Introduction
Glioma is the most common form of aggressive central
nervous system tumor in adults (1).
Despite the fact that therapeutic strategies have advanced during
recent years, patients have a very poor prognosis with a median
survival of 15 months (2,3). Genomic instability and signaling
alternations are major contributors to radiotherapy and
chemotherapy resistance of gliomas, therefore, leading to tumor
recurrence following resection (4).
Therefore, a better understanding of the molecular mechanisms in
glioma tumorigenesis and an identification of novel prognostic
molecular markers may potentially guide the design of functional,
diagnostic and therapeutic strategies to this disease.
Protein tyrosine phosphatase 1B (PTPN1) is a
non-transmembrane protein tyrosine phosphatase, which is located on
the face of the endoplasmic reticulum, and serves a critical role
in regulating the activities of major signaling pathways involved
in different diseases, including obesity, diabetes and cancer
(5,6).
However, to the best of our knowledge, the role of PTPN1 in tumor
progression remains controversial (6). PTPN1 has been reported to be involved in
the progression of different types of tumors by interacting with
numerous oncogenic substrates, including SRC proto-oncogene,
non-receptor tyrosine kinase (Src)/MAPK pathway in breast and lung
cancer (7,8). In addition, it has also been reported to
negatively regulate several oncogenes, including Bcr-Abl and
β-catenin (9,10).
Previous studies have greatly extended our
understanding of the molecular events of gliomagenesis, however,
the function of PTPN1 in the development of gliomas is less
understood.
Materials and methods
Bioinformatics
All the TCGA data were downloaded from the cancer
Genome Browser of the UCSC database (https://genome.ucsc.edu/index.html). Normalized PTPN1
mRNA expression in GBM and LGG with corresponding clinical data
were included in files. In total, 10 non-neoplastic brain samples,
167 GBM samples and 530 LGG samples were analyzed.
Sample tissue collection
Paraffin-embedded samples, including 102 glioma
specimens and 34 matched pairs of resected glioma tissues with
non-neoplastic brain specimens, and fresh tissue, including 25
glioma specimens and 14 non-neoplastic brain specimens, were
resected at the Xijing Hospital and the First Affiliated Hospital
of Xi'an Jiaotong University School of Medicine (Xi'an, China)
between 2008 and 2014. The present study was approved by the Ethics
Committee of Xijing Hospital (Xi'an, China) in cooperation with the
First Affiliated Hospital of Xi'an Jiaotong University School of
Medicine. All patients underwent brain tumor resections and then
were followed up until the death or the end of 2017. None of them
had received radiotherapy or chemotherapy previously. Written
informed consent was obtained from each patient prior to surgery.
The clinicopathological characteristics and the follow-up
information of the 136 patients, including sex, age, WHO grade,
recurrence or not, receiving radiotherapy during the follow-up or
not, receiving chemotherapy during the follow-up or not, the KPS
score, the location of the tumor, living status at the end of the
follow-up are presented in Table I.
The median survival time of the glioma patients is provided in
Table II. We could not provide the
exact details of each individual since they will be used in future
research from Xijing Hospital.
 | Table I.Clinicopathological characteristics
of the glioma patients (n=136). |
Table I.
Clinicopathological characteristics
of the glioma patients (n=136).
|
Characteristics | No. | Percent |
|---|
| Sex |
|
|
|
Male | 74 | 54.4 |
|
Female | 62 | 45.6 |
| Age, years |
|
|
|
Mean | 45.7 |
|
| SD | 16.4 |
|
| WHO grade |
|
|
|
I/II | 87 | 64.0 |
|
III/IV | 49 | 36.3 |
| Recurrence |
|
|
| No | 39 | 28.7 |
|
Yes | 97 | 71.3 |
| Radiotherapy |
|
|
| No | 57 | 41.9 |
|
Yes | 79 | 58.1 |
| Chemotherapy |
|
|
| No | 81 | 59.6 |
|
Yes | 55 | 40.4 |
| Seizures |
|
|
| No | 74 | 54.4 |
|
Yes | 62 | 45.6 |
| KPS |
|
|
|
≤80 | 56 | 41.2 |
|
>80 | 80 | 58.8 |
| Location |
|
|
| Frontal
lobe | 56 | 42.6 |
|
Temporal lobe | 35 | 25.7 |
|
Parietal lobe | 23 | 17.6 |
|
Occipital lobe | 8 |
5.9 |
|
Cerebellum | 6 |
4.4 |
| Spinal
cord | 2 |
1.5 |
| Other
part | 6 |
5.9 |
| Survival
status |
|
|
|
Dead | 74 | 54.4 |
|
Alive | 62 | 45.6 |
 | Table II.Mean and median survival time of
various genotypes in glioma patients. |
Table II.
Mean and median survival time of
various genotypes in glioma patients.
| Genotypes | Mean (month) | Median (month) |
|---|
|
PTPN1− | 51.35 | 48.00 |
|
PTPN1+ | 30.06 | 25.00 |
IHC analysis
Briefly, specimens were cut into sections
(thickness, 5 µm). The sections were deparaffinized and rehydrated
in a graded ethanol series, and washed in distilled water. The
sections were then incubated with anti-PTPN1 antibody (dilution
1:200; cat. no. ab133244; Abcam, Cambridge, UK) overnight at 4°C
and a secondary antibody (cat. no. sp9001; Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd., Beijing, China) for 30 min. For
visualization, diaminobenzidine (DAB; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) was used. A light microscope (Olympus
Corp.) was used to capture images to observe sample staining. To
analyze the expression of PTPN1, the staining was categorized as
previously described (11) and was
confirmed by a pathologist at the First Affiliated Hospital of
Xi'an Jiaotong University School of Medicine.
Copy number analysis
The copy number of PTPN1 was analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) on a
CFX384 Thermal Cycler Dice™ (Bio-Rad Laboratories, Inc.) as
previously described (12). This
method has been well established and widely used in various types
of human cancer (12–14). Specific primers and TaqMan probes were
designed using Primer Express 3.0 (Applied Biosystems; Thermo
Fisher Scientific, Inc.) to amplify PTPN1 and the internal
reference gene β-actin. For the PTP1B gene, the TaqMan probe used
was 5′-6FAM-TAACCCATCTCTGCCCTCTGATTCCTCAG-TAMRA-3′, and the primers
were 5′-GCCATTCATTTTCTCCAAAGTGA-3′ (forward) and
5′-CGACCCGACTTCTAACTTCAGTGT-3′ (reverse). For the β-actin gene, the
probe was
5′-6-carboxyfluorescein-ATGCCCTCCCCCATGCCATCC-tetramethyrhodamine-3′,
and the primers were 5′-TCACCCACACTGTGCCCATCTACGA-3′ (forward) and
5′-TCGGTGAGGATCTTCATGAGGTA-3′ (reverse). Using a PCR protocol
previously described (15), the
samples were run in triplicate, and β-actin was run in parallel, in
order to normalize input DNA. Standard curves were established
using serial dilutions of normal leukocyte DNA. PTPN1 amplification
was defined by a copy number ≥4.
Cell culture and siRNA
transfection
Human glioma cell lines SF295 (cat. no. TCHu 58),
A172 (cat. no. TCHu171) and glioblastoma cell line ‘U87’ of unknown
origin (cat. no. TCHu138) were obtained and authenticated with STR
profile in The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences (www.cellbank.org.cn). Cells were cultured in DMEM
media (cat. no. 10569010; Gibco; Thermo Fisher Scientific, Inc.)
with 10% fetal bovine serum (FBS; Biological Industries) and
maintained in an incubator with 5% CO2 at 37°C. For
transient small interfering (si) RNA transfection, cells were
transfected with siRNA targeting PTPN1 (si-PTPN1-1:
GUCGGAUUAAACUACAUCATT, si-PTPN1-2: UGAUGUAGUUUAAUCCGACTT) and
control siRNA (si-NC), constructed by Shanghai GenePharma Co.,
Ltd.
RNA extraction and RT-qPCR
Total RNA was extracted from fresh samples and cell
lines using TRIzol reagent (Takara Bio, Inc.) and cDNA was prepared
using PrimeScript RT reagent kit (Roche Diagnostics), according to
the manufacturer's protocols. RT-qPCR was performed on a CFX96
Thermal Cycler Dice™ real-time PCR system (Bio-Rad Laboratories,
Inc.) using SYBR™ Green Master Mix (BioTools Pty. Ltd.) under the
following cycling conditions: 3 min at 95°C, followed by 35 cycles
of 10 sec at 95°C and 45 sec at 58°C. The mRNA expression of PTPN1
was normalized to 18S rRNA. Relative mRNA expression was calculated
by using 2−ΔΔCq method (16). Each sample was run in triplicate.
Primers: 18s F: 5′-CGCCGCTAGAGGTGAAATTC-3′, R:
5′-CTTTCGCTCTGGTCCGTCTT-3′, PTPN1 F: 5′-GCCACCCAAACGAATCCT-3′, R:
5′-CGACCCGACTTCTAACTTCAG-3′.
Western blot analysis
Cells were lysed in pre-chilled RIPA buffer
containing protease inhibitors (Sigma-Aldrich; Merck KGaA).
Supernatants were collected and subjected to 10% SDS-PAGE with 200
ng total protein, and transferred onto polyvinylidene fluoride
(PVDF) membranes (Roche Diagnostics). The membranes were
subsequently blocked with 5% non-fat dry milk for 1.5 h at room
temperature. The membranes were incubated with primary antibodies,
anti-PTPN1 (dilution 1:1,500; cat. no. ab133244; Epitomics; Abcam),
anti-phospho-Akt (S473; dilution 1:1,000; cat. no. BS4007; Bioworld
Technology, Inc.), anti-phospho-Akt (T308; dilution 1:1,000; cat.
no. AP0056; Bioworld Technology, Inc.), anti-phospho-Erk1/2
(dilution 1:1,000; cat. no. 4370; Cell Signaling Technology, Inc.),
anti-total-Akt (t-Akt, dilution 1:1,000; cat. no. BS1379; Bioworld
Technology, Inc.), anti-total-Erk1/2 (t-Erk) (dilution 1:1,000;
cat. no. 9102; Cell Signaling Technology, Inc.), anti-c-Myc
(dilution 1:500; cat. no. sc-4084; Santa Cruz Biotechnology, Inc.),
and anti-GAPDH (dilution 1:2,000; cat. no. AW5681; Abgent Biotech
Co., Ltd.). This was followed by incubation with species-specific
HRP-conjugated secondary antibodies (dilution 1:2,000; cat. nos.
130004 and 130023) from OriGene Technologies, Inc. Immunoblotting
signals were visualized using the Western Bright enhanced
chemiluminescence detection system (Advansta, Inc.).
Cell proliferation assay
Cells (1,000 cells/well) were seeded and cultured in
96-well plates for 1, 3, 5 and 7 days. At the indicated
time-points, 20 µl of 0.5 mg/ml MTT (Sigma-Aldrich; Merck KGaA) was
added into the medium and incubated for 4 h, followed by adding 150
µl DMSO for an additional 15 min. A microplate reader (Dynatec
Scientific Labs, Inc.) was used to measure the absorbance at a
wavelength of 570 nm.
Transwell migration assay
Cell migration and invasion assays were assessed by
Transwell (8.0 µm pore size; Corning Inc.). For the cell invasion
assay, Transwell chambers were coated with Matrigel (4X dilution;
15 µl/well; BD Biosciences). Cells were seeded in the upper chamber
at a density of 1×104 cells/ml for the migration assay
and 1×104 cells/ml for the invasion assay in 200 µl of
medium containing 0.5% FBS. The DMEM medium with 20% FBS (1 ml) was
added to the lower chamber. After 12 and 24 h of incubation,
non-migrating/non-invading cells in the upper chamber were removed
with a cotton swab, and migrating/invading cells were subsequently
fixed in 100% methanol and stained with crystal violet solution
(0.5% crystal violet in 2% ethanol) for 15 min. Images were
captured and five fields of each membrane were randomly selected.
The number of migrating/invading cells was expressed as the average
number of cells observed per microscopic (light microscope; Olympus
Corp., Tokyo, Japan) field over the five fields.
Statistical analysis
Student's t-test was performed to compare two
independent groups and two-way analysis of variance (ANOVA), with
Bonferroni post hoc test was used for MTT group comparisons.
One-way analysis of variance (ANOVA), with Dunnett's post hoc test
was used for multiple group comparisons. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results are presented as the mean ± standard deviation. One-way
analysis of variance (ANOVA), with Dunnett's post hoc test was used
for multiple comparisons between groups. The Kaplan-Meier survival
curve and log-rank tests were used to assess the survival of
patients with glioma. Cox regression analysis and logistic
regression were used to evaluate the effects of PTPN1 copy number
on survival and the odds ratio of each characteristic. Calculations
and graphing were performed using SPSS (version 18.0; SPSS, Inc.)
and GraphPad Prism (version 5.01; GraphPad Software, Inc., La
Jolla, CA, USA).
Results
Amplification and overexpression of
PTPN1 in human glioma
An analysis of PTPN1 expression pattern was
performed using RT-qPCR, which indicated that PTPN1 was upregulated
in glioma tissue compared with non-neoplastic brain tissues
(Fig. 1A). Taking into consideration
that gene amplification is a common mechanism for gene
overexpression, an additional RT-qPCR was performed to analyze the
copy number of the PTPN1 gene in 136 gliomas and 34 non-neoplastic
brain specimens. As indicated in Fig.
1B, the copy number of the PTPN1 gene in glioma cases was
significantly higher compared with non-neoplastic brain cases.
Consistent with the RT-qPCR data, it was further confirmed that
PTPN1 expression was significantly upregulated in low-grade gliomas
(LGG) and was even higher in glioblastoma (GBM) compared with
normal brain tissue, according to The Cancer Genome Atlas (TCGA)
database (Fig. 1C). For protein
expression, immunohistochemistry also confirmed that PTPN1
expression was increased in GBM tissue compared with non-neoplastic
brain tissue (Fig. 1D).
PTPN1 amplification and overexpression
is associated with poor prognosis in patients with glioma
To assess the effect of PTPN1 amplification on the
survival of patients with glioma, Kaplan-Meier survival curves were
used, grouped by the aberrant copy number of PTPN1, with the median
copy number as the cut-off value. The data indicated that the
patients with PTPN1 amplification had shorter mean and median
survival times compared with the patients without PTPN1
amplification (Table II). The
differences between the Kaplan-Meier survivals curves were also
analyzed using the log-rank test. In accordance to the
aforementioned, PTPN1 expression was significantly associated with
poor survival among patients with GBM according to the TCGA
database (Fig. 2). Univariate Cox
regression analyses also indicated that there was a significant
association of PTPN1 amplification with poor survival rate in
patients with glioma (Table III).
In addition, multivariate Cox regression analyses suggested that
PTPN1 amplification was an independent risk factor for patients
with glioma with respect to copy number, pathological diagnosis,
World Health Organization (WHO), and relapse (Table III). In addition, PTPN1
amplification was associated with a significantly increased risk of
tumor relapse and age (Table
IV).
 | Table III.Prognostic value of
clinicopathological factors and PTPN1 amplification using
univariate and multivariate Cox regression analysis (n=136). |
Table III.
Prognostic value of
clinicopathological factors and PTPN1 amplification using
univariate and multivariate Cox regression analysis (n=136).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | Hazard Ratio | P-value | Hazard Ratio | P-value |
|---|
| Copy number | 1.88
(1.32–3.10) | 0.01c | 1.69
(1.04–2.73) | 0.03c |
| Pathological
diagnosisa | 2.31
(1.74–2.68) |
<0.01d | 2.03
(1.59–2.59) |
<0.01d |
| WHOb | 4.03
(2.61–6.39) |
<0.01d | 3.82
(2.40–6.08) |
<0.01d |
| KPS | 1.25
(0.86–1.97) | 0.51 | 1.11
(0.71–1.74) | 0.64 |
| Relapse | 4.75
(2.35–7.95) |
<0.01d | 4.10
(2.21–7.61) |
<0.01d |
 | Table IV.Association of PTPN1 copy number with
clinicopathological characteristics in patients with glioma (OR and
95% CI). |
Table IV.
Association of PTPN1 copy number with
clinicopathological characteristics in patients with glioma (OR and
95% CI).
|
| PTPN1 copy
number |
|---|
|
|
|
|---|
|
Characteristics | ORa (95% CI) | P-value |
|---|
| Pathological
diagnosisb | 0.38
(0.12–1.48) | 0.86 |
| Age | 0.52
(0.30–0.90) | 0.02f |
| Sex | 1.43
(0.71–2.87) | 0.31 |
| WHO
gradec | 1.34
(0.65–2.75) | 0.42 |
| Recurrence | 2.35
(1.59–5.49) | 0.03f |
| KPS
scored | 1.07
(0.54–2.14) | 0.85 |
| Survival
statuse | 2.00
(1.00–4.02) | 0.05 |
PTPN1 knockdown inhibits glioma cell
growth
Increased expression of PTPN1 in glioma indicated
that PTPN1 may be a putative oncogene in primary glioma. Firstly,
we assessed the expression of PTPN1 in different glioma cells. As
revealed in Fig. 3A, glioma cell
lines SF295 and A172 exhibited higher PTPN1 expression than U87,
this may due to the different genetic backgrounds of these cell
lines. Thus, the effect of PTPN1 on glioma cell growth was
investigated by knocking down PTPN1 in SF295 and A172 glioma cells.
The knockdown of PTPN1 expression using two different siRNA
sequences, si-PTPN1-1 and 2, was confirmed by RT-qPCR and western
blot analysis (Fig. 3B and C). Glioma
cell proliferation was reduced when PTPN1 expression was inhibited
by specific PTPN1 siRNA compared with control siRNA (si-NC), in
particular si-PTPN1-2 (Fig. 3D).
Downregulated PTPN1 expression
inhibits glioma cell migration and invasion
Due to the fact that invasion and metastasis are the
main causes of cancer-associated mortality (17), the aim of the present study was to
examine the effect of PTPN1 knockdown on cell migration and
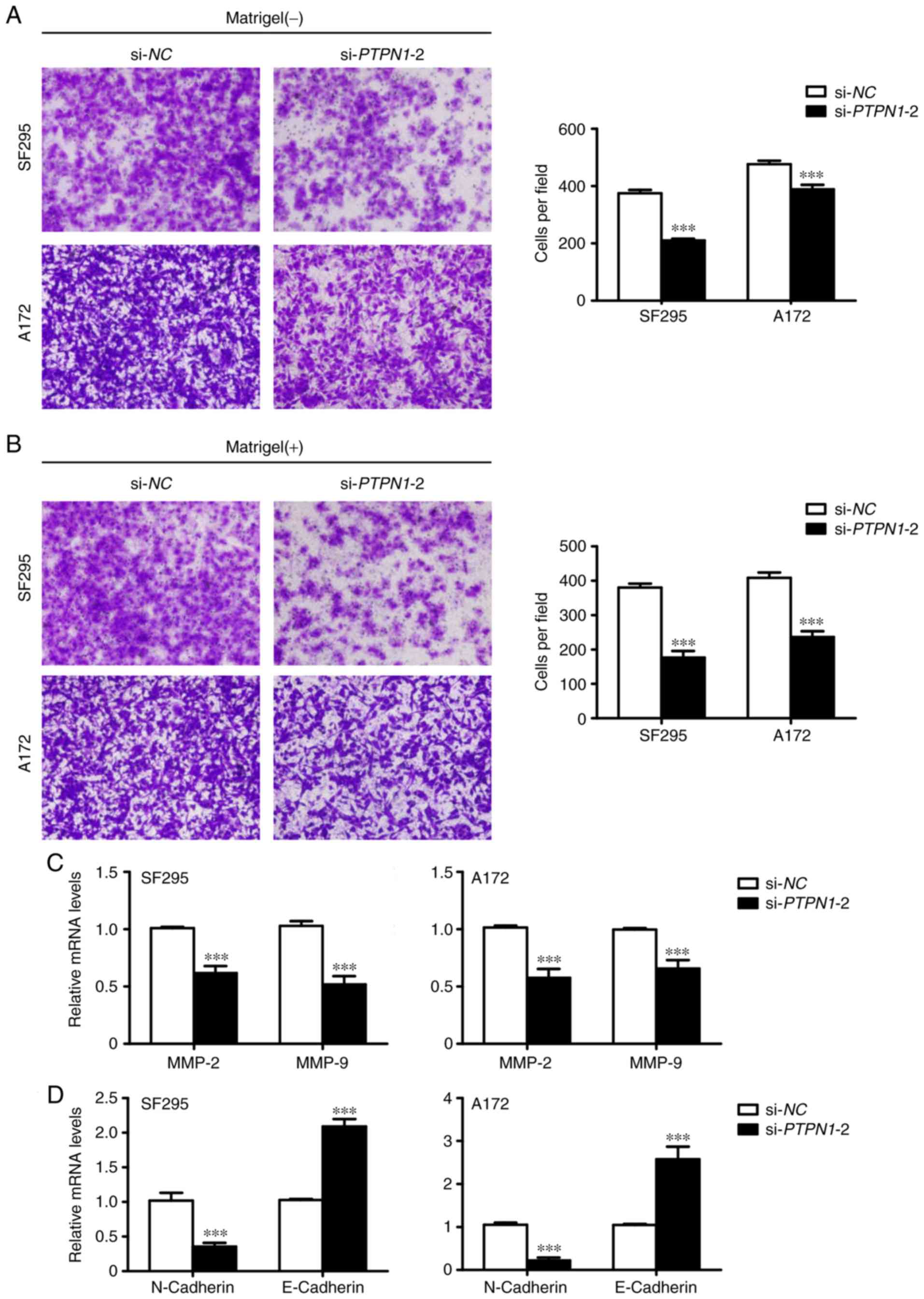
invasion using Transwell assays. Downregulated PTPN1 expression
decreased the number of migrated cells (Fig. 4A). In addition, PTPN1 knockdown also
reduced the number of cells passing through the Matrigel-coated
membrane (Fig. 4B). In addition,
RT-qPCR analysis indicated that the decreased metastasis-associated
phenotypes were due to the reduced expression of MMPs, which are
involved in cancer cell metastasis (18), in glioma cells (Fig. 4C). Therefore, the inhibition of
epithelial-mesenchymal transition (EMT) process by PTPN1
downregulation may contribute to the suppression of glioma cell
metastasis (Fig 4D).
PTPN1 regulates the MAPK/ERK and
PI3K/AKT signaling pathways in glioma
Previous studies have reported that the MAPK/ERK
pathway and its downstream Myc serve an important role in the
development and progression of glioma (19,20).
Therefore, the present study investigated the effect of PTPN1 on
the activities of the MAPK/ERK pathway. As indicated in Fig. 5, PTP1N knockdown suppressed
phosphorylation of MAPK/ERK pathway functional kinase ERK1/2 and
the expression of ERK downstream c-Myc. In addition, the PI3K/AKT
pathway has been reported to regulate glioma metastasis (21,22). PTPN1
expression silencing also suppressed the phosphorylation of AKT in
Thr308 and Ser473 (Fig. 5). These
results indicated that PTPN1 promotes glioma cell proliferation and
metastasis by activating the MAPK/ERK and PI3K/AKT signaling
pathways.
Discussion
PTPN1, as an oncogene, has been reported as a
potential therapeutic target and prognostic marker in breast cancer
and gastric cancer (23,24), however its role in glioma has yet to
be established. Studies involving the underlying mechanism of PTPN1
in gliomagenesis have reported controversial findings on the role
of PTPN1: a number of previous studies have reported PTPN1 as a
potential tumor suppressor gene in glioma (25,26), while
other studies have suggested PTPN1 to serve an oncogenic role in
glioma (27,28). The results of the present study
provided a series of evidence in support of an oncogenic role for
PTPN1 in glioma. Furthermore, high expression of PTPN1 predicted
poor prognosis of patients with GBM, therefore, indicating that
PTPN1 may serve as a prognostic marker and a putative target in the
treatment of glioma.
A previous study indicated that PTPN1 overexpression
may activate Src by reducing phosphorylation at tyrosine 530
(29). Src activation has been
reported to be associated with proliferation, survival and
metastasis in cancer cells, by stimulating multiple signaling
pathways, including the Src/PI3K/Akt and Src/MAPK/ERK signaling
pathways (30). The MAPK/ERK and
PI3K/Akt signaling pathways have been reported to serve an
important role in the proliferation of numerous types of cancer
cells (31–33). EGFR, as an upstream of the MAPK/ERK
and PI3K/AKT signaling pathways, has been reported to be
overexpressed in glioma cells, resulting in proliferation with
downstream effects (34,35). In addition, the activation of the
MAPK/ERK pathway has been revealed to promote the expression of
vascular endothelial growth factor, which is an important
angiogenic factor for glioma (36).
An increasing number of studies have reported that the
hyperactivation of AKT is involved in the migration and invasion of
tumor cells (37,38). In addition, a previous study confirmed
that abnormal activation of the PI3K/AKT signaling pathway may
induce overexpression of oncogene sprouty RTK signaling antagonist
1, leading to inhibited apoptosis and increased proliferation in
human glioma cells (39). Therefore,
it was hypothesized that overexpression of PTPN1 may lead to glioma
progression and metastasis by activating the Src/PI3K/AKT and
Src/MAPK/ERK signaling pathways, and may predict poor prognosis of
patients with glioma. The data of the present study indicated that
PTPN1 silencing inhibits phosphorylation of ERK and AKT in glioma
cells. In addition, PTPN1 knockdown inhibited the EMT process,
which has been demonstrated to serve a key role during the early
steps of invasion and metastasis of epithelial malignancies
(40), by increasing the expression
of epithelial cell marker E-cadherin and reducing the expression of
mesenchymal marker N-cadherin. These results indicated that
downregulation of PTPN1 may contribute to the inhibition of glioma
cell migration and invasion. These insights into the molecular
mechanisms may provide potential treatment options for a subset of
cancers, including glioma, via inhibition of PTPN1.
Notably, PTPN1 may play an important role in the
transformation of LGG to GBM, which was supported by analyzing LGG
and GBM data in TCGA database. In fact, the expression of certain
biomarkers undergo change when the progression of LGG to GBM occurs
(41). However, we did not have
enough LGG patient samples or cell lines to verify our hypothesis.
Thus, further studies are required to investigate the role of PTPN1
in the conversion of LGG to GBM. In addition, for comparisons with
SF295 and A172, we did not have an appropriate non-cancerous
negative control since we failed to have a non-malignant glial cell
line in our in vitro study, and this was also a limitation
of this study.
In summary, the present study demonstrated PTPN1 is
overexpressed in glioma compared with matched normal brain tissue
by immunohistochemistry (IHC). In addition, PTPN1 overexpression
was associated with the poor survival of patients. PTPN1 knockdown
was indicated to suppress glioma cell growth, migration and
invasion. Therefore, PTPN1 was revealed to promote glioma cell
proliferation and invasion through the MAPK/ERK and PI3K/AKT
pathways. The data of the present study, to the best of our
knowledge, was the first to verify the oncogenic role of PTPN1 in
glioma tumorigenesis and investigate the molecular mechanism of
PTPN1 in the promotion of glioma cell proliferation and survival.
Overexpression of PTPN1 has been reported to be associated with
shorter survival of patients and glioma metastasis. Therefore,
PTPN1 may serve as a potential prognostic marker and a possible
therapeutic target against glioma.
Acknowledgements
This research was supported by the First Affiliated
Hospital of Xi'an Jiaotong University School of Medicine and Xijing
Hospital of the Fourth Military Medical University (the First
Affiliated Hospital of Fourth Military Medical University).
Funding
No funding was received.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
JK and YZ designed the research and approved the
final version of the manuscript to be published. TJ as well as DL
analyzed the data and wrote the manuscript. TJ, TY and FL conducted
the research. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Written informed consent was obtained from all of
the patients before the surgery. The present study was approved by
the Ethics Committee of Ankang Central Hospital, First Affiliated
Hospital of Xi'an Jiaotong University and Xijing Hospital.
Patient consent for publication
Identifying information, including names, initials,
date of birth or hospital numbers, images or statements were not
included in the manuscript. Written informed consent was obtained
from all of the patients prior to treatment.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wen PY, Macdonald DR, Reardon DA,
Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert
MR, Lassman AB, et al: Updated response assessment criteria for
high-grade gliomas: Response assessment in neuro-oncology working
group. J Clin Oncol. 28:1963–1972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mittal S, Pradhan S and Srivastava T:
Recent advances in targeted therapy for glioblastoma. Expert Rev
Neurother. 15:935–946. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huse JT and Holland EC: Targeting brain
cancer: Advances in the molecular pathology of malignant glioma and
medulloblastoma. Nat Rev Cancer. 10:319–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seely BL, Staubs PA, Reichart DR, Berhanu
P, Milarski KL, Saltiel AR, Kusari J and Olefsky JM: Protein
tyrosine phosphatase 1B interacts with the activated insulin
receptor. Diabetes. 45:1379–1385. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lessard L, Stuible M and Tremblay ML: The
two faces of PTP1B in cancer. Biochim Biophys Acta. 1804:613–619.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cortesio CL, Chan KT, Perrin BJ, Burton
NO, Zhang S, Zhang ZY and Huttenlocher A: Calpain 2 and PTP1B
function in a novel pathway with Src to regulate invadopodia
dynamics and breast cancer cell invasion. J Cell Biol. 180:957–971.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Wu Y, Zhu S, Liang W, Wang Z, Wang
Y, Lv T, Yao Y, Yuan D and Song Y: PTP1B promotes cell
proliferation and metastasis through activating src and ERK1/2 in
non-small cell lung cancer. Cancer Lett. 359:218–225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LaMontagne KR Jr, Hannon G and Tonks NK:
Protein tyrosine phosphatase PTP1B suppresses p210 bcr-abl-induced
transformation of rat-1 fibroblasts and promotes differentiation of
K562 cells. Proc NatI Acad Sci USA. 95:14094–14099. 1998.
View Article : Google Scholar
|
|
10
|
Balsamo J, Leung T, Ernst H, Zanin MK,
Hoffman S and Lilien J: Regulated binding of PTP1B-like phosphatase
to N-cadherin: Control of cadherin-mediated adhesion by
dephosphorylation of beta-catenin. J Cell Biol. 134:801–813. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Chen W, Zhang X, Hu L, Tang G,
Kong J and Wang Z: E26 transformation (ETS)-specific related
transcription factor-3 (ELF3) orchestrates a positive feedback loop
that constitutively activates the MAPK/Erk pathway to drive thyroid
cancer. Oncol Rep. 41:570–578. 2019.PubMed/NCBI
|
|
12
|
Wu G, Mambo E, Guo Z, Hu S, Huang X,
Gollin SM, Trink B, Ladenson PW, Sidransky D and Xing M: Uncommon
mutation, but common amplifications of the PIK3CA gene in thyroid
tumors. J Clin Endocrinol Metab. 90:4688–4693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawano O, Sasaki H, Okuda K, Yukiue H,
Yokoyama T, Yano M and Fujii Y: PIK3CA gene amplification in
Japanese non-small cell lung cancer. Lung Cancer. 58:159–160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mambo E, Gao X, Cohen Y, Guo Z, Talalay P
and Sidransky D: Electrophile and oxidant damage of mitochondrial
DNA leading to rapid evolution of homoplasmic mutations. Proce Natl
Acad Sci USA. 100:1838–1843. 2003. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guha A, Feldkamp MM, Lau N, Boss G and
Pawson A: Proliferation of human malignant astrocytomas is
dependent on Ras activation. Oncogene. 15:2755–2765. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Annibali D, Whitfield JR, Favuzzi E,
Jauset T, Serrano E, Cuartas I, Redondo-Campos S, Folch G,
Gonzàlez-Juncà A, Sodir NM, et al: Myc inhibition is effective
against glioma and reveals a role for Myc in proficient mitosis.
Nat Commun. 5:46322014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tu M, Wange W, Cai L, Zhu P, Gao Z and
Zheng W: IL-13 receptor alpha2 stimulates human glioma cell growth
and metastasis through the Src/PI3K/Akt/mTOR signaling pathway.
Tumour Biol. 37:14701–14709. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Chen T, Zhang J, Mao Q, Li S,
Xiong W, Qiu Y, Xie Q and Ge J: Notch1 promotes glioma cell
migration and invasion by stimulating beta-catenin and NF-kappaB
signaling via AKT activation. Cancer Sci. 103:181–190. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taliaferro-Smith L, Nagalingam A, Knight
BB, Oberlick E, Saxena NK and Sharma D: Integral role of PTP1B in
adiponectin-mediated inhibition of oncogenic actions of leptin in
breast carcinogenesis. Neoplasia. 15:23–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Liu B, Chen X, Su L, Wu P, Wu J
and Zhu Z: PTP1B expression contributes to gastric cancer
progression. Med Oncol. 29:948–956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reichardt W, Jung V, Brunner C, Klein A,
Wemmert S, Romeike BF, Zang KD and Urbschat S: The putative
serine/threonine kinase gene STK15 on chromosome 20q13.2 is
amplified in human gliomas. Oncol Rep. 10:1275–1279.
2003.PubMed/NCBI
|
|
26
|
Mondol AS, Tonks NK and Kamata T: Nox4
redox regulation of PTP1B contributes to the proliferation and
migration of glioblastoma cells by modulating tyrosine
phosphorylation of coronin-1C. Free Radic Biol Med. 67:285–291.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akasaki Y, Liu G, Matundan HH, Ng H, Yuan
X, Zeng Z, Black KL and Yu JS: A peroxisome proliferator-activated
receptor-gamma agonist, troglitazone, facilitates caspase-8 and −9
activities by increasing the enzymatic activity of protein-tyrosine
phosphatase-1B on human glioma cells. J Biol Chem. 281:6165–6174.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Petri MK, Koch P, Stenzinger A,
Kuchelmeister K, Nestler U, Paradowska A, Steger K, Brobeil A,
Viard M and Wimmer M: PTPIP51, a positive modulator of the MAPK/Erk
pathway, is upregulated in glioblastoma and interacts with 14–3-3β
and PTP1B in situ. Histol Histopathol. 26:1531–1543.
2011.PubMed/NCBI
|
|
29
|
Zhu S, Bjorge JD and Fujita DJ: PTP1B
contributes to the oncogenic properties of colon cancer cells
through Src activation. Cancer Res. 67:10129–10137. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen T, George JA and Taylor CC: Src
tyrosine kinase as a chemotherapeutic target: Is there a clinical
case? Anticancer Drugs. 17:123–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Zhang Z, Li R, Mao F, Sun W, Chen
J, Zhang H, Bartsch JW, Shu K and Lei T: ADAM12 induces EMT and
promotes cell migration, invasion and proliferation in pituitary
adenomas via EGFR/ERK signaling pathway. Biomed Pharmacother.
97:1066–1077. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du MR, Zhou WH, Yan FT, Zhu XY, He YY,
Yang JY and Li DJ: Cyclosporine A induces titin expression via
MAPK/ERK signalling and improves proliferative and invasive
potential of human trophoblast cells. Hum Reprod. 22:2528–2537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang HY, Chang HF, Tsai MJ, Chen JS and
Wang MJ: 6-Mercaptopurine attenuates tumor necrosis factor-alpha
production in microglia through Nur77-mediated transrepression and
PI3K/Akt/mTOR signaling-mediated translational regulation. J
Neuroinflammation. 13:782016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Butowski NA and Chang SM: Glial tumors:
The current state of scientific knowledge. Clin Neurosurg.
53:106–113. 2006.PubMed/NCBI
|
|
35
|
Purow BW, Sundaresan TK, Burdick MJ, Kefas
BA, Comeau LD, Hawkinson MP, Su Q, Kotliarov Y, Lee J, Zhang W and
Fine HA: Notch-1 regulates transcription of the epidermal growth
factor receptor through p53. Carcinogenesis. 29:918–925. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo G, Yao W, Zhang Q and Bo Y: Oleanolic
acid suppresses migration and invasion of malignant glioma cells by
inactivating MAPK/ERK signaling pathway. PLoS One. 8:e720792013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hollander MC, Blumenthal GM and Dennis PA:
PTEN loss in the continuum of common cancers, rare syndromes and
mouse models. Nat Rev Cancer. 11:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chai C, Song LJ, Han SY, Li XQ and Li M:
MicroRNA-21 promotes glioma cell proliferation and inhibits
senescence and apoptosis by targeting SPRY1 via the PTEN/PI3K/AKT
signaling pathway. CNS Neurosci Ther. 24:369–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang L, Wang Z, Liu C, Gong Z, Yang Y,
Kang H, Li Y and Hu G: TrkB promotes laryngeal cancer metastasis
via activation PI3K/AKT pathway. Oncotarget. 8:108726–108737. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ceccarelli M, Barthel FP, Malta TM,
Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh
A, Pagnotta SM, et al: Molecular profiling reveals biologically
discrete subsets and pathways of progression in diffuse glioma.
Cell. 164:550–563. 2016. View Article : Google Scholar : PubMed/NCBI
|