI-1640 medium
supplemented with 10% FBS was used as a control.
Cell apoptosis analysis by flow
cytometry
Jurkat cells were plated into a 6-well plate at a
concentration of 3×105 cells in 1 ml RPMI-1640 medium
supplemented with 10% FBS. Following incubation with TAIII (0, 2
and 8 µM) at 37°C for 24 h, the cells were harvested and washed
twice with ice-cold PBS. Subsequently, the cells were suspended in
suspended in 400 µl 1X binding buffer (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) and double-stained with Annexin V-fluorescein
isothiocyanate/propidium iodide (Annexin V-FITC/PI; Nanjing KeyGen
Biotech Co., Ltd.) for 15 min in the dark at room temperature. The
apoptosis cells were analyzed using a FACSCanto™ II Flow Cytometer
with CellQuest software V 7.5.3 (BD Biosciences; Becton, Dickinson
and Company, Franklin Lakes, NJ, USA). The apoptotic rate was
calculated as the percentage of early apoptotic cells plus the
percentage of late apoptotic cells.
Observation of cell ultrastructure
under transmission electron microscopy (TEM)
Jurkat cells were incubated with TAIII (0 and 8 µM)
at 37°C for 24 h. Subsequently, cells were harvested, washed twice
with PBS, fixed with 3% glutaraldehyde for 24 h at 4°C. Following
washing three times with ice-cold PBS, Jurkat cells were
progressively dehydrated in 30% ethanol for 5–10 min, 50% ethanol
for 5–10 min, 70% ethanol for 5–10 min, 90% ethanol for 10–15 min,
100% ethanol for 10–15 min and embedded in Epon812 resin. Finally,
the specimens were sliced into serial ultrathin sections
(thickness, 100 nm), and stained in uranyl acetate for 15–30 min
and lead citrate for 5–10 min at room temperature. The specific
ultrastructure features were observed under a TEM (JEM-2800; JEOL,
Ltd., Tokyo, Japan; ×4,000 magnification).
Visualization of MDC-labeled
autophagic vacuoles
MDC staining of autophagic vacuoles was performed
for autophagy analysis (20).
Jurkat cells growing on coverslips were pretreated with TAIII (0, 2
and 8 µM) for 24 h at 37°C. Following treatments, the cells were
stained with 50 µM MDC in PBS for 10 min at 37°C, and then washed
three times with PBS to remove excess MDC and subsequently analyzed
under an inverted fluorescence microscopy (F-7000; Hitachi, Ltd.,
Tokyo, Japan; ×400 magnification). Fluorescence of MDC was measured
at 365 nm excitation filter, 395 nm spectroscope and 420 nm
absorption filter.
Detection of intracellular mean
fluorescence intensity of MDC
Jurkat cells were plated into a 6-well plate at a
concentration of 3×105 cells in 1 ml RPMI-1640 medium
supplemented with 10% FBS. The cells were pretreated with TAIII (0,
2 and 8 µM) at 37°C for 24 h. Subsequently, the cells were
harvested by centrifugation at 3,000 × g for 3 min at room
temperature and suspended with 1 ml RPMI-1640 medium supplemented
with 10% FBS. Following this, MDC (50 µM) was added to each
group and dyed for 10 min in the dark at 37°C. After staining,
the cells were collected by centrifugation at 3,000 × g for 3 min
at room temperature, washed twice with ice-cold PBS and suspended
with 1 ml ice-cold PBS. Immediately after that, the cell-associated
mean fluorescence intensity (MFI) was detected at 488 nm excitation
wavelength by a FACSCanto™ II Flow Cytometer with CellQuest
software V 7.5.3.
Western blot assay
The cells were plated into a 6-well plate at a
concentration of 3×105 and cultured to 70–80% confluence
at 37°C for 24 h. The cells were harvested and washed with PBS
twice, and then scraped and lysed in Radioimmunoprecipitation Assay
buffer (Beyotime Institute of Biotechnology, Shanghai, China).
Protein concentrations were determined by Bicinchoninic Acid
Protein Assay kit (Beyotime Institute of Biotechnology). Protein
samples (40 µg), including Beclin 1 (50 kDa), Akt (56 kDa), p-Akt
(56 kDa), mTOR (289 kDa), p-mTOR (289 kDa), PI3K (82 kDa), p-PI3K
(82 kDa) and GAPDH (37 kDa), were separated with 8% SDS-PAGE
(Beyotime Institute of Biotechnology). Bcl-2 (26 kDa), Bax (21 kDa)
and LC3-I/LC3-II (14/16 kDa) were separated with 15% SDS-PAGE.
Subsequently, the proteins were transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked by 5% skimmed dry milk in TBS containing
0.2% Tween 20 at room temperature for 2 h followed by an overnight
incubation with specific antibodies at 4°C. The primary antibodies
were rabbit polyclonal antibodies against Bcl-2 (1:500), Bax
(1:500), Beclin 1 (1:500), LC3-I/LC3-II (1:500), Akt (1:500), p-Akt
(1:500), mTOR (1:500), p-mTOR (1:500), PI3K (1:500), p-PI3K (1:500)
and GAPDH (1:1,000). Following three washes in TBS/Tween buffer,
the membranes were incubated in horseradish peroxidase-labeled goat
anti-rabbit immunoglobulin G (1:5,000; cat. no. TA130015; OriGene
Technologies, Inc., Beijing, China) for 2 h at 37°C. Detection was
performed using the FluorChem FC2 gel imaging system
(ProteinSimple, San Jose, CA, USA). Each band density was
quantified using Image J V 1.8.0 image processing program (National
Institutes of Health, Bethesda, MD, USA) and normalized by GAPDH
for their respective lanes.
Data analysis
Statistical analyses were conducted using SPSS 21.0
software (IBM Corp., Armonk, NY, USA). The normality of data was
analyzed by the Shapiro-Wilk test, and it was determined that the
data were normally distributed. One-way analysis of variance was
used to analyze differences between ≥3 groups, followed by a Least
Significant Difference multiple range test for post-hoc
comparisons. Data are expressed as the means ± standard deviations.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TAIII suppresses proliferation of
Jurkat cells
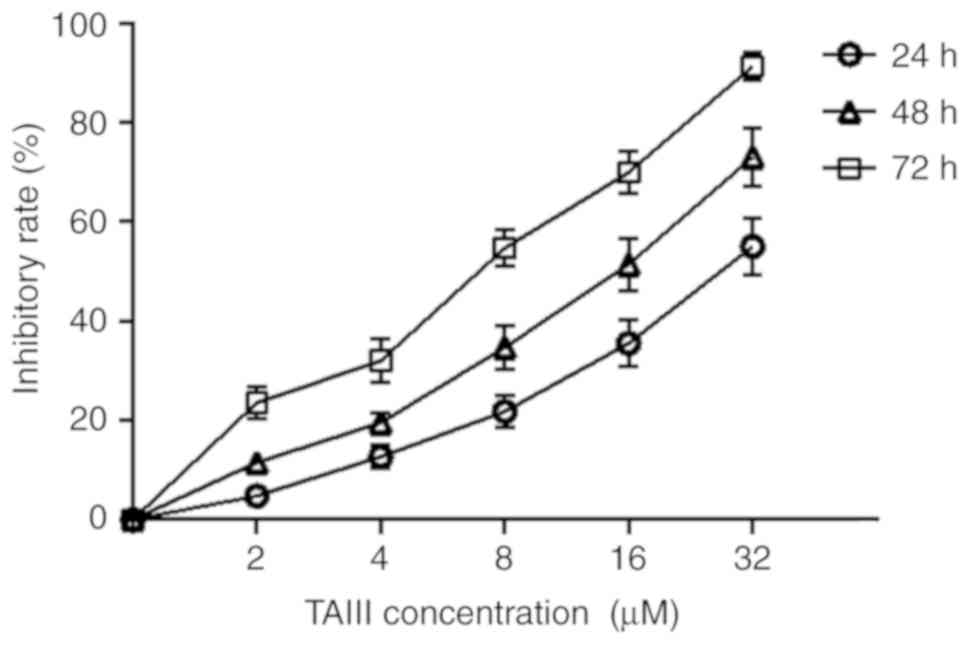
The CCK-8 assay was conducted to evaluate the
cytotoxicity of TAIII on the proliferation of Jurkat cells. As
depicted in Fig. 1, Jurkat cells
were sensitive to TAIII and TAIII inhibited the viability of Jurkat
cells in a time- and dose-dependent manner in vitro. The
inhibitory rate of TAIII on growth of Jurkat cells was 4.34±0.31,
13.67±0.78 and 22.15±1.04%, respectively, after the cells were
treated with 2 µM TAIII for 24, 48 and 72 h.
TAIII induces apoptosis of Jurkat
cells
As depicted in Fig.
2A-C, after treatment with 0, 2 or 8 µM TAIII for 24 h, the
total apoptosis rate of Jurkat cells was 4.63±1.47, 27.07±2.57 and
55.13±5.17%, respectively. Additionally, TAIII induced apoptosis of
Jurkat cells in a dose-dependent manner (Fig. 2D).
Autophagy detection by TEM
TEM was performed to detect the micro-morphological
change of Jurkat cells. Results demonstrated that TAIII induces
Jurkat cells to generate autophagy. As depicted in Fig. 3, Jurkat cells not treated with TAIII
exhibited the normal ultrastructural morphology of nuclei,
cytoplasm and organelles. The most prominent morphological change
in TAIII-treated cells was the formation of numerous autophagic
vacuoles in the cytoplasm, and the giant cytophagosomes filled with
degraded organelles and autolysosomes were frequently observed.
Observation of vacuolization in
cytoplasm by inverted fluorescence microscope
MDC-labeled autophagic vacuoles were observed by
inverted fluorescence microscope. As depicted in Fig. 4, MDC-labeled autophagic vacuoles
appeared as distinct dot-like structures distributing in cytoplasm
or in perinuclear regions. The TAIII-treated group exhibited
increased fluorescent density and more MDC-labeled particles,
compared with the control group, indicating that TAIII induced the
formation of the MDC-labeled vacuoles. Additionally, it was
determined that vacuolization in the cytoplasm progressively became
larger and denser when the concentration of TAIII increased.
MDC accumulation increases in Jurkat
cells following TAIII treatment
Furthermore, flow cytometry was used to detect
intracellular MDC MFI. The results implied that the MFI of MDC in
TAIII-treated Jurkat cells increased in a dose-dependent manner,
compared with untreated Jurkat cells. As depicted in Fig. 5, Jurkat cells treated with TAIII (2
and 8 µM) enhanced the MDC MFI by 1.31- and 1.76-fold, compared
with the control group.
TAIII increases Bax and decreases
Bcl-2 expression in Jurkat cells
To elucidate the molecular mechanism underlying the
apoptosis induced by TAIII, the protein expression levels of Bcl-2
and Bax genes, apoptosis-associated molecules, were evaluated by
western blot assay. The results demonstrated that the expression of
Bax increases significantly while the expression of Bcl-2 decreases
after Jurkat cells incubation with different concentrations of
TAIII for 24 h (Fig. 6).
TAIII upregulates Beclin 1 and LC3-II
expression in Jurkat cells
To clarify the mechanism underlying the autophagy
induced by TAIII, western blotting was conducted to assess the
effect of TAIII on the expression of Beclin 1 and LC3-II, which
serve key roles in autophagy (18,20).
As depicted in Fig. 7A and B, TAIII
treatment caused the conversion of LC3 from the cytoplasmic form
(LC3-I) into the autophagosomic form (LC3-II), while also
increasing the expression of Beclin 1, in Jurkat cells in a
concentration-dependent manner.
 | Figure 7.TAIII promotes Beclin 1 and LC3-II
expression via PI3K/Akt/mTOR signaling in Jurkat cells. Jurkat
cells were treated with TAIII at different concentrations (0, 2 and
8 µM) for 24 h and the expression of Beclin 1, LC3-II, p-PI3K,
p-AKT and p-mTOR was analyzed by western blot analysis. (A) Western
blot analysis of Beclin 1 and LC3-II. (B) Analysis of Beclin 1 and
LC3-II expression. (C) Western blot analysis of PI3K, p-PI3K,
T-Akt, p-Akt, mTOR and p-mTOR. (D) Analysis of p-PI3K, p-Akt and
p-mTOR expression. All results are expressed as the mean ± standard
deviation of triplicate experiments. *P<0.05 and **P<0.01 vs.
0 µM TAIII. TAIII, Timosaponin A-III; PI3K, phosphoinositide
3-kinase; mTOR, mechanistic target of rapamycin kinase; p-,
phospho-. |
TAIII promotes Jurkat cells autophagy
via PI3K/Akt/mTOR signaling
It is known that the PI3K/Akt/mTOR signaling pathway
serves a suppressive role in autophagy (21). In the present study, the activity of
the PI3K/Akt/mTOR signaling pathway was investigated by TAIII
treatment in Jurkat cells. As depicted in Fig. 7C and D, western blotting
demonstrated that the levels of p-PI3K, p-Akt and p-mTOR are
notably reduced in TAIII treatment groups, compared with the
control group. However, there were no notable changes in the total
amount of PI3K, Akt and mTOR. These results indicated that
PI3K/Akt/mTOR signaling is involved in Jurkat cells autophagy
induced by TAIII.
Discussion
TAIII, one of the main active constituents of
Chinese medicinal herb anemarrhena asphodeloides bunge, has been
credited with effective anticancer activities, including
suppressing proliferation, enhancing apoptosis, activating
autophagy and reversing multidrug resistance (14,16,17).
Sy et al (16) reported that
TAIII-induced autophagy in human cervical HeLa cells, followed by
mitochondria-dependent apoptotic cell death. It was demonstrated
that TAIII reverses multi-drug resistance in human chronic
myelogenous leukemia K562/ADM cells via downregulation of MDR1 and
MRP1 expression via inhibiting the PI3K/Akt signaling pathway
(17). Kang et al (18) determined that TAIII inhibits the
proliferation of human colon cancer HCT-15 cells with cell cycle
arrest and induction of apoptosis. In the present study, results
indicated that TAIII exerts a significant inhibitory effect on
Jurkat cells in a time- and dose-dependent manner, and induces
tumor cell apoptosis via downregulating Bcl-2 expression.
Additionally, Jurkat cells autophagy was promoted by the inhibition
PI3K/Akt/mTOR signaling. Overall, the results demonstrated that
TAIII stimulates cell apoptosis and autophagy of Jurkat cells in
vitro.
Considering that apoptosis induction is one of the
key features of antitumor drugs in tumor therapy, it is important
to examine the effects of TAIII on apoptosis induction and tumor
growth. Apoptosis, also known as type I programmed cell death, is
characterized by morphological changes, including cell shrinkage,
chromatin condensation and membrane bleeding, without disruption of
the plasma membrane; additionally, apoptosis is triggered by two
pathways, the death receptor-mediated extrinsic pathway and the
mitochondrial-involved intrinsic pathway (21–24).
Bcl-2 family members are critical players in mitochondrial-involved
intrinsic apoptosis (25). Bax and
Bcl-2 are the most characterized apoptosis regulators in
mitochondrial-associated apoptosis; furthermore, Bax is the first
identified pro-apoptotic protein member of the Bcl-2 protein
family, possessing promoting apoptosis activity (26). In the present study, data
demonstrated that TAIII induces apoptosis of Jurkat cells in a
dose-dependent manner (Fig. 2).
However, consistent with the ability of TAIII to kill cells via
apoptosis processes, TAIII increased the expression of Bax and
decreased the expression of Bcl-2 in a dose-dependent manner
(Fig. 6), indicating that the
upregulated Bax and downregulated Bcl-2 expression may trigger
TAIII-induced apoptosis of Jurkat cells.
Furthermore, for the first time, the present study
also provides experimental evidence to demonstrate the activation
of an autophagic program in Jurkat cells following treatment with
TAIII. Autophagy is a genetically programmed and evolutionarily
conserved process, and macro-, micro-, and chaperone-mediated
autophagy are three primary autophagy forms, with macro-autophagy
being the most prevalent form (27). Autophagy can regulate physiological
and pathophysiological cell death, and the basic function of
autophagy is to sustain survival and maintain cell homeostasis
through organelles and proteins recycling (28,29).
Increasing evidence indicate that autophagy is important in human
cancer suppression and extensive attention has been paid to its
role in tumor therapy (30,31). Previous studies indicated that TAIII
is a pronounced activator of autophagy (15,16,32).
In the present study, TEM determined the formation of
autophagosomes, which are the representative characteristics of
autophagy (Fig. 3). Furthermore,
numerous MDC-labeled autophagic vacuoles were observed in
TAIII-treated Jurkat cells under an inverted fluorescence
microscope (Fig. 4), and flow
cytometry analysis illustrated that the MDC accumulation increased
notably in TAIII treatment groups in a dose-dependent manner
(Fig. 5).
In order to further examine the mechanism by which
TAIII regulated autophagy, various molecular studies were
performed. Beclin 1, a mammalian orthologue of the yeast Apg6/Vps30
gene and a subunit of the class III PI3-kinase complex, is the
first mammalian gene that was identified to be able to induce
autophagy (33). LC3-II has been
identified as a marker for autophagosome formation (8). Therefore, the expression levels of
Beclin 1 and LC3-II in TAIII-treated Jurkat cells were measured. As
depicted in Fig. 7, TAIII treatment
upregulated the expression of Beclin 1 and LC3-II in Jurkat cells
in a concentration-dependent manner. The results demonstrated that
TAIII induces the expression of the autophagy-associated proteins
Beclin 1 and LC3-II, while reducing the proliferation of Jurkat
cells in vitro. There have been a substantial amount of
studies indicating that PI3K/Akt/mTOR signaling are key axes
involved in anticancer drug-induced autophagy (8,34,35).
Further investigation was performed, with a conclusion that the
PI3K/Akt/mTOR signaling pathway was inactivated by TAIII through
suppressing the expression of p-PI3K, p-Akt and p-mTOR. While the
preliminary observations are promising, more comprehensive and
detailed studies are required to be conducted, for example, animal
models in vivo or more cellular experiments, including a
colony form assay, and other apoptosis indicators, including a DNA
fragmentation assay.
In conclusion, the present results demonstrate that
TAIII possesses antitumor activity and its antineoplastic action is
associated with inhibition of proliferation, and induction of
apoptosis and autophagy in Jurkat cells. Furthermore, the results
demonstrated that the anticancer activity of TAIII in Jurkat cells
may be through regulating apoptosis-associated proteins, including
Bax and Bcl-2, and autophagy-associated proteins, including Beclin
1 and LC3-II. Furthermore, TAIII promoted Jurkat cells autophagy
through the PI3K/Akt/mTOR pathway. Finally, the present data may
provide a novel approach for the development of T-ALL therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by Shandong Province
medical science and technology development project (grant no.
2016WS0701), the Science and Technology Benefiting Project of
Qingdao City (15-9-2-83-nsh), the Science and Technology Project of
Yantai (grant no. 2016WS003), and the Scientific researching fund
projects of Yantai Yu Huang Ding Hospital (grant no. 201504).
Availability of data and materials
The datasets analyzed during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HW performed the experiments, analyzed the results
and wrote the manuscript. RD and WWF were responsible for the
collection and assembly of data, and the data analysis. XCZ and AML
revised the manuscript and performed supplementary experiments. WDW
was responsible for design of the present study and financial
support. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bassan R, Bourquin JP, DeAngelo DJ and
Chiaretti S: New approaches to the management of adult acute
lymphoblastic leukemia. J Clin Oncol. Sep 21–2018.(Epub ahead of
print). JCO2017773648. doi: 10.1200/JCO.2017.77.3648. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brassesco MS, Pezuk JA, Cortez MA, Bezerra
Salomão K, Scrideli CA and Tone LG: TLE1 as an indicator of adverse
prognosis in pediatric acute lymphoblastic leukemia. Leuk Res.
74:42–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patrick K and Vora A: Update on biology
and treatment of T-cell acute lymphoblastic leukaemia. Curr Opin
Pediatr. 27:44–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matloub Y, Stork L, Asselin B, Hunger SP,
Borowitz M, Jones T, Bostrom B, Gastier-Foster JM, Heerema NA,
Carroll A, et al: Outcome of children with standard-risk T-lineage
acute lymphoblastic leukemia - Comparison among different treatment
strategies. Pediatr Blood Cancer. 63:255–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaynon PS, Angiolillo AL, Carroll WL,
Nachman JB, Trigg ME, Sather HN, Hunger SP and Devidas M: Long-term
results of the children's cancer group studies for childhood acute
lymphoblastic leukemia 1983–2002: A Children's Oncology Group
Report. Leukemia. 24:285–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silverman LB, Stevenson KE, O'Brien JE,
Asselin BL, Barr RD, Clavell L, Cole PD, Kelly KM, Laverdiere C,
Michon B, et al: Long-term results of Dana-Farber Cancer Institute
ALL Consortium protocols for children with newly diagnosed acute
lymphoblastic leukemia (1985–2000). Leukemia. 24:320–334. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang W, Wang WT, Fang K, Chen ZH, Sun YM,
Han C, Sun LY, Luo XQ and Chen YQ: MIR-708 promotes phagocytosis to
eradicate T-ALL cells by targeting CD47. Mol Cancer. 17:122018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
You P, Wu H, Deng M, Peng J, Li F and Yang
Y: Brevilin A induces apoptosis and autophagy of colon
adenocarcinoma cell CT26 via mitochondrial pathway and
PI3K/AKT/mTOR inactivation. Biomed Pharmacother. 98:619–625. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang YH, Sun ZL, Fan MS, Li ZX and Huang
CG: Anti-diabetic effects of TongGuanWan, a Chinese traditional
herbal formula, in C57BL/KsJ-db/db mice. Planta Med. 78:18–23.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee B, Jung K and Kim DH: Timosaponin
AIII, a saponin isolated from Anemarrhena asphodeloides,
ameliorates learning and memory deficits in mice. Pharmacol Biochem
Behav. 93:121–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim SM, Jeong JJ, Kang GD, Kim KA, Choi HS
and Kim DH: Timosaponin AIII and its metabolite sarsasapogenin
ameliorate colitis in mice by inhibiting NF-κB and MAPK activation
and restoringTh17/Treg cell balance. Int Immunopharmacol.
25:493–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee B, Trinh HT, Jung K, Han SJ and Kim
DH: Inhibitory effects of steroidal timosaponins isolated from the
rhizomes of Anemarrhena asphodeloides against passive
cutaneous anaphylaxis reaction and pruritus. Immunopharmacol
Immunotoxicol. 32:357–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang GJ, Lin LC, Chen CF, Cheng JS, Lo YK,
Chou KJ, Lee KC, Liu CP, Wu YY Su W, et al: Effect of Timosaponin
A-III, from Anemarrhenae asphodeloides Bunge (Liliaceae), on
calcium mobilization in vascular endothelial and smooth muscle
cells and on vascular tension. Life Sci. 71:1081–1090. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang HL, Chiang WL, Hsiao PC, Chien MH,
Chen HY, Weng WC, Hsieh MJ and Yang SF: Timosaponin AIII mediates
caspase activation and induces apoptosis through JNK1/2 pathway in
human promyelocytic leukemia cells. Tumour Biol. 36:3489–3497.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lok CN, Sy LK, Liu F and Che CM:
Activation of autophagy of aggregation- prone ubiquitinated
proteins by timosaponin A-III. J Biol Chem. 286:31684–31696. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sy LK, Yan SC, Lok CN, Man RY and Che CM:
Timosaponin A-III induces autophagy preceding mitochondria-mediated
apoptosis in HeLa cancer cells. Cancer Res. 68:10229–10237. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JR, Jia XH, Wang H, Yi YJ, Wang JY
and Li YJ: Timosaponin A-III reverses multi-drug resistance in
human chronic myelogenous leukemia K562/ADM cells via
downregulation of MDR1 and MRP1 expression by inhibiting PI3K/Akt
signaling pathway. Int J Oncol. 48:2063–2070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang YJ, Chung HJ, Nam JW, Park HJ, Seo
EK, Kim YS, Lee D and Lee SK: Cytotoxic and antineoplastic activity
of timosaponin A-III for human colon cancer cells. J Nat Prod.
74:701–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nho KJ, Chun JM and Kim HK: Induction of
mitochondria-dependent apoptosis in HepG2 human hepatocellular
carcinoma cells by timosaponin A-III. Environ Toxicol Pharmacol.
45:295–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Biederbick A, Kern HF and Elsasser HP:
Monodansylcadaverine (MDC) is a specific in vivo marker for
autophagic vacuoles. Eur J Cell Biol. 66:3–14. 1995.PubMed/NCBI
|
|
21
|
Kwak CH, Lee SH, Lee SK, Ha SH, Suh SJ,
Kwon KM, Chung TW, Ha KT, Chang YC, Lee YC, et al: Induction of
apoptosis and antitumor activity of eel skin mucus, containing
lactose-binding molecules, on human leukemic K562 cells. Mar Drugs.
13:3936–3949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scarfò L and Ghia P: Reprogramming cell
death: BCL2 family inhibition in hematological malignancies.
Immunol Lett. 155:36–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi L, Ren K, Fang F, Zhao DH, Yang NJ and
Li Y: Over expression of BCL2 and low expression of caspase 8
related to TRAIL resistance in brain cancer stem cells. Asian Pac J
Cancer Prev. 16:4849–4852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Christodoulou MI, Kontos CK, Halabalaki M,
Skaltsounis AL and Scorilas A: Nature promises new anticancer
agents: Interplay with the apoptosis-related BCL2 gene family.
Anticancer Agents Med Chem. 14:375–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Zhou M, Ouyang J, Geng Z and Wang
Z: Tetraarsenictetrasulfide and arsenic trioxide exert synergistic
effects on induction of apoptosis and differentiation in acute
promyelocytic leukemia cells. PLoS One. 10:e01303432015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cingeetham A, Vuree S, Dunna NR, Gorre M,
Nanchari SR, Edathara PM, Meka P, Annamaneni S, Digumarthi R, Sinha
S and Satti V: Influence of BCL2-938C>A and BAX-248G>A
promoter polymorphisms in the development of AML: Case-control
study from South India. Tumour Biol. 36:7967–7976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilde L, Tanson K, Curry J and
Martinez-Outschoorn U: Autophagy in cancer: A complex relationship.
Biochem J. 475:1939–1954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mizushima N: The pleiotropic role of
autophagy: from protein metabolism to bactericide. Cell Death
Differ. 12 (Suppl 2):S1535–S1541. 2005. View Article : Google Scholar
|
|
30
|
Altman BJ, Jacobs SR, Mason EF, Michalek
RD, MacIntyre AN, Coloff JL, Ilkayeva O, Jia W, He YW and Rathmell
JC: Autophagy is essential to suppress cell stress and to allow
BCR-Abl-mediated leukemogenesis. Oncogene. 30:1855–1867. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gong A, Ye S, Xiong E, Guo W, Zhang Y,
Peng W, Shao G, Jin J, Zhang Z, Yang J, et al: Autophagy
contributes to ING4-induced glioma cell death. Exp Cell Res.
319:1714–1723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang N, Feng Y, Zhu M, Siu FM, Ng KM and
Che CM: A novel mechanism of XIAP degradation induced by
Timosaponin AIII in hepatocellular carcinoma. Biochim Biophys Acta.
1833:2890–2899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the
beclin 1 autophagy gene. J Clin Invest. 112:1809–20. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Y, Cai X, Zong B, Feng R, Ji Y, Chen G
and Li Z: Qianlie Xiaozheng decoction induces autophagy in human
prostate cancer cells via inhibition of the Akt/mTOR pathway. Front
Pharmacol. 9:2342018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin H, Zhang C, Zhang H, Xia YZ, Zhang CY,
Luo J, Yang L and Kong LY: Physakengose G induces apoptosis via
EGFR/mTOR signaling and inhibits autophagic flux in human
osteosarcoma cells. Phytomedicine. 42:190–198. 2018. View Article : Google Scholar : PubMed/NCBI
|