Introduction
Oral lichen planus (OLP) is an inflammatory disorder
of the skin and mucous membranes. Extraoral mucosal lesions have
been reported in the esophagus, conjunctiva, bladder, nasal mucosa,
larynx, pharynx, stomach, anal mucosa, vulva, vagina and penis
(1,2). Some patients with OLP develop
extraoral manifestations simultaneously (1). Vulvo-vaginal-gingival syndrome is a
variant of mucosal lichen planus characterized by erosions and
desquamation of the vulva, vagina and gingiva (3).
One of the potential complications of OLP is
malignant transformation. Patients with OLP have an increased risk
of developing oral squamous cell carcinomas (OSCC), which led the
World Health Organization (WHO) to classify OLP as a potentially
malignant disorder (4). A number of
studies have reported an association between OLP and a premalignant
state, with a malignant transformation rate of 0–12.5% (5–10).
Lichen planus is known to occur in patients with
liver disease, particularly hepatitis C. A close association of
lichen planus and hepatitis C virus (HCV) infection has been
reported in Japan and certain southern European regions (11,12).
In multiple meta-analyses, patients with lichen planus have an
∼5-fold higher risk, compared to the controls, of being
HCV-seropositive (odds ratio: 2.5–4.5) (13–15).
An association between HCV infection and OSCC has
been reported (16–19). The prevalence of HCV infection in
OSCC with multiple primary carcinomas was previously reported
(20). In addition, HCV infection
has been found to be an independent factor in the development of
multiple primary carcinomas in patients with OSCC (21).
Carrozzo et al(22) reported a case of oral verrucous
carcinoma arising in a patient with OLP and HCV infection.
Verrucous carcinoma is a rare type of low-grade and
well-differentiated SCC, with less potential for lymph node
metastasis compared to other oral carcinomas and is known to occur
in the larynx, pyriform sinus, nasal cavity, skin and esophagus,
the oral cavity being the most common site (23).
In this study, we present a case of synchronous oral
verrucous carcinoma and esophageal SCC in a patient with
HCV-related liver cirrhosis. This oral verrucous carcinoma is a
malignant change arising in OLP-coexisting vulvo-vaginal-gingival
syndrome.
Case report
In June 1994, a 71-year-old Japanese females
presented at the Kurume University Hospital (Fukuoka, Japan) with a
burning pain in the oral cavity on eating and drinking. She first
experienced the pain and a burning sensation in the oral mucosa
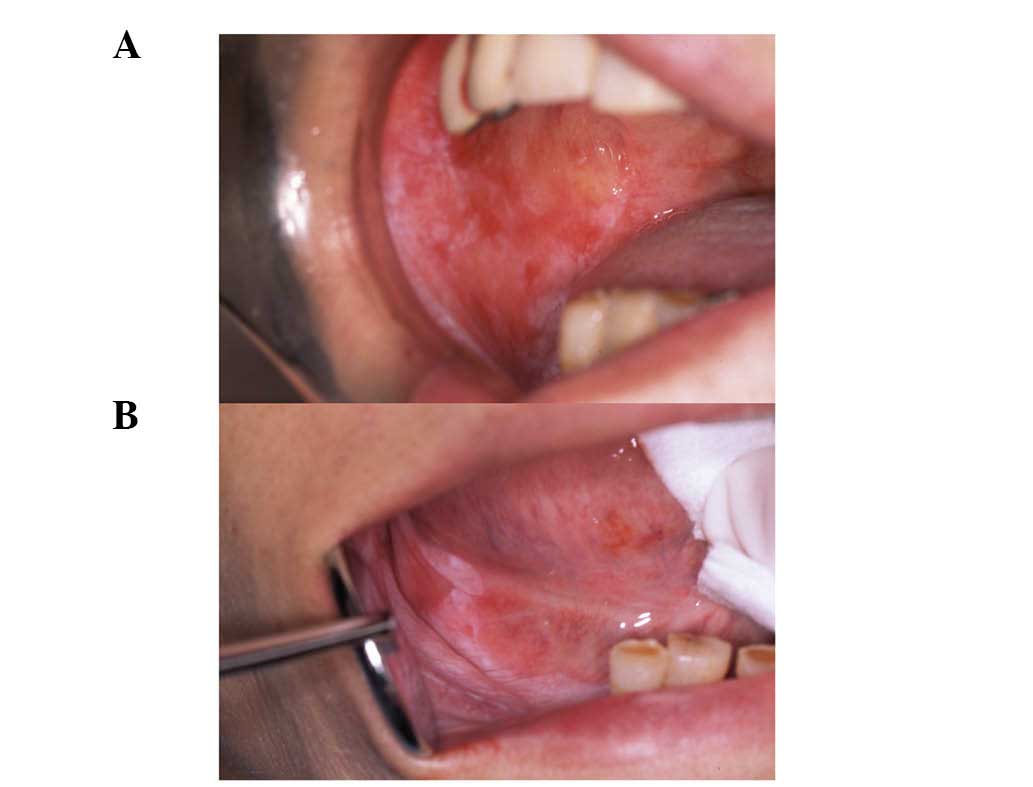
approximately 12 months before. The lesions were examined and were
identified as erosive and atrophic types, affecting the bilateral
buccal mucosa, mandibular alveolar mucosa, tongue, gingiva, palate,
oral floor and the lower lip (Fig.
1).
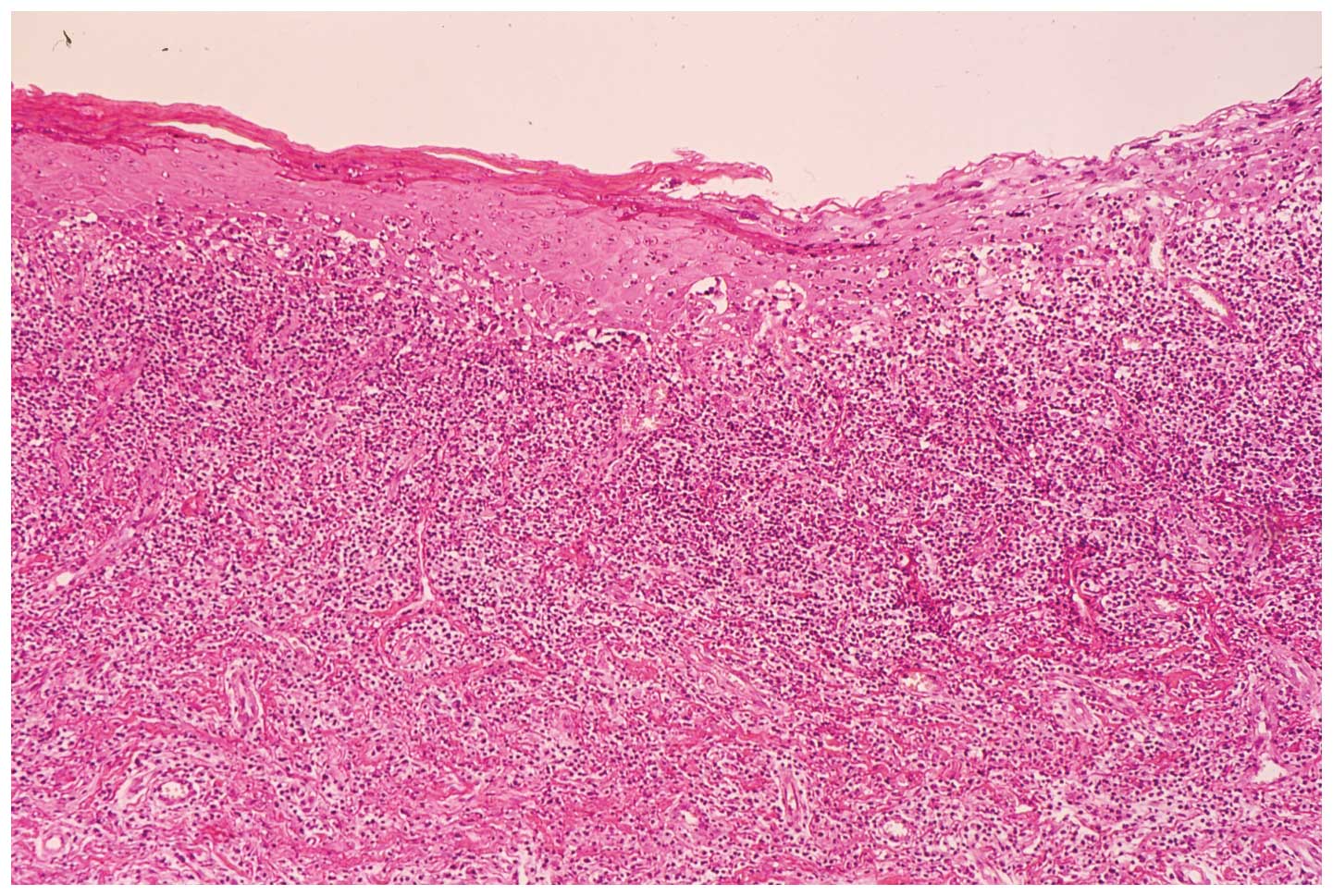
Two biopsy specimens of the buccal mucosa and
mandibular alveolar mucosa were characterized by hyperparakeratosis
with the thickening of the granular layer, a subepithelial band of
infiltration of lymphocytes and liquefied degeneration of the basal
cell layer. Histopathological findings were consistent with a
diagnosis of OLP (Fig. 2).
The patient was unaware of being infected with HCV
until she underwent a serological examination at our hospital. The
serum HCV RNA levels, quantified using the Roche Amplicor Monitor
assay (Roche Diagnostic Systems, Inc., Indianapolis, IN, USA) and
HCV genotype were 2200 KIU/ml and 1 b, respectively. Serum levels
were negative for hepatitis B surface antigen (HBsAg). The patient
underwent ultrasonographic (US) examination and computed tomography
(CT). Alternative potential predictors of progression of liver
cirrhosis were applied, including serum albumin, total bilirubin,
prothrombin time and the platelet count. The patient was diagnosed
with liver cirrhosis. On detailed examination, no systemic disease
other than chronic liver disease was confirmed.
Laboratory data in 1994 were: aspartate
aminotransferase 62 U/l; alanine aminotransferase 39 U/l;
γ-glutamyl transpeptidase 26 U/l; total protein 7.8 g/dl; albumin
3.7 g/dl; red blood cell count 384×104/mm; white blood
cell count 4400 mm; hemoglobin 11.8 g/dl; platelet count
15.9×104/mm; fasting plasma glucose 97 mg/dl; hemoglobin
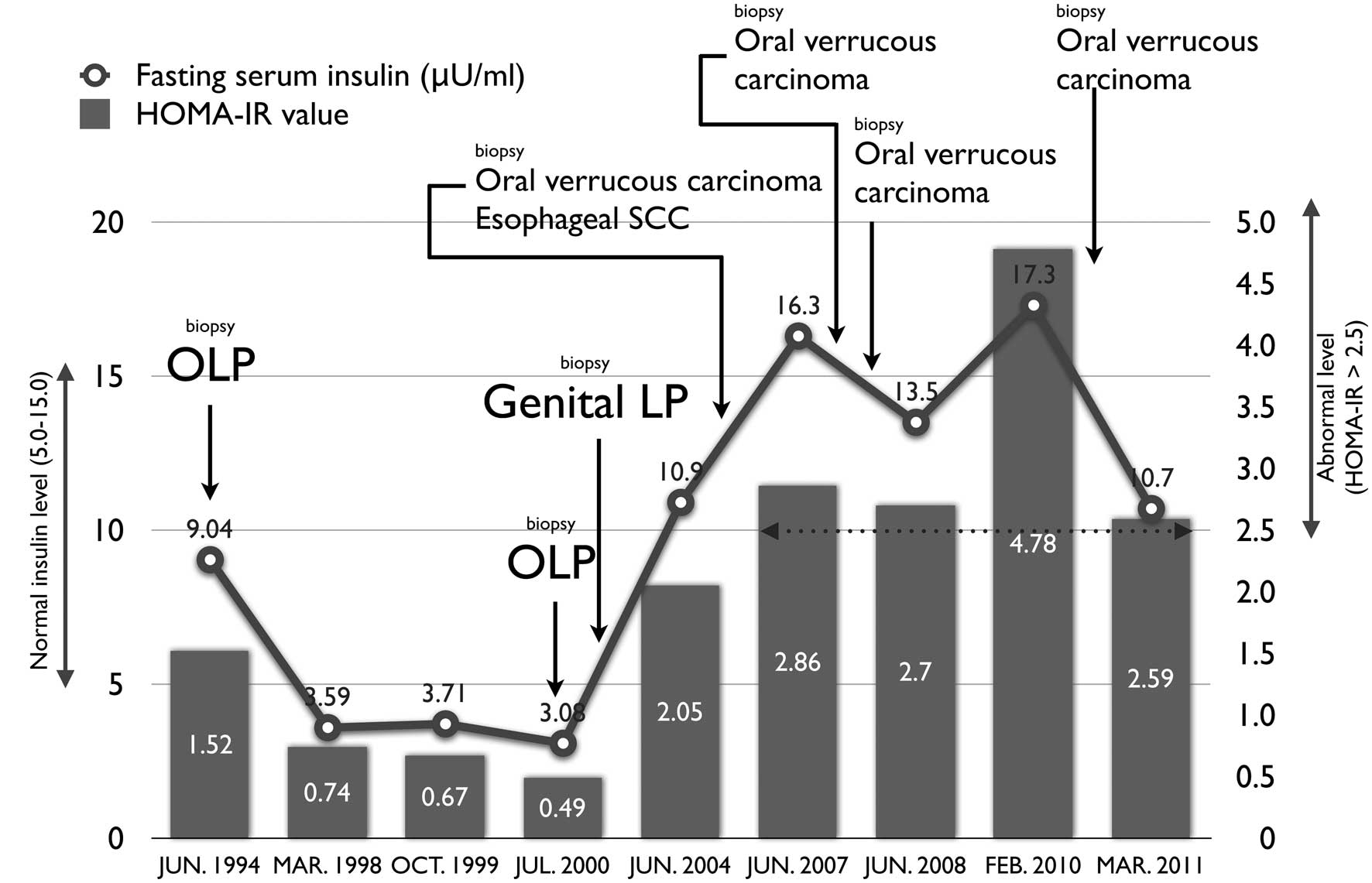
A1c 5.8; fasting serum insulin 9.04 μU/ml and homeostasis model
assessment-insulin resistance (HOMA-IR) 4.78. Insulin resistance
was evaluated using the homeostasis model assessment (HOMA-IR)
method, using the formula: HOMA-IR = fasting glucose (mg/dl) ×
fasting insulin (μU/ml)/405.
Body mass index (BMI) of the patient was 20.93
kg/m2 (height 1.45 m, weight 44 kg). BMI was used as an
index of obesity and was calculated as body weight in kilograms
divided by the square of height in meters (kg/m2). No
habitual alcohol drinking or smoking was confirmed. No history of
blood transfusion or tattooing was confirmed, while her family
history was not contributory.
Treatment was initiated with a topical
corticosteroid and vitamins. Glycyrrhizin was administered daily
from January 1995 by intravenous injection. Subsequently, the
patient controlled the oral lesions by applying a topical
corticosteroid for oral pain. We monitored her condition every 3–4
months by conducting an oral medical examination, blood tests and
abdominal echography performed by an oral surgery specialist and a
hepatologist.
In March 2001, although the patient did not complain
of sensation in the genital mucosa, she was examined by a
gynecologist to determine whether genital lichen planus was
present. The physical findings comprised primarily erythema of the
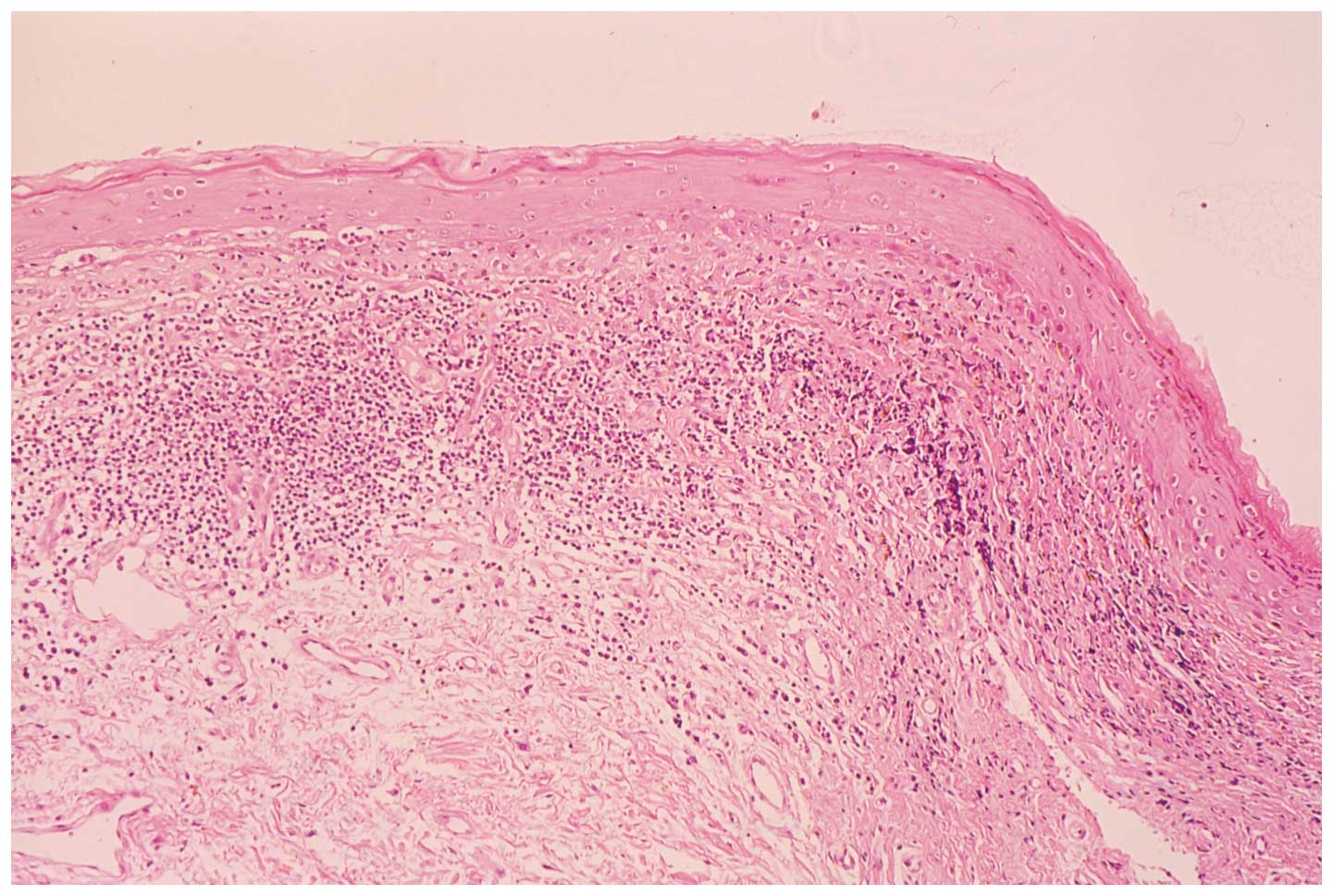
inner labia minora. A biopsy specimen of the vulval mucosa was
characterized by a subepithelial band of infiltrating lymphocytes
and liquefied degeneration of the basal cell layer (Fig. 3). The clinicopathological diagnosis
of the patient was vulvo-vaginal-gingival syndrome.
In November, 2004, the 81-year-old patient underwent
a routine examination of the upper gastrointestinal tract to
confirm the presence or absence of esophageal varices associated
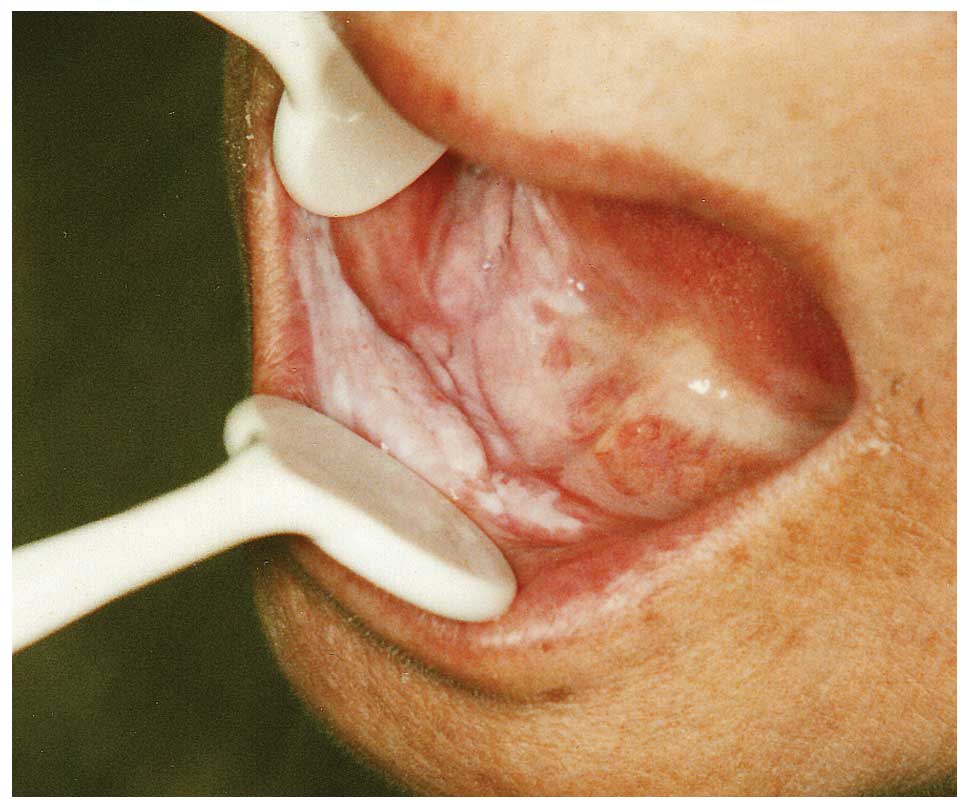
with her liver cirrhosis. An erosive lesion of the esophagus was
observed. In addition, we observed a red-and-white papillary and
exophytic mass, 15×13 mm in diameter, in the mandibular alveolar
and the right buccal mucosa and the right lower lip (Fig. 4). Ultrasonographic examination of
the cervical region did not show lymph node metastasis. The
pathological diagnosis was SCC of the esophagus (0-IIc, T1a) and
verrucous carcinoma of the oral cavity (T1N0M0, stage I). There was
no continuity between the esophageal and oral cancer. The patient
underwent surgical resection of the oral verrucous carcinoma in
December, 2004 and endoscopic submucosal dissection of the
esophageal cancer in March, 2005.
The oral verrucous carcinoma recurred in September,
2007 and February 2008 and was resected surgically. In addition,
the patient (then 87-year-old) received radiotherapy in August,
2010, subsequent to recurrence of the oral cancer. Following
radiotherapy of 45 Gy, the tumor disappeared completely. The
patient’s condition was considered stable, under treatment in
August, 2012, two years later. Fig.
5 shows the clinical course of the patient.
Discussion
Several studies have demonstrated that carcinoma may
arise from OLP (5–10). In their study, Gandolfo et
al(5) reported that HCV
infection increased the risk of OSCC in patients with OLP by a
factor of 3.16 (95% CI, 0.80–12.5). Smoking, atrophic-erosive
forms, gender and age, diet and candidiasis are factors, other than
HCV infection, associated with a malignant change of OLP (7).
In this study, we have presented a case of verrucous
carcinoma that developed in a patient with OLP who suffered from
HCV-related liver cirrhosis, 10 years after the initial diagnosis.
Simultaneously, the patient developed esophageal SCC besides oral
cancer. The patient had lichen planus affecting not only the oral,
but also the genital mucosa.
In their study, Sikuler et al(24) evaluated the association between HCV
infection and extrahepatic malignancies. Extrahepatic malignancies
were found in 14.6% of anti-HCV-positive patients. Lee et
al(25) prospectively studied
the risk of HCV infection on hepatic and extrahepatic deaths. Their
study demonstrated significant associations between anti-HCV
seropositivity and increased mortality from extrahepatic cancers
with multivariate-adjusted hazard ratios of 4.08 for the esophagus,
4.19 for the prostate and 8.22 for the thyroid. Furthermore, they
showed that persistent HCV infection (anti-HCV and HCV RNA
seropositive) markedly increased the mortality rate caused by
hepatic and extrahepatic diseases.
In the present case, the factors thought to be
responsible for the development of malignant transformation are the
long-lasting presence of symptomatic OLP, persistent HCV infection,
advanced age and hyperinsulinemia. We reported previously that
insulin resistance might be involved in the development of multiple
primary cancers in patients with OSCC and HCV infection (21), and might cause OLP and extrahepatic
manifestations (26,27). The prevalence of extra-hepatic
malignant tumors was significantly higher in patients with OLP
(29.4%), compared to patients without (4.3%). Hyperinsulinemia may
induce extrahepatic malignant tumors, as well as hepatocellular
carcinoma (HCC), thus high insulin levels could promote the
selective growth of cancer cells (28). HCV-associated insulin resistance
induces hepatic steatosis, resistance to anti-viral treatment,
hepatic fibrosis and esophageal varices, hepatocarcinogenesis and
proliferation of HCC, and extrahepatic manifestations (29).
The patient in this report developed carcinoma when
the insulin level was high, thus we believe that this should be
measured regularly in patients with HCV-associated OLP.
Lichen planus is known to develop in mucosal
tissues, with the exception of the oral mucosa (1–3). Eisen
(1) evaluated extraoral involvement
in a large series of patients with OLP and reported that extraoral
manifestations included cutaneous LP in 16% of patients (93/584)
and genital LP in 19% of 399 women and 4.6% of 174 men. Previously,
we showed extraoral involvement in female patients with OLP of
41.7% (10/24) vulvar and 8.3% (2/24) cutaneous LP (30). It is important for patients with OLP
to be monitored for the presence of extra-oral lichen planus, such
as in the genital mucous membrane and the gastrointestinal tract,
since it is more likely to progress to a malignant
transformation.
In conclusion, we reported a case of oral verrucous
carcinoma arising from OLP-coexisting vulvo-vaginal-gingival
syndrome and esophageal SCC in a patient with HCV-related liver
cirrhosis. Success in the detection and treatment of multiple
primary cancers at early stages requires close cooperation between
various medical specialists.
Abbreviations:
|
OLP
|
oral lichen planus
|
|
SCC
|
squamous cell carcinoma
|
|
OSCC
|
oral squamous cell carcinoma
|
|
HCV
|
hepatitis C virus
|
|
HCC
|
hepatocellular carcinoma
|
|
anti-HCV
|
HCV antibody
|
|
HBsAg
|
hepatitis B surface antigen
|
Acknowledgements
This study was supported in part by a
Grant-in-Aid for Scientific Research (C) (no. 22592354) from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan.
References
|
1.
|
Eisen D: The evaluation of cutaneous,
genital, scalp, nail, esophageal, and ocular involvement in
patients with oral lichen planus. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 88:431–436. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Le Cleach L and Chosidow O: Clinical
practice. Lichen planus. N Engl J Med. 366:723–732. 2012.
|
|
3.
|
Pelisse M, Leibowitch M, Sedel D and
Hewitt J: A new vulvovaginogingival syndrome. Plurimucous erosive
lichen planus. Ann Dermatol Venereol. 109:797–798. 1982.(In
French).
|
|
4.
|
Warnakulasuriya S, Johnson NW and van der
Waal I: Nomenclature and classification of potentially malignant
disorders of the oral mucosa. J Oral Pathol Med. 36:575–580. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Gandolfo S, Richiardi L, Carrozzo M, et
al: Risk of oral squamous cell carcinoma in 402 patients with oral
lichen planus: a follow-up study in an Italian population. Oral
Oncol. 40:77–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lodi G, Scully C, Carrozzo M, Griffiths M,
Sugerman PB and Thongprasom K: Current controversies in oral lichen
planus: report of an international consensus meeting. Part 2.
Clinical management and malignant transformation. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 100:164–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gonzalez-Moles MA, Scully C and
Gil-Montoya JA: Oral lichen planus: controversies surrounding
malignant transformation. Oral Dis. 14:229–243. 2008. View Article : Google Scholar
|
|
8.
|
Bermejo-Fenoll A, Sánchez-Siles M,
López-Jornet P, Camacho-Alonso F and Salazar-Sánchez N: A
retrospective clinicopathological study of 550 patients with oral
lichen planus in south-eastern Spain. J Oral Pathol Med.
39:491–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Warnakulasuriya S, Kovacevic T, Madden P,
Coupland VH, Sperandio M, Odell E and Møller H: Factors predicting
malignant transformation in oral potentially malignant disorders
among patients accrued over a 10-year period in South East England.
J Oral Pathol Med. 40:677–683. 2011.
|
|
10.
|
Bombeccari GP, Guzzi G, Tettamanti M,
Giannì AB, Baj A, Pallotti F and Spadari F: Oral lichen planus and
malignant transformation: a longitudinal cohort study. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 112:328–334. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nagao Y, Sata M, Tanikawa K, Itoh K and
Kameyama T: Lichen planus and hepatitis C virus in the northern
Kyushu region of Japan. Eur J Clin Invest. 25:910–914. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lodi G, Giuliani M, Majorana A, Sardella
A, Bez C, Demarosi F and Carrassi A: Lichen planus and hepatitis C
virus: a multicentre study of patients with oral lesions and a
systematic review. Br J Dermatol. 151:1172–1181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shengyuan L, Songpo Y, Wen W, Wenjing T,
Haitao Z and Binyou W: Hepatitis C virus and lichen planus: a
reciprocal association determined by a meta-analysis. Arch
Dermatol. 145:1040–1047. 2009.PubMed/NCBI
|
|
14.
|
Lodi G, Pellicano R and Carrozzo M:
Hepatitis C virus infection and lichen planus: a systematic review
with meta-analysis. Oral Dis. 16:601–612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Petti S, Rabiei M, De Luca M and Scully C:
The magnitude of the association between hepatitis C virus
infection and oral lichen planus: meta-analysis and case control
study. Odontology. 99:168–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nagao Y, Sata M, Tanikawa K, Itoh K and
Kameyama T: High prevalence of hepatitis C virus antibody and RNA
in patients with oral cancer. J Oral Pathol Med. 24:354–360. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nagao Y, Sata M, Itoh K, et al: High
prevalence of hepatitis C virus antibody and RNA in patients with
head and neck squamous cell carcinoma. Hepatol Res. 7:206–212.
1997.
|
|
18.
|
Nobles J, Wold C, Fazekas-May M, Gilbert J
and Friedlander PL: Prevalence and epidemiology of hepatitis C
virus in patients with squamous cell carcinoma of the head and
neck. Laryngoscope. 114:2119–2122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hunt J, Hagan J, Nobles J, Wold C,
Fazekas-May M, Gilbert J and Friedlander PL: Outcome analysis of
patients with squamous cell carcinoma of the head and neck and
hepatitis C virus. Laryngoscope. 115:1882–1886. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yoshida M, Nagao Y, Sata M, Kusukawa J and
Kameyama T: Multiple primary neoplasms and hepatitis C virus
infection in oral cancer patients. Hepatol Res. 9:75–81. 1997.
View Article : Google Scholar
|
|
21.
|
Nagao Y and Sata M: High incidence of
multiple primary carcinomas in HCV-infected patients with oral
squamous cell carcinoma. Med Sci Monit. 15:CR453–CR459.
2009.PubMed/NCBI
|
|
22.
|
Carrozzo M, Carbone M, Gandolfo S, Valente
G, Colombatto P and Ghisetti V: An atypical verrucous carcinoma of
the tongue arising in a patient with oral lichen planus associated
with hepatitis C virus infection. Oral Oncol. 33:220–225. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Spiro RH: Verrucous carcinoma, then and
now. Am J Surg. 176:393–397. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Sikuler E, Shnaider A, Zilberman D,
Hilzenrat N, Shemer-Avni Y, Neumann L and Buskila D: Hepatitis C
virus infection and extrahepatic malignancies. J Clin
Gastroenterol. 24:87–89. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lee MH, Yang HI, Lu SN, et al: Chronic
hepatitis C virus infection increases mortality from hepatic and
extrahepatic diseases: a community-based long-term prospective
study. J Infect Dis. 206:469–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Nagao Y, Kawaguchi T, Tanaka K, Kumashiro
R and Sata M: Extrahepatic manifestations and insulin resistance in
an HCV hyperendemic area. Int J Mol Med. 16:291–296.
2005.PubMed/NCBI
|
|
27.
|
Nagao Y, Kawasaki K and Sata M: Insulin
resistance and lichen planus in patients with HCV-infectious liver
diseases. J Gastroenterol Hepatol. 23:580–585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jee SH, Ohrr H, Sull JW, Yun JE, Ji M and
Samet JM: Fasting serum glucose level and cancer risk in Korean men
and women. JAMA. 293:194–202. 2005. View Article : Google Scholar
|
|
29.
|
Kawaguchi T and Sata M: Importance of
hepatitis C virus-associated insulin resistance: therapeutic
strategies for insulin sensitization. World J Gastroenterol.
28:1943–1952. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Nagao Y, Tomonari R, Kage M, Komai K,
Tsubone K, Kamura T and Sata M: The possible intraspousal
transmission of HCV in terms of lichen planus. Int J Mol Med.
10:569–573. 2002.PubMed/NCBI
|