Introduction
Retinoblastoma (RB) is the most common type of
intraocular malignant tumor among children, and it seriously
affects the vision of these children and is associated with a poor
patient prognosis (1). The main
clinical manifestation is a white pupil, and the pathogenesis is
widely accepted to be due to mutations in the two copies of the
RB gene (2). RB has always
been considered to be the ideal model for studying tumor genetics
and tumor pathogenesis (1). The
RB gene was the first to be identified as a tumor-suppressor
gene in humans. With continued research, novel methods for the
effective prevention and treatment of the disease may be identified
through a deeper exploration into the pathogenesis of RB at the
molecular biology level (3).
microRNAs (miRNAs/miRs) are a family of mature
non-coding RNA molecules composed of 21–25 nucleotides that can
modulate target gene expression by cleaving the target mRNA or
inhibiting protein synthesis to cause post-transcriptional gene
silencing (4). As gene regulators,
miRNAs can affect a variety of cellular pathways and functions, and
early studies showed that miRNAs have an impact on gene expression
during development, cell death and proliferation, and formation of
the immune and nervous systems (5).
The current understanding is that miRNAs play an important role in
the occurrence of many diseases (5). A previous study demonstrated that
miRNAs are expressed in human tumor cells and are classified into
tumor-suppressor genes or oncogenes according to their roles in
tumor cell transformation and gene expression (6). miRNA genes are located in the fragile
sites of the human genome, thus they may be mutated easily in the
cancer genome as it accumulates damage (4–6).
miRNAs play a role in the regulation of proliferation,
differentiation and apoptosis of tumor cells (4).
The phosphoinositide 3-kinase (PI3K) signaling
pathway plays a role in the formation of a variety of tumors.
Activation of PI3K signaling leads to phosphorylation of protein
kinase B (AKT) and activation of the downstream signaling pathway,
as well as regulation of cell growth, reproduction, migration and
apoptosis (7). Members of the PI3K
signaling pathway are frequently observed to be abnormally
expressed in a variety of solid tumors (7). Mutations in the helical domain of the
protein of the phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit α (PIK3CA) gene, in exon 9, and mutations in the
kinase domain in exon 20 may upregulate PI3K signaling and
facilitate tumorigenesis (8). It
has been reported that increased copy number and mutation of the
PIK3CA gene occur in lung cancer, suggesting that the two types of
genetic alterations may play a role in the formation of RB
(8). PIK3CA is an oncogene that has
been confirmed in recent years, and mutations in PIK3CA may be
involved in the regulation of carcinogenesis (9). Mutations in the PIK3CA gene have been
identified in ~30% of solid tumors, and the mutation of this gene
can increase the kinase activity of the enzyme, activate AKT,
reduce apoptosis and contact inhibition, promote tumorigenesis and
increase tumor invasiveness (10).
A study by Liu et al (11)
indicated that miR-363-3p inhibits papillary thyroid carcinoma
progression by targeting PIK3CA. The present study was designed to
ascertain whether miR-363-3p regulates the PIK3CA signaling pathway
in RB and if it exerts anticancer effects in regards to this
disease.
Materials and methods
Patient samples
The serum samples of patients and normal controls
were collected from May 2016 to December 2016 at the Xi'an
Traditional Chinese Medicine Hospital (Xi'an, Shaanxi, China)
(Table I). The peripheral blood (10
ml) of all samples was centrifuged at 1,000 × g for 10 min at 4°C,
and then serum was collected. Serum was immediately frozen in
liquid nitrogen, and stored at −80°C. The study protocol was
approved by the Medical Ethics Committee of Xi'an Traditional
Chinese Medicine Hospital (Xi'an, Shaanxi, China).
 | Table I.Characteristic of the patients with
retinoblastoma (RB). |
Table I.
Characteristic of the patients with
retinoblastoma (RB).
| Variables | Normal control
subjects (n=6) | RB patients
(n=6) |
|---|
| Mean age (years) | 57.9±2.5 | 55.8±3.8 |
| Sex |
|
|
|
Female | 3 | 3 |
| Male | 3 | 3 |
| Edmondson grade |
|
|
| I | 0 |
|
| II | 0 |
|
| III | 0 | 1 |
| IV | 0 | 3 |
| V | 0 | 2 |
RNA isolation and quantitative
real-time PCR (RT-qPCR) analysis and microarray analysis
Total RNA was extracted from serum and cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA
synthesis was carried out using a RT kit (Takara Biotechnology
Ltd.). MicroRNA-363-3p expression was quantified by using SYBR
Premix Ex TaqTM (Takara) under ABI 7500 Fast Sequence Detection
System (Applied Biosystems Prism; Thermo Fisher Scientific,
Inc.).
Microarray analysis was performed using Illumina,
HT-12 v4.0 platform (Bencos Research Solutions). Total RNA was
extracted from serum and cells using Trizol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Gene expression analysis was
performed commercially using Illumina, HT-12 v4.0 platform and
expression of genes was analyzed using R 3.1.2 (www.r-project.org).
Cell, plasmids and transfections
Human RB WERI-Rb-1 cell line (Shanghai Cell Bank,
Chinese Academy of Sciences) was cultured in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) and
1% penicillin-streptomycin at 37°C in a humidified 5%
CO2 incubator. MicroRNA-363-3p plasmid and negative
plasmid were purchased from Shanghai Gene-Pharma Co. The
microRNA-363-3p plasmid (50 ng) and negative plasmid (50 ng) were
transfected into WERI-Rb-1 cells using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions.
Cell growth assay
Cell proliferation (1×103 cells/well) was
assessed using the MTT assay (0.5 mg/ml; Sigma-Aldrich; Merck KGaA)
for 4 h at 37°C and DMSO was added into the cells for 20 min at
37°C. Absorbance was then recorded using a microplate reader
(Bio-Rad) at 490 nm.
Cell apoptosis
The cells (1×106 cells/well) were washed
with PBS and cells were resuspended with dilute binding buffer (BD
Biosciences). Cells were stained with 5 µl Annexin V and 5 µl
propidium iodide (PI) (BD Biosciences) for 15 min at room
temperature. Cell apoptosis were detected by LSRII flow cytometer
(BD Biosciences) and analyzed by FlowJo 3.1 (Tree Star, Ashland,
OR, USA).
Cell invasion assays
The cells (1×106 cells/well) were seeded
into the upper chamber of Matrigel-coated inserts and RPMI-1640
medium containing 10% FBS was added to the lower chamber at 37°C in
a humidified 5% CO2 incubator for 48 h. The lower
surface cells were fixed in 70% ethanol for 30 min and stained with
0.1% crystal violet for 10 min. Cells were observed under an X71
inverted microscope (Olympus Corporation).
Western blot analysis
Total protein was extracted from the cells using
RIPA Kit and protein concentrations of the sample were quantified
using a BCA protein quantification kit. An amount of 40 µg protein
was separated by 8–12% SDS-PAGE and transferred to a PVDF membrane.
The membrane was blocked with 5% skim milk in TBST for 1 h at room
temperature and incubated with primary antibodies: anti-Bax (cat.
no. sc-6236, dilution 1:1,000, Santa Cruz Biotechnology),
anti-PIK3CA (cat. no. 4249, dilution 1:2,000, Cell Signaling
Technology, Inc.), anti-PDK1 (cat. no. sc-376586, dilution 1:1,000,
Santa Cruz Biotechnology), anti-p-Akt (cat. no. 4060, dilution
1:2,000, Cell Signaling Technology, Inc.) and GAPDH (cat. no. 5174,
dilution 1:5,000, Cell Signaling Technology, Inc.) at 4°C
overnight. Membrane was washed with TBST and incubated with
anti-rabbit (cat. no. sc-2030) or anti-mouse (cat. no. sc-2031)
secondary antibodies (dilution 1:5,000, Santa Cruz Biotechnology)
for 2 h at room temperature. Protein blank was detected using the
enhanced chemiluminescence (ECL) method and analyzed using Image
Lab 3.0 (Bio-Rad Laboratories, Inc.).
Caspase-3/9 activities
Total protein was extracted from the cells
(1×106 cells/well) using the RIPA Kit and protein
concentrations of the sample were quantified by the BCA protein
quantification kit. A total of 5 µg of protein was used to measure
caspase-3/9 activities using caspase-3/9 apoptosis activities
(C1115/ C1158). Absorbance was then recorded using a microplate
reader (Bio-Rad Laboratories) at 405 nm.
Statistical analysis
Data are presented as mean ± SD (n=3). All date were
analyzed by using ANOVA by Tukey's post test or two-tailed
Student's t test. P<0.05 was accepted as statistically
significant.
Results
miR-363-3p expression in serum from
patients with RB
To investigate the role of miRNAs in RB development
and progression, the expression levels of miRNAs in RB patient
serum were examined. The results of cDNA microarray analysis
revealed that miR-363-3p was downregulated in the serum of patients
with RB compared with the controls (Fig. 1A). RT-qPCR analysis showed that
miR-363-3p serum expression was downregulated in RB compared with
the controls (Fig. 1B).
Overexpression of miR-363-3p reduces
RB cell proliferation and invasion
The impact of overexpression of miR-363-3p on cell
growth and invasion was investigated using RB cells. As presented
in Fig. 2A, transfection with
miR-363-3p mimics significantly increased miR-363-3p expression.
Overexpression of miR-363-3p significantly reduced cell growth and
invasion of RB cells compared with the control group (Fig. 2B-D). In addition, overexpression of
miR-363-3p significantly increased the apoptosis rate of RB cells
compared with the control group (Fig.
2E and F). These findings indicate that miR-363-3p expression
may influence RB development and progression, and may be involved
in RB cell growth and apoptosis.
Overexpression of miR-363-3p induces
BAX protein expression and caspase-3/9 activity
BAX protein expression and caspase-3/9 activity were
analyzed in RB cells with miR-363-3p overexpression. Overexpression
of miR-363-3p significantly induced BAX protein expression and
caspase-3/9 activity compared with the control group (Fig. 3).
Overexpression of miR-363-3p
suppresses PIK3CA, pyruvate dehydrogenase kinase 1 (PDK1) and
phosphorylated (p)-AKT protein expression
To investigate whether miR-363-3p targets the PIK3CA
signaling pathway in RB, PIK3CA, PDK1 and p-AKT protein expression
levels were measured in RB cells with miR-363-3p overexpression.
The predicted interaction between miR-363-3p and the target site in
the 3′ untranslated region of PIK3CA is presented in Fig. 4A. As shown in Fig. 4B-E, overexpression of miR-363-3p
significantly suppressed PIK3CA, PDK1 and p-AKT protein expression
in RB cells compared with the control group.
 | Figure 4.Overexpression of miR-363-3p
suppresses PIK3CA, PDK1 and p-Akt protein expression in human RB
WERI-Rb-1 cells. (A) Predicted interaction between miR-363-3p and
the target site in the PIK3CA 3′-UTR. (B) PIK3CA, PDK1 and p-Akt
protein expression by western blotting assay. (C-E) PIK3CA, PDK1
and p-Akt protein expression by statistical analysis. Control,
negative control group; miR-363-3p, miR-363-3p-overexpressing
group. ##P<0.01 compared with the negative control
group. RB, retinoblastoma; PIK3CA,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α;
PDK1, pyruvate dehydrogenase kinase 1; p-AKT, phosphorylated
protein kinase B. |
Knockdown of miR-363-3p promotes RB
cell growth and invasion
The effects of miR-363-3p knockdown on RB cell
growth and invasion were investigated. As presented in Fig. 5A, transfection with anti-miR-363-3p
inhibitor (Anti-363-3p) significantly reduced the expression of
miR-363-3p. Knockdown of miR-363-3p significantly promoted RB cell
growth and invasion compared with the control group (Fig. 5B-D). Knockdown of miR-363-3p
significantly reduced the cell apoptosis rate of the RB cells
compared with the control group (Fig.
5E and F).
Knockdown of miR-363-3p suppresses BAX
protein expression and caspase-3/9 activity, and induces PIK3CA,
PDK1 and p-AKT protein expression
Knockdown of miR-363-3p significantly induced BAX
protein expression and caspase-3/9 activity in RB cells, compared
with the control group (Fig. 6A-D).
In addition, knockdown of miR-363-3p significantly induced PIK3CA,
PDK1 and p-AKT protein expression in RB cells, compared with the
control group (Fig. 6E-H).
 | Figure 6.Downregulation of miR-363-3p
suppresses Bax protein expression and caspase-3/9 activity, and
induces PIK3CA, PDK1 and p-Akt protein expression in human RB
WERI-Rb-1 cells. (A and B) Caspase-3/9 activity. Bax protein
expression by (C) statistical analysis and (D) western blotting
analysis. (E) PIK3CA, PDK1 and p-Akt protein expression by western
blotting assays. (F-H) PIK3CA, PDK1 and p-Akt protein expression by
statistical analysis. Control, negative control group; Anti-363-3p,
miR-363-3p downregulation group. ##P<0.01 compared
with the negative control group. RB, retinoblastoma; PIK3CA,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α;
PDK1, pyruvate dehydrogenase kinase 1; p-AKT, phosphorylated
protein kinase B. |
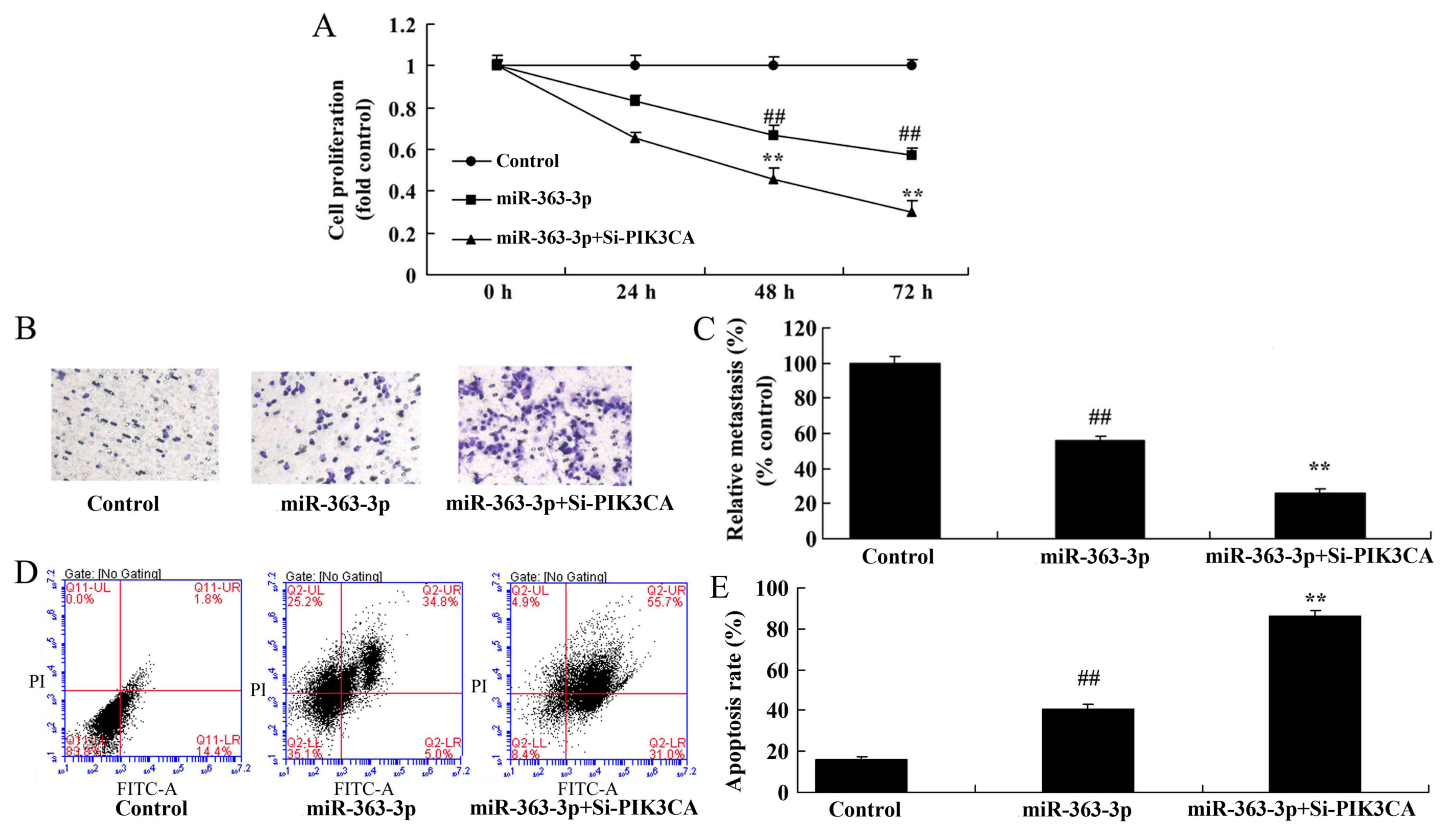
Knockdown of PIK3CA increases the
anticancer effects of miR-363-3p on PIK3CA, PDK1 and p-AKT protein
expression
The role of PIK3CA in the anticancer effect of
miR-363-3p in regards to RB cell proliferation and invasion was
investigated. si-PIK3CA further significantly suppressed PIK3CA,
PDK1 and p-AKT protein expression in RB cells with miR-363-3p
overexpression compared with cells with overexpression of
miR-363-3p alone (Fig. 7A-D).
Inhibition of PIK3CA significantly induced BAX protein expression
and caspase-3/9 activity in RB cells with miR-363-3p overexpression
compared with cells with overexpression of miR-363-3p alone
(Fig. 7E-H).
 | Figure 7.Silencing of PIK3CA (Si-PIK3CA)
increases the anticancer effect of miR-363-3p on PIK3CA, PDK1 and
p-Akt protein expression in human RB WERI-Rb-1 cells. (A) PIK3CA,
PDK1 and p-Akt protein expression by western blot analysis. (B-D)
PIK3CA, PDK1 and p-Akt protein expression by statistical analysis.
(E and F) Caspase-3/9 activity. Bax protein expression by (G)
statistical analysis and (H) western blot analysis. Control,
negative control group; miR-363-3p, miR-363-3p-overexpressing
group; miR-363-3p+Si-PIK3CA, miR-363-3p overexpression and
Si-PIK3CA group. ##P<0.01 compared with the negative
control group. **P<0.01 compared with the
miR-363-3p-overexpressing group. RB, retinoblastoma; PIK3CA,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α;
PDK1, pyruvate dehydrogenase kinase 1; p-AKT, phosphorylated
protein kinase B. |
Knockdown of PIK3CA increases the
anticancer effects of miR-363-3p on RB cell proliferation and
invasion
Inhibition of PIK3CA expression using si-PIK3CA
significantly increased the inhibition of cell growth and invasion
induced by miR-363-3p overexpression in RB cells, compared with
cells with overexpression of miR-363-3p alone (Fig. 8A-C). si-PIK3CA significantly
increased the induction of apoptosis by miR-363-3p overexpression,
compared with the cells with overexpression of miR-363-3p alone
(Fig. 8D and E). These results
suggest that PIK3CA may play a significant role in modulating the
effects of miR-363-3p on RB cell growth.
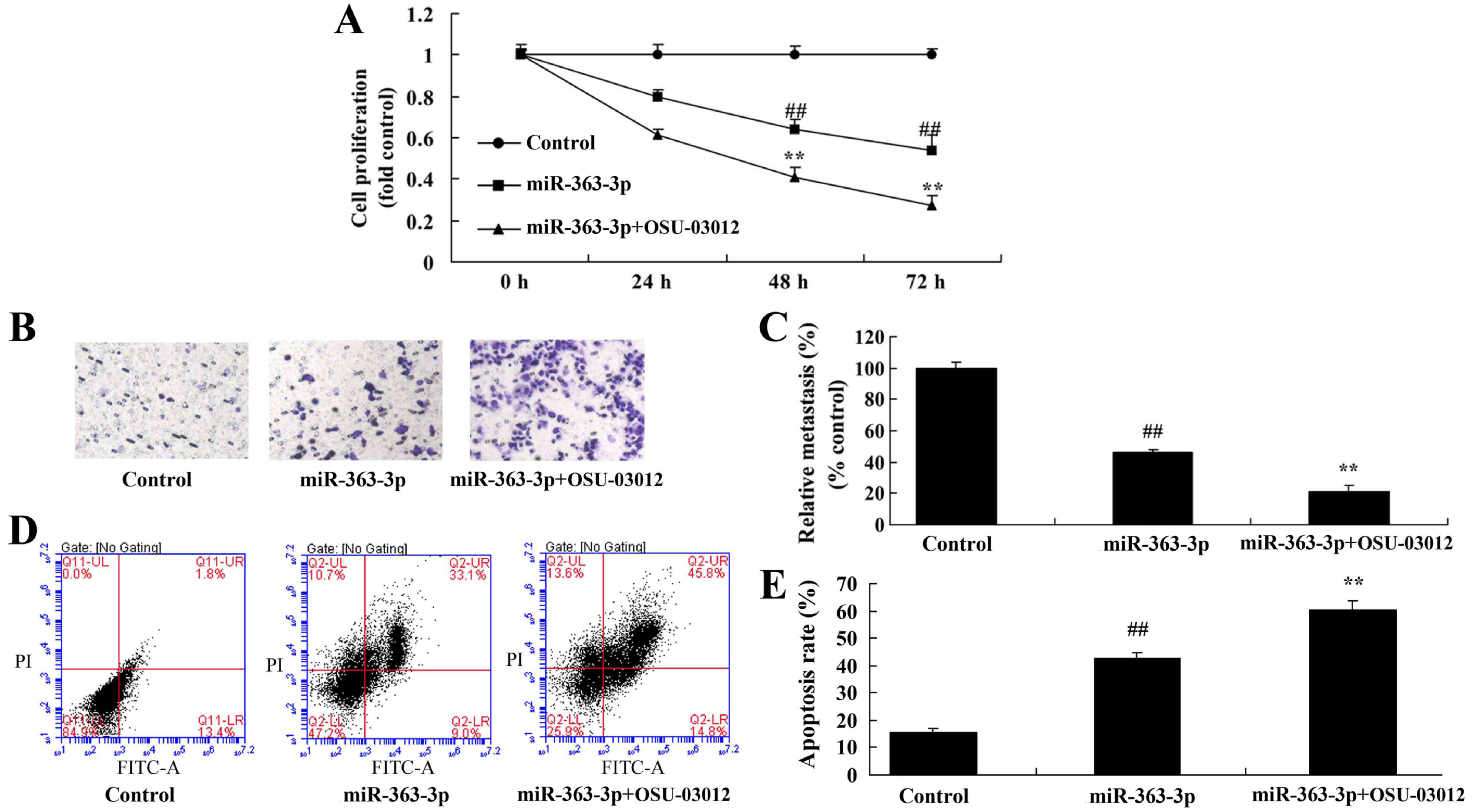
Treatment with a PDK1 inhibitor
accelerates the anticancer effects of miR-363-3p on p-AKT protein
expression
To further determine whether PDK1 participates in
the anticancer effects of miR-363-3p on RB cell proliferation and
invasion, a PDK1 inhibitor (OSU-03012; 1 µM) was used to reduced
PDK1 protein expression. As presented in Fig. 9A-C, treatment with the PDK1
inhibitor suppressed PDK1 expression and increased the suppression
of p-AKT in RB cells with overexpression of miR-363-3p, compared
with cells that were not treated with the inhibitor. The PDK1
inhibitor also further increased BAX protein expression and
caspase-3/9 activity in RB cells with overexpression of miR-363-3p,
compared with untreated cells with overexpression of miR-363-3p
alone (Fig. 9D-G).
Treatment with a PDK1 inhibitor
accelerates the anticancer effects of miR-363-3p on RB cell
proliferation and invasion
The PDK1 inhibitor further suppressed RB cell growth
and invasion in RB cells with overexpression of miR-363-3p compared
with untreated cells with overexpression of miR-363-3p alone
(Fig. 10A-C). Additionally, the
PDK1 inhibitor increased the promotion of apoptosis by miR-363-3p
compared with untreated cells with overexpression of miR-363-3p
alone (Fig. 10D and E).
Discussion
RB is the most common type of intraocular malignancy
among children, with poor prognosis, seriously affecting patient
vision and even threatening their lives. The pathogenesis is
generally considered to be the second mutation of the RB
gene (4). With the progress of
research, through a deeper exploration of the pathogenic molecular
biology of RB and other tumors, it has been suggested that RB is
associated with changes in multiple signaling pathways, but the
specific pathogenesis of this disease remains uncertain (12). The findings of the present study
revealed that miR-363-3p expression was upregulated in serum from
patients with RB. A study by Liu et al (11) showed that miR-363-3p inhibits cell
growth of papillary thyroid carcinoma. The findings of the current
study are limited by the small sample size of patients and normal
samples, which was 6. More clinical cases will be investigated in
future studies.
Bcl-2 is an inhibitor of apoptosis. It is an
endogenous inhibitor of mitochondrial membrane permeability
(13). Expressed in proliferating
cells, Bcl-2 plays an important role in the maintenance of cell
metabolism (14). Chromosome
translocation can lead to upregulation of Bcl-2, blocking the
activation of the protease chain reaction and prolonging cell
survival through stopping the release of cytochrome c from
the mitochondria, so that the balance of cell proliferation and
apoptosis is disturbed, leading to the development of tumors
(15). Studies have shown that
activation of pyruvate dehydrogenase kinase 1 (PDK1) by
phosphorylation induces the apoptosis of cells by affecting
mitochondrial damage and regulating Bcl-2, Bcl-xL and survivin
(15,16). In the present study, overexpression
of miR-363-3p reduced RB cell proliferation and invasion.
The P110a-PI3K catalytic subunit of the
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
(PIK3CA) gene has been studied in human cancer for more than
15 years (17). Several studies
have shown that PI3K kinase activity is closely related to the key
oncogenic protein P110a, which plays an important role in tumor
formation (8,17,18).
The phosphoinositide 3-kinase (PI3K) pathway is activated by PIK3CA
mutation or amplification (8,17).
Activated PI3K generates a second messenger to activate a series of
downstream protein kinases, including AKT, leading to further
signal transmission, and therefore, the PDK signaling pathway has a
significant effects on cell proliferation, apoptosis, migration,
vesicular transport and malignant transformation of cells and many
other pathophysiological processes (18). The PI3K/AKT/PKB signal transduction
pathway is particularly important for the modulation of apoptosis
(19). In the present study,
overexpression of miR-363-3p suppressed PIK3CA, PDK1 and p-AKT
protein expression. Liu et al (11) showed that miR-363-3p inhibits cell
growth of papillary thyroid carcinoma through the PIK3CA/AKT
signaling pathway.
PDK1 is a 63-kDa serine/threonine protein kinase
that includes a C-terminal platelet-leukocyte C-kinase substrate
homology domain and an N-terminal kinase domain (20). The PH domain combines with the PI3K
product inositol triphosphate, targeting PDK1 to the membrane and
activating AKT, thereby acting on a variety of downstream
substrates such as NF-кB, caspase-9 and endothelial nitric oxide
synthase, and subsequently affecting cell growth, migration,
apoptosis and angiogenesis, as well as other biological effects
(21). PDK1-mediated PI3K/AKT
signaling pathways have been associated with multiple types of
malignant tumor (18). In the
present study, inhibition of PIK3CA or PDK1 was found to accelerate
the anticancer effects of miR-363-3p on RB through PI3K/AKT
signaling. Based on the results of the present study, the mechanism
of miR-363-3p/PI3K/AKT signaling in RB is unclear. Therefore, more
signaling pathways, including GSK-3, FOXO1 and mTORC1, will be
investigated in further studies.
In summary, the results of this study revealed that
overexpression of miR-363-3p reduced the proliferation and invasion
of RB cells through suppression of the PIK3CA/PI3K/AKT pathway.
These findings suggest that enhancing the expression of a single
miRNA, miR-363-3p may significantly improve the efficacy of
treatment for RB and possibly other solid tumors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LJ designed the experiments; XM, XL, JT and RW
performed the experiment; XM and LJ analyzed the data; XM wrote the
manuscript. All authors read, reviewed and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Medical
Ethics Committee of Xi'an Traditional Chinese Medicine Hospital
(Xi'an, Shaanxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Kommoss S, du Bois A, Ridder R, Trunk MJ,
Schmidt D, Pfisterer J and Kommoss F; AGO-OVAR: Independent
prognostic significance of cell cycle regulator proteins p16(INK4a)
and pRb in advanced-stage ovarian carcinoma including optimally
debulked patients: A translational research subprotocol of a
randomised study of the arbeitsgemeinschaft gynaekologische
onkologie ovarian cancer study group. Br J Cancer. 96:306–313.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lumbroso-Le Rouic L, Aerts I, Hajage D,
Lévy-Gabriel C, Savignoni A, Algret N, Cassoux N, Bertozzi AI,
Esteve M, Doz F and Desjardins L: Conservative treatment of
retinoblastoma: A prospective phase II randomized trial of
neoadjuvant chemotherapy followed by local treatments and
chemothermotherapy. Eye (Lond). 30:46–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeMichele A, Clark AS, Tan KS, Heitjan DF,
Gramlich K, Gallagher M, Lal P, Feldman M, Zhang P, Colameco C, et
al: CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced
breast cancer: Phase II activity, safety, and predictive biomarker
assessment. Clin Cancer Res. 21:995–1001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin J, Bryar P, Mets M, Weinstein J,
Jones A, Martin A, Vanin EF, Scholtens D, Costa FF, Soares MB and
Laurie NA: Differentially expressed miRNAs in retinoblastoma. Gene.
512:294–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei Y, Sun J and Li X: MicroRNA-215
enhances invasion and migration by targeting retinoblastoma tumor
suppressor gene 1 in high-grade glioma. Biotechnol Lett.
39:197–205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu SS, Wang YS, Sun YF, Miao LX, Wang J,
Li YS, Liu HY and Liu QL: Plasma microRNA-320, microRNA-let-7e and
microRNA-21 as novel potential biomarkers for the detection of
retinoblastoma. Biomed Rep. 2:424–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi L, Zhu F, Li SH, Si LB, Hu LK and Tian
H: Retinoblastoma binding protein 2 (RBP2) promotes
HIF-1α-VEGF-induced angiogenesis of non-small cell lung cancer via
the Akt pathway. PLoS One. 9:e1060322014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao W, Chen X and He M: Inhibition of the
Jagged/Notch pathway inhibits retinoblastoma cell proliferation via
suppressing the PI3K/Akt, Src, p38MAPK and Wnt/β-catenin signaling
pathways. Mol Med Rep. 10:453–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eo SH, Kim JH and Kim SJ: Induction of
G2/M arrest by berberine via activation of PI3K/Akt and p38 in
human chondrosarcoma cell line. Oncol Res. 22:147–157. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gui F, Hong Z, You Z, Wu H and Zhang Y:
MiR-21 inhibitor suppressed the progression of retinoblastoma via
the modulation of PTEN/PI3K/AKT pathway. Cell Biol Int.
40:1294–1302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Li Q, Li R, Ren P and Dong S:
MicroRNA-363-3p inhibits papillary thyroid carcinoma progression by
targeting PIK3CA. Am J Cancer Res. 7:148–158. 2017.PubMed/NCBI
|
|
12
|
Friedman DN, Lis E, Sklar CA, Oeffinger
KC, Reppucci M, Fleischut MH, Francis JH, Marr B, Abramson DH and
Dunkel IJ: Whole-body magnetic resonance imaging (WB-MRI) as
surveillance for subsequent malignancies in survivors of hereditary
retinoblastoma: A pilot study. Pediatr Blood Cancer. 61:1440–1444.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song W, Liu MG, Zhang JB, Zhang JJ, Sun MM
and Yu QK: Mechanism of action of EBV, Bcl-2, p53, c-Myc and Rb in
non-Hodgkin's lymphoma. Eur Rev Med Pharmacol Sci. 20:1093–1097.
2016.PubMed/NCBI
|
|
14
|
Singh L, Pushker N, Saini N, Sen S, Sharma
A, Bakhshi S, Chawla B and Kashyap S: Expression of pro-apoptotic
Bax and anti-apoptotic Bcl-2 proteins in human retinoblastoma. Clin
Exp Ophthalmol. 43:259–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
La Thangue NB: A mismatched role for
Bcl-2. Nat Cell Biol. 7:101–102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai JH, Fleming KE, Ly TY, Pasternak S,
Godlewski M, Doucette S and Walsh NM: Pure versus combined Merkel
cell carcinomas: Immunohistochemical evaluation of cellular
proteins (p53, Bcl-2, and c-kit) reveals significant overexpression
of p53 in combined tumors. Hum Pathol. 46:1290–1296. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang G, Cao X, Lai S, Luo X, Feng Y, Xia
X, Yen PM, Gong J and Hu J: PI3K stimulates DNA synthesis and
cell-cycle progression via its p55PIK regulatory subunit
interaction with PCNA. Mol Cancer Ther. 12:2100–2109. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomosugi M, Sowa Y, Yasuda S, Tanaka R, te
Riele H, Ikawa H, Koyama M and Sakai T: Retinoblastoma
gene-independent G1 phase arrest by flavone, phosphatidylinositol
3-kinase inhibitor, and histone deacetylase inhibitor. Cancer Sci.
103:2139–2143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei TY, Juan CC, Hisa JY, Su LJ, Lee YC,
Chou HY, Chen JM, Wu YC, Chiu SC, Hsu CP, et al: Protein arginine
methyltransferase 5 is a potential oncoprotein that upregulates G1
cyclins/cyclin-dependent kinases and the phosphoinositide
3-kinase/AKT signaling cascade. Cancer Sci. 103:1640–1650. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vora SR, Juric D, Kim N, Mino-Kenudson M,
Huynh T, Costa C, Lockerman EL, Pollack SF, Liu M, Li X, et al: CDK
4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K
inhibitors. Cancer Cell. 26:136–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choy E, Hornicek F, MacConaill L, Harmon
D, Tariq Z, Garraway L and Duan Z: High-throughput genotyping in
osteosarcoma identifies multiple mutations in
phosphoinositide-3-kinase and other oncogenes. Cancer.
118:2905–2914. 2012. View Article : Google Scholar : PubMed/NCBI
|