Introduction
Biologically active steroids such as estrogens and
androgens are locally produced in breast cancer tissues by
sex-steroid producing enzymes. Among these enzymes, aromatase is an
important therapeutic target in estrogen receptor (ER)-positive
breast cancers. However, de novo or acquired resistance to
aromatase inhibitors (AIs) are often experienced and this is a key
problem to be solved (1). On the
other hand, androgen-producing enzymes such as 17β-hydroxysteroid
dehydrogenase type 5 [17βHSD5, also known as aldo-keto reductase
family 1 member C3 (AKR1C3); androstenedione to testosterone] and
5α-reductase type 1 [5αRed1; testosterone to dihydrotestosterone
(DHT), biologically active androgens] are also frequently expressed
in breast carcinoma tissues. These enzymes contribute to
intratumoral androgen synthesis (2)
and 5αRed1 is known as a potent regulator of in situ DHT
production (3). Because the androgen
receptor (AR) is also frequently expressed in breast cancer cells
(4,5),
it has been considered that intratumoral androgens have pivotal
roles in the progression of breast cancers. However, the
significance of androgen in breast cancer has not been fully
understood and opposite findings have often been reported. For
instance, numerous studies have pointed out that AR expression is
associated with better clinical outcomes (6), while androgen action was recently
highlighted in a distinct subset of ER-negative breast cancers
[namely molecular apocrine subtype or luminal AR subtype (7,8)] or
ER-positive breast cancers which have become resistant to AIs
(9). It is therefore important to
study the action of androgens in breast cancer.
The tumor microenvironment is composed not only of
tumor cells but also contains various stromal cells including
macrophages, leukocytes and fibroblasts, which, together with
cross-talks between tumor cells and other cellular components, is
strongly associated with tumor progression (10). Tumor-associated macrophages (TAMs) are
well known as the primary components of the tumor microenvironment
and promote cancer progression (11–16).
Monocytes in the blood are attracted to tumor tissues via soluble
factors released from tumor tissues, where they differentiate into
TAMs (17). Based on their function,
macrophages are classified into M1 or M2 phenotypes. M1 macrophages
are driven by cytokines such as tumor necrosis factor α (TNFα) and
interferon γ (IFNγ), while M2 macrophages are driven by interleukin
(IL)-4 and IL-13 (18). Each
macrophage type displays different functions and expression
profiles of cytokines and cell-surface markers. While M1
macrophages express CD80 and CD86 and have tumor-suppressive and
pro-inflammatory effects, M2 macrophages express CD163, are the
predominant phenotype of TAMs in human solid tumors (18,19) and
contribute to tumor malignancy by promoting tumor growth,
metastasis, angiogenesis, epithelial-mesenchymal transition (EMT)
and drug resistance in breast cancers (11–16).
Previous studies have reported that a high infiltration of M2
macrophages is correlated with lymph node metastasis, higher
histological grade and cell proliferation, and contributes to worse
prognosis in breast cancers (13,19).
It has been reported that AR in
monocytes/macrophages plays key roles in the greater prevalence and
severity of atherosclerosis in men (20,21).
Moreover, it has also been reported that some macrophages express
AR and that androgen/AR signaling in macrophages inhibits cutaneous
wound healing (22). These findings
suggest that androgen acts upon macrophages affecting various human
diseases, although its role in human tumors, including breast
cancers, remains unclear. We therefore focused on the relationship
between androgen action in intratumoral macrophages and progression
of breast cancers, with the purpose of a better understanding of
the biological and/or clinical significance of androgens in breast
cancers.
Materials and methods
Patients and tissues
A total of 116 specimens of invasive breast
carcinomas were obtained from Japanese female patients who had
undergone surgical treatment from 2007 to 2008 at Tohoku University
Hospital, Sendai, Japan. The clinical outcome was evaluated by
disease-free and breast cancer-specific survival. Disease-free
survival was defined as the time from the date of surgery to that
of the first locoregional recurrence or distant metastasis within
the follow-up time and the median was 59 (range 3–84) months.
Breast cancer-specific survival was defined as the time from
surgery to death from breast cancer and follow-up time was 61 (from
3–84) months. All specimens had been fixed with 10% formalin
neutral buffer solution and embedded in paraffin wax. Experiments
and analyses were performed in accordance with the Helsinki
declaration and the research protocol of this study was approved by
the Ethics Committee at the Tohoku University Graduate School of
Medicine (approval no. 2016-1-697, 2019-1-219). This is a
retrospective study and, therefore, informed consents had not been
obtained.
Immunohistochemistry
The information regarding the antibodies is listed
in Table SI. Immunohistochemistry
for CD163, 5αRed1 and Ki67 was carried out using a Histofine kit
(Nichirei Bio, Inc., Tokyo, Japan) as described previously
(23,24). The antigen-antibody complex was
visualized with 3,3′-diaminobenzidine (DAB) and hematoxylin was
used for counterstaining. Immunohistochemistry for ER and
progesterone receptor (PR) was carried out with Ventana Benchmark
XT system (Roche Diagnostics Japan). For human epidermal growth
factor receptor 2 (HER2), the HercepTest (Dako) was used.
Double immunohistochemistry
Immunoreactivity of AR was firstly visualized with
DAB as described above, and then antigen retrieval was carried out
again and immunoreactivity of CD163 was visualized with nitro blue
tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP).
Scoring of immunoreactivity
5αRed1 immunoreactivity was detected in the
cytoplasm of breast carcinoma cells and considered positive when
the cases had more than 10% of positive carcinoma cells (3). Infiltration of CD163-positive
macrophages was scored into 4 categories (0, no focal areas; 1,
small scattered focal areas; 2, numerous larger focal areas; 3,
very numerous large focal areas) according to a previous study
(25), and finally dichotomized for
statistical analyses (0, 1: Low infiltration and 2, 3: High
infiltration). ER, PR and Ki67 immunoreactivity were detected in
the nucleus of carcinoma cells and the percentage of positive cells
(labeling index; LI) was calculated by evaluating in more than
1,000 cells. According to a previous report (26), cases with LI of more than 1% were
considered positive for ER and PR. HER2 immunoreactivity was
determined according to the grading system proposed in HercepTest
(Dako).
Cell lines and chemicals
Mouse breast cancer cell line 4T1 and mouse
macrophage cell line RAW264.7 were obtained from American Type
Culture Collection (ATCC) and RIKEN Bioresource Center (Japan),
respectively. These cells were cultured in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA) with 10% fetal bovine serum (FBS)
(Biosera) and incubated at 37°C under 5% CO2. In
experiments using androgen, these cells were cultured in phenol
red-free RPMI-1640 with 10% dextran-coated charcoal-stripped
FBS.
Coculture experiment
The 4T1 cells were cocultured with RAW264.7 cells
using ThinCerts™ (pore size 0.4 µm, Greiner bio-one). RAW264.7
cells were seeded in the bottom plate (3×105 cells/well)
and allowed to attach for 24 h. Then, the 4T1 cells were placed
onto an upper Transwell (2×105 cells/well) and
cocultured with RAW264.7 cells for 3 days.
Quantitative real-time PCR
Total RNA (500 ng) was extracted using TRI Reagent
(Molecular Research Center, Inc.) and cDNA was synthesized using a
ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo Co.
Ltd.). Real-time PCR was performed using the THUNDERBIRD™
SYBR® qPCR Mix (Toyobo Co. Ltd.) and LightCycler nano
system (Roche Diagnostics Japan). Thermocycling conditions were as
follows: 95°C for 60 sec (initial denaturation), followed by 45
cycles at 95°C for 15 sec and 60°C for 30 sec. The sequences for
the PCR primer sets are listed in Table
SII. Relative mRNA levels of Arg-1, Ar, Srd5a1 and
Akr1c6 were summarized as the ratio to Rpl13a.
Plasmid construction
Open reading frame of Ar and FLAG-tagged
aldo-keto reductase family 1 member C6 (Akr1c6, mouse
testosterone-producing enzyme) were amplified via PCR using KOD FX
Neo (Toyobo Co. Ltd.) and cloned into pIRES2-AcGFP1 vector
(Clontech Laboratories) and pcDNA3.1 (−) vector (Invitrogen; Thermo
Fisher Scientific, Inc.), respectively, using DNA Ligation Kit
<Mighty Mix> (Takara Bio) (pIRES-mAR and pAKR1C6-FLAG).
Thermocycling conditions were as follows: 94°C for 2 min, followed
by 35 cycles at 98°C for 10 sec, 62°C for 30 sec and 68°C for 90
sec. The sequences for the PCR primer sets are listed in Table SII.
Plasmid transfection and establishment
of stable clones
Since 4T1 and RAW264,7 cells do not express
Akr1c6 and Ar, respectively, we tried to establish
their sublines which stably express these genes in order to
investigate the roles of androgens in intratumoral macrophages.
According to the manufacturer's instructions for transfection,
RAW264.7 cells were transfected with pIRES-mAR or pIRES2-AcGFP1
using GenomONE™-Neo (Ishihara Sangyo Kaisya, Ltd.) and 4T1 cells
were transfected with pAKR1C6-FLAG (4T1-AKR1C6) or pcDNA3.1(−)
(4T1-CT) using Avalanche®-Everyday Transfection Reagent
(APRO Science). These cells were cultured in media containing 400
µg/ml G418 (Wako), and stable clones expressing AR (RAW-AR), AKR1C6
(4T1-AKR1C6) and corresponding negative control clones (RAW-CT and
4T1-CT) were established.
Western blotting
Western blotting was performed according to previous
reports (27,28) and the information regarding the
primary antibodies is listed in Table
SI. The cells were lysed using M-PER Mammalian Protein
Extraction Reagent (Pierce Biotechnology) containing Halt Protease
Inhibitor Cocktail (Sigma-Aldrich; Merck KGaA). The protein
extracts (10 µg) were separated by SDS-PAGE (10% acrylamide gel)
and then transferred to Hybond P polyvinylidene difluoride membrane
(GE Healthcare). The membrane was blocked in 5% non-fat milk in
TBS-T for 1 h at room temperature and then incubated with primary
antibodies at 4°C overnight. The blots were incubated with the
appropriate HRP-conjugated secondary antibody (anti-mouse;
1:10,000, cat. no. NA931 or anti-rabbit; 1:10,000, cat. no. NA934,
GE Healthcare) for 1 h at room temperature. Detection of
antibody-protein complexes on the membrane was visualized using
ECL-Prime Western blotting detection reagents (GE Healthcare) and
LAS-4000 image analyzer (Fuji Photo Film Co.).
Synthetic androgen R1881
treatment
RAW-CT and RAW-AR cells (1×105
cells/well) were cultured in a 12-well plate with or without
synthetic androgen R1881 (10 nM) for 48 h.
Sphere-forming assay
A sphere-forming assay was performed using
EZ-BindShut®II micro plate (IWAKI). The 4T1 cells
(1×105 cells/well) were cocultured in a well with RAW-CT
or RAW-AR cells (3.33×104 cells/well) with or without
R1881 (10 nM). After 3 days, spheres were photographed under a
microscope and the size of 100 spheres was measured using ImageJ
1.53e (https://imagej.nih.gov/ij/).
Cell proliferation assay
The 4T1-AKR1C6 or 4T1-CT cells were seeded in a
96-well plate (3,000 cells/well) and the cell proliferation was
measured by the WST-8 method using a Cell Counting Kit-8 (Dojindo
Molecular Technologies) for 4 days. Absorbance was determined using
a Bio-Rad iMark plate reader (Bio-Rad Laboratories Inc.).
Tumor-bearing mouse model
The protocols of all animal experiments were
submitted, reviewed and approved in advance by the Institutional
Animal Care and Use Committee at Tohoku University (2019MdA-172).
The experiments were carried out according to ARRIVE guidelines and
Guidelines for Animal Experiments at Tohoku University. A total of
11 4-week-old wild-type female BALB/c mice were obtained from CLEA
Japan (Tokyo, Japan) and randomly assigned to two types of
tumor-bearing models as described below with no inclusion criteria.
They were housed in a specific pathogen-free facility with free
access to water and MR stock food and environmental enrichment. In
the present study, 4T1 and RAW264.7 sublines were subcutaneously
injected in both the left and right side of mice in different
combinations in order to avoid individual differences. In the first
experiment (n=6), 4T1-CT or 4T1-AKR1C6 cells (5×105
cells) were subcutaneously injected together with RAW-AR cells
(1.25×105 cells) into the left or right side of the back
of mice, respectively, using 150 µl Matrigel (Corning, Inc.). In
the mirror experiment (n=5), 4T1-AKR1C6 cells (5×105
cells) were injected together with either RAW-CT or RAW-AR cells
(1.25×105 cells) into the left or right side of the back
of mice, respectively. Mice were monitored for body weight and
tumor volume changes twice a week. Tumor volume was measured using
a digital caliper at least twice a week and calculated by modified
ellipsoid formula 1/2 (length × width2). Tumors were
allowed to grow in mice until they reached the volume of 2,000
mm3 or 10 weeks from injection had elapsed, whichever
came first. No adverse events were observed and mice were
euthanized 4 weeks after injection by cervical dislocation under
anesthesia using isoflurane (4%) and thereafter tumors were
resected, weighted, and then fixed in 10% formalin for
immunohistochemistry.
Statistical analyses
Statistical analyses were performed using JMP pro
14.0.0 (SAS Institute). χ2 test or Mann-Whitney U test
were applied for correlation between the infiltration of
CD163-positive macrophages and clinicopathological factors. Breast
cancer-specific and disease-free survival curves were generated by
Kaplan-Meier method and statistical significance was evaluated by a
log-rank test. In the in vitro and in vivo
experiments, the statistical analyses were performed using paired
or unpaired t-test. The data are presented as the mean ± SD. In
this study, P<0.05 was considered as indicative of statistical
significance.
Results
Expression of AR in macrophages within
breast carcinoma tissues
We first examined whether macrophages in breast
carcinoma tissues expressed AR or not. By double
immunohistochemistry for AR and CD163, a marker of M2 macrophages,
the macrophages with immunoreactivity for both CD163 (blue) in the
cell membrane and AR (brown) in the nuclei were sporadically
observed in the stroma (Fig. 1A).
 | Figure 1.Immunohistochemistry for CD163,
androgen receptor (AR) and 5α-reductase type 1 (5αRed) in human
breast carcinoma tissues. (A) Representative image of double
immunohistochemistry for CD163 (deep blue, NBT/BCIP) and AR (brown,
DAB). Bar, 100 µm (low magnification) and 10 µm (high
magnification). (B and C) Representative image of CD163-positive
macrophages infiltration (B, high infiltration; C, low
infiltration). (D and E) Representative image of 5αRed1
immunostaining, showing immunoreactivity in the cytoplasm of breast
cancer cells (D, positive case; E, negative case). Scale bar, 100
µm. |
We next immunolocalized CD163 as well as 5αRed1 in
116 breast carcinoma tissues in order to address the clinical
significance of androgen action on macrophages in breast cancer.
CD163 immunoreactivity was observed in the macrophages (Fig. 1B and C) and 34% (39 out of 116 cases)
were categorized as high infiltration. 5αRed1 immunoreactivity was
observed in the cytoplasm of breast carcinoma cells (Fig. 1D and E) and 56% (65 out of 116 cases)
were considered positive for 5αRed1. Correlation between macrophage
infiltration and clinicopathological parameters is presented in
Table I. Macrophage infiltration was
positively correlated with lymph node metastasis (P=0.0057),
histological grade (P<0.0001) and Ki67 labeling index (LI)
(P<0.0001), while it was negatively correlated with ER
(P=0.0020) and PR (P=0.0014). No significant correlation was
detected between macrophage infiltration and 5αRed1
immunoreactivity (P=0.74). When we compared the correlation between
macrophage infiltration and clinicopathological parameters in the
5αRed1-negative group (n=51) and the 5αRed1-positive group (n=65)
(Table II), macrophage infiltration
was correlated with lymph node metastasis (P=0.011), PR (P=0.0061)
and HER2 (P=0.015) only in the 5αRed1-positive group. On the other
hand, it was negatively correlated with ER only in the
5αRed1-negative group (P=0.001), while it was correlated with
histological grade and Ki67 LI in both the 5αRed1-positive and
-negative group (histological grade; P=0.0013 in the
5αRed1-negative group and P=0.0069 in the 5αRed1-positive group,
Ki67 LI; P=0.0017 in the 5αRed1-negative group and P=0.0020 in the
5αRed1-positive group). Furthermore, it is of interest that a
significant correlation between macrophage infiltration and these
parameters was detected only in the 5αRed1-positive group when the
cases were limited to the ER-positive group.
 | Table I.Association between CD163-positive
macrophages and clinicopathological parameters in 116 breast
carcinomas. |
Table I.
Association between CD163-positive
macrophages and clinicopathological parameters in 116 breast
carcinomas.
|
| CD163 positive
macrophages |
|
|---|
|
|
|
|
|---|
|
| Low (n=77) | High (n=39) | P-value |
|---|
| Agea (years) | 55 (27–87) | 58 (37–76) | 0.3700 |
| Menopause |
|
|
|
|
Premenopause | 30 | 11 | 0.2500 |
|
Postmenopause | 47 | 28 |
|
| Stage |
|
|
|
| I | 49 | 18 | 0.1000 |
| II | 19 | 11 |
|
|
III | 9 | 10 |
|
| Pathological T
factor |
|
|
|
|
pT1 | 56 | 22 | 0.0770 |
|
pT2-4 | 21 | 17 |
|
| Lymph node
metastasis |
|
|
|
|
Negative | 59 | 20 | 0.0057 |
|
Positive | 18 | 19 |
|
| Histological
grade |
|
|
|
| 1
(well) | 39 | 6 |
<0.0001 |
| 2
(intermediate) | 30 | 16 |
|
| 3
(poor) | 8 | 17 |
|
| ER |
|
|
|
|
Negative | 9 | 14 | 0.0020 |
|
Positive | 68 | 25 |
|
| PR |
|
|
|
|
Negative | 17 | 20 | 0.0014 |
|
Positive | 60 | 19 |
|
| HER2 |
|
|
|
|
Negative | 69 | 30 | 0.0680 |
|
Positive | 8 | 9 |
|
| 5αRed1 |
|
|
|
|
Negative | 33 | 18 | 0.7400 |
|
Positive | 44 | 21 |
|
| Ki67 LI
a (%) | 8 (1–44) | 21 (1–60) |
<0.0001 |
 | Table II.Association between CD163-positive
macrophages and clinicopathological parameters according to 5αRed1
and ER status in 116 breast carcinomas. |
Table II.
Association between CD163-positive
macrophages and clinicopathological parameters according to 5αRed1
and ER status in 116 breast carcinomas.
|
| All cases
(n=116) | ER-positive cases
(n=93) |
|---|
|
|
|
|
|---|
| Parameters | 5αRed1-negative
(n=51) | 5αRed1-positive
(n=65) | 5αRed1-negative
(n=41) | 5αRed1-positive
(n=52) |
|---|
| Age | 0.42 | 0.73 | 0.53 | 0.61 |
| Menopause | 0.14 | 0.81 | 0.40 | 0.69 |
| Stage | 0.20 | 0.15 | 0.67 | 0.26 |
| Pathological T
factor | 0.14 | 0.29 | 0.28 | 0.36 |
| Lymph node
metastasis | 0.18 | 0.011 | 0.63 | 0.043 |
|
|
| (Positive) |
| (Positive) |
| Histological
grade | 0.0013 | 0.0069 | 0.14 | 0.0011 |
|
| (Positive) | (Positive) |
| (Positive) |
| ER | 0.0010 | 0.23 | – | – |
|
| (Negative) | (Negative) |
|
|
| PR | 0.082 | 0.0061 | 0.18 | 0.0059 |
|
|
| (Negative) |
| (Negative) |
| HER2 | 0.92 | 0.015 | 0.23 | 0.0079 |
|
|
| (Positive) |
| (Positive) |
| Ki67 LI (%) | 0.0017 | 0.0020 | 0.091 | 0.0009 |
|
| (Positive) | (Positive) |
| (Positive) |
We next examined the correlation between macrophage
infiltration and clinical outcomes of breast cancer patients. As
shown in Fig. 2A and B, macrophage
infiltration was significantly correlated with an increased risk of
recurrence (P=0.0049) and worse prognosis (P=0.016). In contrast,
when we analyzed according to 5αRed1 status, macrophage
infiltration was significantly correlated with increased risk of
recurrence (P=0.01, Fig. 2C) and
worse prognosis (P=0.045, Fig. 2E)
only in the 5αRed1-positive group and no significant correlation
was detected in the 5αRed1-negative group (Fig. 2D and F). This tendency was still
observed when the cases were limited to the ER-positive group
(Fig. 2G-I), although breast
cancer-specific survival curve could not be generated because no
patients had died in the ER-positive/5αRed1-negative group.
Androgen is necessary for the
pro-proliferative effects of TAMs
We suggested that androgens may have important roles
in macrophage-induced breast cancer progression by
immunohistochemical analyses for CD163 and 5αRed1 as described
above. We then tested this hypothesis in in vitro
experiments using mouse breast cancer and macrophage cell lines,
4T1 and RAW264.7.
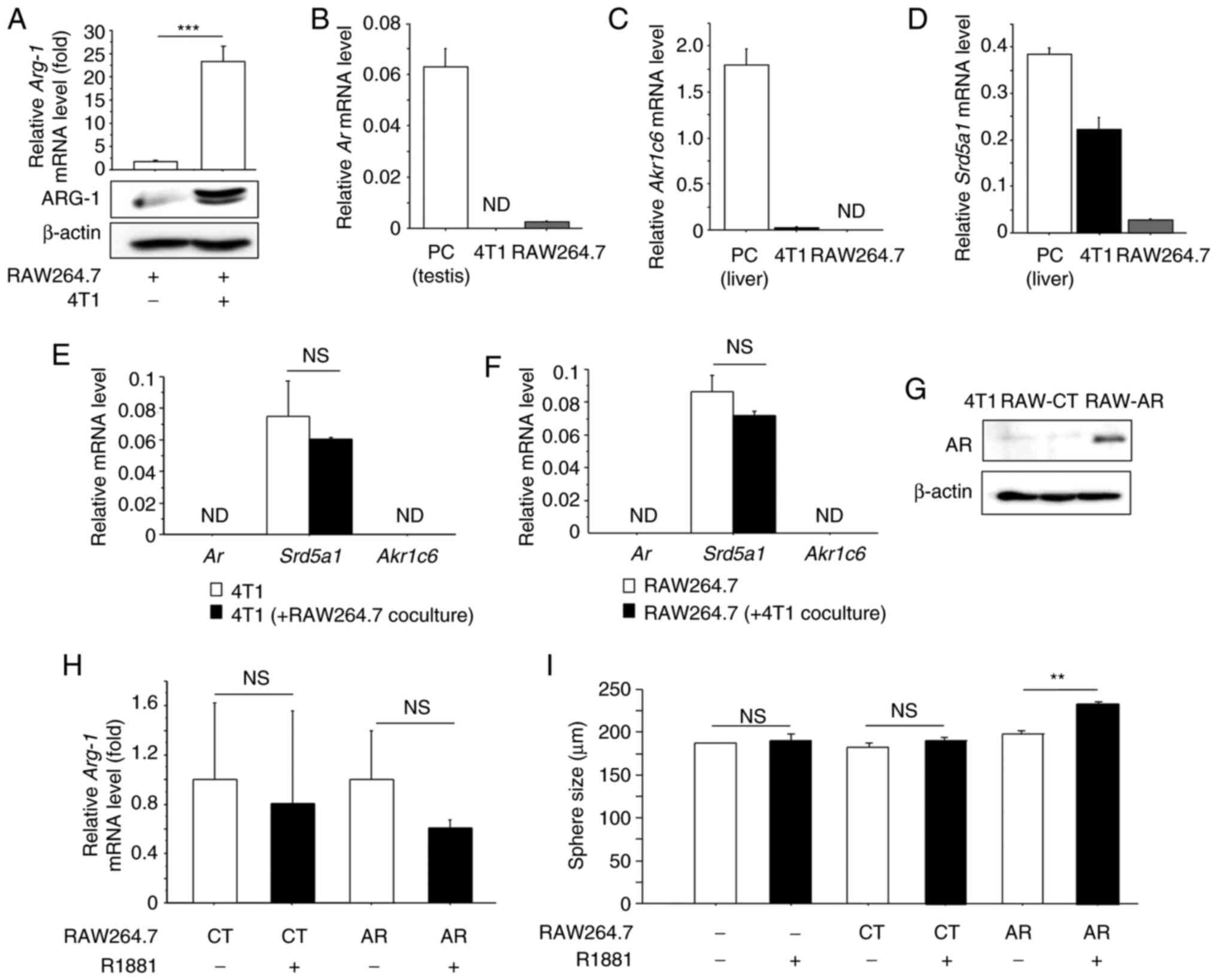
When RAW264.7 cells interacted with 4T1 cells in a
coculture chamber, mRNA and protein expression of Arg-1, an
M2 macrophage marker was significantly increased when compared to
the control (monoculture group), indicating that RAW264.7 cells
were successfully polarized into an M2 phenotype (Fig. 3A). We next investigated mRNA
expression, in 4T1 and RAW264.7 cells, of mouse AR (Ar) as
well as 5αRed1 (Srd5a1) and Akr1c6, the ortholog of
human AKR1C3 and involved in androgen synthesis in the mouse
(29). Real-time PCR analysis showed
that Ar (Fig. 3B) and
Akr1c6 (Fig. 3C) mRNA was
almost negligible in both cell lines, while Srd5a1 mRNA was
detected in both (Fig. 3D). In
addition, the mRNA level of these genes was almost comparable in
coculture condition (Fig. 3E and F).
To investigate the effects of androgens on macrophages, we
generated RAW264.7 sublines expressing AR (RAW-AR) and the
corresponding negative control (RAW-CT) (Fig. 3G). When we treated RAW-CT and RAW-AR
cells with synthetic androgen R1881, mRNA expression of M2
macrophage marker Arg-1 was not affected by R1881 (Fig. 3H). We confirmed that AR protein was
not detected in 4T1 cells (Fig. 3G),
and 4T1 cells were cocultured with RAW-CT or RAW-AR cells and cell
proliferation was investigated by sphere-forming assay. As shown in
Fig. 3I, sphere size was
significantly increased by R1881 when the cells were cocultured
with RAW-AR (P<0.01), while R1881 had no effect on monocultured
4T1 cells or those cocultured with RAW-CT.
Androgen acts on macrophages to
enhance breast cancer progression in a tumor-bearing mouse
model
In order to further address whether androgen acts on
macrophages to promote breast cancer progression, we performed
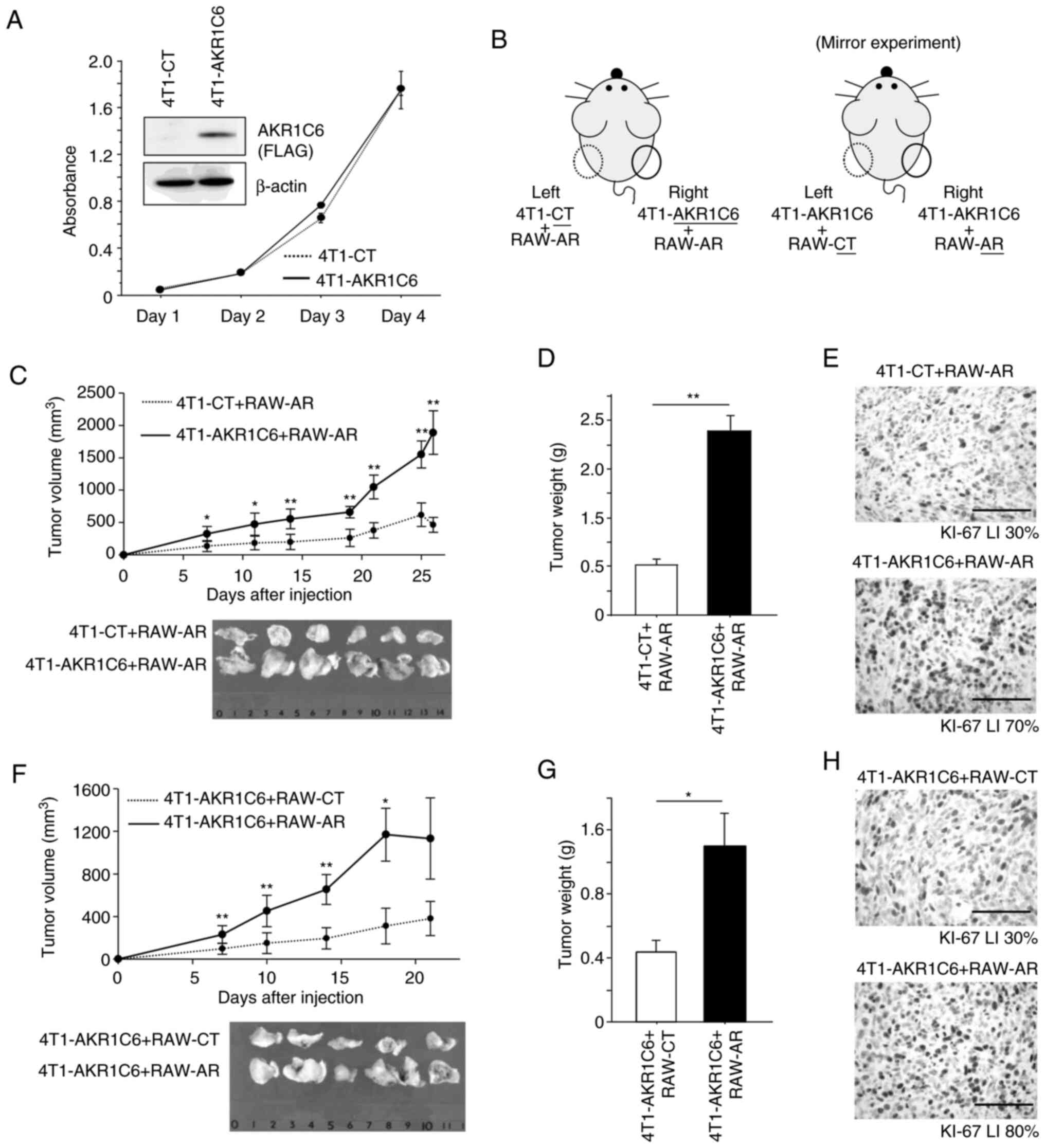
in vivo experiments using tumor-bearing mice. We generated
4T1 cells stably expressing AKR1C6 (4T1-AKR1C6) using pAKR1C6-FLAG
vector or control vector (Fig. 4A).
When we compared in vitro cell proliferation of these cells,
it was comparable in 4T1-AKR1C6 and 4T1-CT cells (Fig. 4A). 4T1-CT or 4T1-AKR1C6 cells were
subcutaneously injected together with RAW-AR cells on the left or
right side of the back of mice, respectively (Fig. 4B; left image). The tumors on the right
side (4T1-AKR1C6+RAW-AR) grew more rapidly compared with those on
the left side (4T1-CT+RAW-AR) (Fig. 4C
and D). In addition, immunohistochemistry demonstrated that
Ki67 LI was higher in the tumor on the right side (70%) compared to
those on the left side (30%) (Fig.
4E). We then performed the mirror experiment, where 4T1-AKR1C6
cells were subcutaneously injected together with RAW-CT and RAW-AR
cells on the left or right side of the back of mice, respectively
(Fig. 4B; right image). The tumors on
the right side (4T1-AKR1C6+RAW-AR) grew more rapidly compared with
those on the left side (4T1-AKR1C6+RAW-CT) (Fig. 4F and G). Ki67 LI was higher in the
tumors on the right side (80%) compared to those on the left side
(30%) (Fig. 4H).
Discussion
This is the first report demonstrating expression of
the androgen receptor (AR) in macrophages in human solid tumors,
including breast carcinomas. To date, the focus of study for the
action of androgen in breast cancers has been mainly its mediation
by AR in breast carcinoma cells, while AR in stromal cells has not
been explored. On the other hand, AR signaling in cancer stromal
cells has been implicated in prostate carcinomas and associated
with higher proliferation and invasion of prostate carcinoma cells
(30,31). Moreover, expression of AR in
macrophages has been so far reported in several human diseases. For
instance, macrophages with AR expression contribute to the greater
prevalence and severity of atherosclerosis in men, by upregulating
cholesteryl ester content (20). In
addition, testosterone has been reported to induce TNFα, NO and
IL-6 in a polycystic ovary syndrome rat model and contribute to the
pathogenesis of polycystic ovary syndrome (32). Therefore, it is reasonably speculated
that androgens affect breast cancer progression through AR, not
only in breast cancer cells but also intratumoral macrophages.
Breast carcinoma cells express androgen-producing
enzymes such as 5α-reductase type 1 (5αRed1) and locally produce
dihydrotestosterone (DHT), a bioactive androgen (2). We therefore hypothesized that
intratumoral androgens possibly affect macrophages which express AR
in a paracrine-manner. Then, we correlated macrophage infiltration
with characteristics of breast carcinomas in all pathological
samples. Macrophage infiltration was positively correlated with
lymph node metastasis, higher histological grade and Ki67 LI while
negatively correlated with the estrogen receptor (ER) and
progesterone receptor (PR) in all 116 cases, showing agreement with
previous reports (13,19). When we further analyzed them according
to 5αRed1 status, correlation between macrophage infiltration and
clinicopathological parameters showed differences between
5αRed1-positive and 5αRed1-negative groups, and this tendency was
more prominent when the cases were limited to ER-positive breast
cancers. Macrophage infiltration was in particular correlated with
a more aggressive phenotype of breast carcinomas with lymph node
metastasis, higher histological grade, PR negativity, HER2
positivity and higher Ki67 LI in the 5αRed1-positive group but not
in the 5αRed1-negative group. These findings indicate that the
action of androgen in macrophages is important for the
pro-tumorigenic roles of macrophages in breast cancers, especially
in ER-positive breast cancers. This may be partly explained by the
high diversity of cytokine receptor expression in breast
carcinomas. For instance, it has been reported that the expression
profile of cytokine receptor genes is quite different between the
luminal type and basal-type breast cancer cell lines. Rearranged
during transfection (RET), a receptor for glial cell line-derived
neurotrophic factor (GDNF), is highly expressed in luminal-type
breast cancer cell lines (33) and
while production of GDNF is accelerated by androgens in testicular
peritubular cells (34). Therefore, a
future challenge will be to identify the soluble factors regulated
by androgens in macrophages and their receptors in breast carcinoma
tissues.
In the present study, macrophage infiltration was
significantly correlated with increased risk of recurrence and
worse prognosis, similarly as previous reports (35,36).
Interestingly, this correlation was observed in the 5αRed1-positive
group but not in the 5αRed1-negative group, suggesting that
androgen action in intratumoral macrophages contributes to breast
cancer progression and worse prognosis. Similar results were
obtained even when the cases were limited to the ER-positive group,
who had received adjuvant endocrine therapy. Although endocrine
therapy targeting estrogen synthesis or ER has successfully
improved clinical outcome of ER-positive breast cancer patients,
de novo or acquired resistance is still frequently
experienced in the clinical setting. We previously reported that
intratumoral androgen production is increased following aromatase
inhibitor (AI) treatment (37), and
increased androgen signaling in breast cancer cells possibly causes
resistance to AI (9). In addition,
macrophages infiltrate more in metastatic breast carcinoma tissues
than in primary breast carcinoma tissues (15). Considering our present findings and
these previous reports, androgen action in macrophages as well as
breast carcinoma cells seems to play pivotal roles for acquisition
of endocrine resistance. Our recent findings may thus provide
important clues to overcome endocrine resistance.
We next constructed in vitro and in
vivo experiments using mouse breast cancer and macrophage cell
lines, 4T1 and RAW264.7. Real-time PCR analysis showed that
Ar and Akr1c6 mRNA was negligible in both 4T1 and
RAW264.7 cells. However, cell lines could not necessarily mimic the
microenvironment of human breast carcinoma tissues. We demonstrated
the expression of AR in breast cancer-associated macrophages by
double-immunohistochemistry, and androgen-producing enzymes such as
17βHSD5 and 5αRed1 are frequently expressed in human breast
carcinomas and contribute to intratumoral androgen synthesis
(2,3).
We therefore tried to mimic this situation using mouse cell lines
by establishing AR- and AKR1C6-overexpressing sublines. In the
sphere-forming assay using 4T1 and RAW-AR cell lines, which stably
express AR, sphere size was significantly increased by androgens
when 4T1 cells were cocultured with RAW-AR cells. Because 4T1 cells
did not express AR, increased sphere size was due to androgen
action in RAW-AR cells. Furthermore, in vivo experiments
using a tumor-bearing mouse model showed that tumor volume as well
as Ki67 LI were increased significantly when androgens were
stability produced in breast cancer cells and AR was expressed in
macrophages. Intratumoral macrophages produce numerous kinds of
soluble factors and they are strongly linked to pro-tumorigenic
roles for macrophages (38), although
little is known about androgen-regulated soluble factors in
intratumoral macrophages. However, some cytokines such as TNFα and
IL-6 are induced by androgens in macrophages in both pathological
and physiological conditions (17,22,39) and
finasteride, a potent 5α-reductase inhibitor suppresses TNFα
secretion from prostatic macrophages (40). In breast cancer, TNFα enhances
invasiveness of breast cancer cells through matrix
metalloproteinase (MMP) induction in macrophages (41). Moreover, IL-6 expression in
macrophages isolated from hepatocellular carcinoma (HCC) is
markedly upregulated by interaction with HepG2 HCC cell line and
IL-6 promotes stemness of HCC via the signal transducer and
activator of transcription (STAT)3 pathway (42). Recently, AR signaling in macrophages
has been shown to promote migration and invasion of prostate cancer
cell lines by increased triggering receptor expressed on myeloid
cells-1 (TREM-1) signaling, regulating the expression of its
downstream cytokines such as CCL2, CCL7, CXCL8 and CCL13 (31). On the other hand, there was no
significant correlation between macrophage infiltration and 5αRed1
in human breast carcinoma tissues and R1881 did not alter
Arg-1 mRNA expression in RAW-AR cells. Taken together, these
results show that androgen action may not necessarily correlate
with macrophage polarization but androgen activates macrophages by
inducing soluble factors which have pro-tumorigenic roles in human
malignancies. Further examination is needed to identify
androgen-induced soluble factors in intratumoral macrophages, in
order to explore the relationships between androgens and the breast
cancer microenvironment.
One of the limitations of the present study is the
lack of quantitative data on AR-positive macrophages due to the
relatively weak immunoreactivity of AR in macrophages, making it
impossible for us to directly evaluate the significance of androgen
action upon them. Alternative quantitative methods such as flow
cytometry will be required in the future. Moreover, we did not
compare the tumor formation of 4T1-CT and 4T1-AKR1C6 cells without
RAW264.7 cells, although we concluded that androgen action may not
correlate with macrophage polarization as described above. In
addition, the effect of finasteride, 5α-reductase inhibitor on
intratumoral macrophages was not investigated in the present study.
Further in vivo examination is needed to reveal in more
detail the action of androgen in macrophages. Finally, in
vitro and in vivo experiments were conducted using 4T1
mouse breast cancer cells, which do not express ER and we could not
experimentally verify the role of androgen action of macrophages in
ER-positive breast cancers. The reason why we used 4T1 is that the
mouse models of ER-positive breast cancer cells are lacking
(43) and 4T1 cells are widely used
for mouse breast cancer models in many previous studies (44–46). In
addition, we could not differentiate human monocytic THP-1 cells
into the M2 phenotype by coculture with human breast cancer cell
lines including MCF-7 cells, which express high levels of ER.
Therefore, the present study is the starting point of further
investigation into novel androgen action focusing on intratumoral
macrophages in breast carcinoma tissues, which may help
understanding the complex breast cancer microenvironment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was partly supported by JSPS KAKENHI Grant
Number 19K09065 and 19K07410.
Availability of data and materials
All data and material presented in this article are
available from the corresponding author upon reasonable
request.
Author's contributions
MY performed cell and animal experiments and
prepared the manuscript. KT contributed to the study conception and
design and performed statistical analysis. MS performed the
immunohistochemistry. AS assisted with the animal experiments. YM
and YO gave skillful suggestions in regards to the
immunohistochemistry. MM and HS collected the clinical samples and
clinicopathological information. TS performed the pathological
analysis. All authors read and approved the final manuscript and
agreed to be accountable for all aspects of the work in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The study using clinical samples was approved by the
Ethics Committee at Tohoku University Graduate School of Medicine
(approval no. 2016-1-697, 2019-1-219). Experiments and analyses
were performed in accordance to the Helsinki declaration. This is a
retrospective study and therefore informed consent had not been
obtained. All animal experiments were performed in accordance with
ARRIVE guidelines with the approval of the Institutional Animal
Care and Use Committee at Tohoku University (2019MdA-172).
Patient consent for publication
Not applicable.
Competing interests
The authors have no competing interests.
References
|
1
|
Luqmani YA and Alam-Eldin N: Overcoming
resistance to endocrine therapy in breast cancer: New approaches to
a nagging problem. Med Princ Pract. 25 (Suppl 2):S28–S40. 2016.
View Article : Google Scholar
|
|
2
|
Suzuki T, Darnel AD, Akahira JI, Ariga N,
Ogawa S, Kaneko C, Takeyama J, Moriya T and Sasano H:
5alpha-reductases in human breast carcinoma: Possible modulator of
in situ androgenic actions. J Clin Endocrinol Metab. 86:2250–2257.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki T, Miki Y, Moriya T, Akahira J,
Ishida T, Hirakawa H, Yamaguchi Y, Hayashi S and Sasano H:
5Alpha-reductase type 1 and aromatase in breast carcinoma as
regulators of in situ androgen production. Int J Cancer.
120:285–291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hall RE, Aspinall JO, Horsfall DJ, Birrell
SN, Bentel JM, Sutherland RL and Tilley WD: Expression of the
androgen receptor and an androgen-responsive protein,
apolipoprotein D, in human breast cancer. Br J Cancer.
74:1175–1180. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuenen-Boumeester V, Van der Kwast TH,
Claassen CC, Look MP, Liem GS, Klijn JG and Henzen-Logmans SC: The
clinical significance of androgen receptors in breast cancer and
their relation to histological and cell biological parameters. Eur
J Cancer. 32A:1560–1565. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vera-Badillo FE, Templeton AJ, de Gouveia
P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, Seruga B, Tannock IF,
Ocana A and Amir E: Androgen receptor expression and outcomes in
early breast cancer: A systematic review and meta-analysis. J Natl
Cancer Inst. 106:djt3192014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farmer P, Bonnefoi H, Becette V,
Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J,
Cameron D, Goldstein D, et al: Identification of molecular apocrine
breast tumours by microarray analysis. Oncogene. 24:4660–4671.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujii R, Hanamura T, Suzuki T, Gohno T,
Shibahara Y, Niwa T, Yamaguchi Y, Ohnuki K, Kakugawa Y, Hirakawa H,
et al: Increased androgen receptor activity and cell proliferation
in aromatase inhibitor-resistant breast carcinoma. J Steroid
Biochem Mol Biol. 144:513–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Palma M, Biziato D and Petrova TV:
Microenvironmental regulation of tumour angiogenesis. Nat Rev
Cancer. 17:457–474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vasiliadou I and Holen I: The role of
macrophages in bone metastasis. J Bone Oncol. 2:158–166. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gan L, Qiu Z, Huang J, Li Y, Huang H,
Xiang T, Wan J, Hui T, Lin Y, Li H and Ren G: Cyclooxygenase-2 in
tumor-associated macrophages promotes metastatic potential of
breast cancer cells through Akt pathway. Int J Biol Sci.
12:1533–1543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J, Li X, Liu X and Liu Y: The role of
tumor-associated macrophages in breast carcinoma invasion and
metastasis. Int J Clin Exp Pathol. 8:6656–6664. 2015.PubMed/NCBI
|
|
14
|
Li J, He K, Liu P and Xu LX: Iron
participated in breast cancer chemoresistance by reinforcing IL-6
paracrine loop. Biochem Biophys Res Commun. 475:154–160. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Wang J, Zhang M, Xuan Q, Wang Z,
Lian X and Zhang Q: Jagged1 promotes aromatase inhibitor resistance
by modulating tumor-associated macrophage differentiation in breast
cancer patients. Breast Cancer Res Treat. 166:95–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gelsomino L, Giordano C, Camera GL, Sisci
D, Marsico S, Campana A, Tarallo R, Rinaldi A, Fuqua S, Leggio A,
et al: Leptin signaling contributes to aromatase inhibitor
resistant breast cancer cell growth and activation of macrophages.
Biomolecules. 10:5432020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sousa S, Brion R, Lintunen M, Kronqvist P,
Sandholm J, Mönkkönen J, Kellokumpu-Lehtinen PL, Lauttia S,
Tynninen O, Joensuu H, et al: Human breast cancer cells educate
macrophages toward the M2 activation status. Breast Cancer Res.
17:1012015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCrohon JA, Death AK, Nakhla S, Jessup W,
Handelsman DJ, Stanley KK and Celermajer DS: Androgen receptor
expression is greater in macrophages from male than from female
donors. A sex difference with implications for atherogenesis.
Circulation. 101:224–226. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang CK, Pang H, Wang L, Niu Y, Luo J,
Chang E, Sparks JD, Lee SO and Chang C: New therapy via targeting
androgen receptor in monocytes/macrophages to battle
atherosclerosis. Hypertension. 63:1345–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC,
Lin WJ and Chang C: Monocyte/macrophage androgen receptor
suppresses cutaneous wound healing in mice by enhancing local
TNF-alpha expression. J Clin Invest. 119:3739–3751. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamaguchi M, Takagi K, Sato A, Miki Y,
Miyashita M, Sasano H and Suzuki T: Rac1 activation in human breast
carcinoma as a prognostic factor associated with therapeutic
resistance. Breast Cancer. 27:919–928. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi M, Takagi K, Narita K, Miki Y,
Onodera Y, Miyashita M, Sasano H and Suzuki T: Stromal CCL5
promotes breast cancer progression by interacting with ccr3 in
tumor cells. Int J Mol Sci. 22:19182021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamlin IM: Possible host resistance in
carcinoma of the breast: A histological study. Br J Cancer.
22:383–401. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hammond ME, Hayes DF, Wolff AC, Mangu PB
and Temin S: American society of clinical oncology/college of
american pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Oncol Pract. 6:195–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takagi K, Miki Y, Onodera Y, Ishida T,
Watanabe M, Sasano H and Suzuki T: ARHGAP15 in human breast
carcinoma: A potent tumor suppressor regulated by androgens. Int J
Mol Sci. 19:E8042018. View Article : Google Scholar
|
|
28
|
Sato A, Takagi K, Miki Y, Yoshimura A,
Hara M, Ishida T, Sasano H and Suzuki T: Cytochrome c1 as a
favorable prognostic marker in estrogen receptor-positive breast
carcinoma. Histol Histopathol. 34:1365–1375. 2019.PubMed/NCBI
|
|
29
|
Vergnes L, Phan J, Stolz A and Reue K: A
cluster of eight hydroxysteroid dehydrogenase genes belonging to
the aldo-keto reductase supergene family on mouse chromosome 13. J
Lipid Res. 44:503–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niu Y, Altuwaijri S, Yeh S, Lai KP, Yu S,
Chuang KH, Huang SP, Lardy H and Chang C: Targeting the stromal
androgen receptor in primary prostate tumors at earlier stages.
Proc Natl Acad Sci USA. 105:12188–12193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cioni B, Zaalberg A, van Beijnum JR, Melis
MHM, van Burgsteden J, Muraro MJ, Hooijberg E, Peters D, Hofland I,
Lubeck Y, et al: Androgen receptor signalling in macrophages
promotes TREM-1-mediated prostate cancer cell line migration and
invasion. Nat Commun. 11:44982020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Figueroa F, Davicino R, Micalizzi B,
Oliveros L and Forneris M: Macrophage secretions modulate the
steroidogenesis of polycystic ovary in rats: Effect of testosterone
on macrophage pro-inflammatory cytokines. Life Sci. 90:733–739.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Levano KS, Jung EH and Kenny PA: Breast
cancer subtypes express distinct receptor repertoires for
tumor-associated macrophage derived cytokines. Biochem Biophys Res
Commun. 411:107–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen LY, Brown PR, Willis WB and Eddy EM:
Peritubular myoid cells participate in male mouse spermatogonial
stem cell maintenance. Endocrinology. 155:4964–4974. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gwak JM, Jang MH, Kim DI, Seo AN and Park
SY: Prognostic value of tumor-associated macrophages according to
histologic locations and hormone receptor status in breast cancer.
PLoS One. 10:e01257282015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klingen TA, Chen Y, Aas H, Wik E and
Akslen LA: Tumor-associated macrophages are strongly related to
vascular invasion, non-luminal subtypes, and interval breast
cancer. Hum Pathol. 69:72–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takagi K, Miki Y, Nagasaki S, Hirakawa H,
Onodera Y, Akahira J, Ishida T, Watanabe M, Kimijima I, Hayashi S,
et al: Increased intratumoral androgens in human breast carcinoma
following aromatase inhibitor exemestane treatment. Endocr Relat
Cancer. 17:415–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang L and Zhang Y; Tumor-associated
macrophages, : From basic research to clinical application. J
Hematol Oncol. 10:582017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schneider CP, Schwacha MG, Samy TS, Bland
KI and Chaudry IH: Androgen-mediated modulation of macrophage
function after trauma-hemorrhage: Central role of
5alpha-dihydrotestosterone. J Appl Physiol (1985). 95:104–112.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao R, Wang X, Jiang C, Shi F, Zhu Y,
Yang B, Zhuo J, Jing Y, Luo G, Xia S and Han B: Finasteride
accelerates prostate wound healing after thulium laser resection
through DHT and AR signalling. Cell Prolif. 51:e124152018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hagemann T, Robinson SC, Schulz M, Trümper
L, Balkwill FR and Binder C: Enhanced invasiveness of breast cancer
cell lines upon co-cultivation with macrophages is due to TNF-alpha
dependent up-regulation of matrix metalloproteases. Carcinogenesis.
25:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wan S, Zhao E, Kryczek I, Vatan L,
Sadovskaya A, Ludema G, Simeone DM, Zou W and Welling TH:
Tumor-associated macrophages produce interleukin 6 and signal via
STAT3 to promote expansion of human hepatocellular carcinoma stem
cells. Gastroenterology. 147:1393–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mohibi S, Mirza S, Band H and Band V:
Mouse models of estrogen receptor-positive breast cancer. J
Carcinog. 10:352011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pulaski BA and Ostrand-Rosenberg S: Mouse
4T1 breast tumor model. Curr Protoc Immunol. 20:Chapter 20: Unit
20.2. 2001.PubMed/NCBI
|
|
45
|
Cho HJ, Jung JI, Lim DY, Kwon GT, Her S
and Park JH and Park JH: Bone marrow-derived, alternatively
activated macrophages enhance solid tumor growth and lung
metastasis of mammary carcinoma cells in a Balb/C mouse orthotopic
model. Breast Cancer Res. 14:R812012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jang JY, Lee JK, Jeon YK and Kim CW:
Exosome derived from epigallocatechin gallate treated breast cancer
cells suppresses tumor growth by inhibiting tumor-associated
macrophage infiltration and M2 polarization. BMC Cancer.
13:4212013. View Article : Google Scholar : PubMed/NCBI
|