Introduction
Colorectal cancer (CRC), one of the most common
malignancies in the Western world (1), is considered to develop from benign
precursor lesions (i.e., adenomatous polyps) through the
ademona-carcinoma sequence (2).
This term refers to a progressive malignant transformation, the
phenotypic expression of underlying stepwise genetic alterations,
according to the model developed by Fearon and Vogelstein (3). Notwithstanding the existence of
certain genetically different tumors following alternative
tumorigenic pathways (with various precursor-serrated adenomas)
(4), this model remains the
dominant carcinogenic mechanism of CRC.
However, the hypothesis of another tumorigenic
pathway, that of de novo carcinogenesis, suggesting the
development of a tumor from normal (or intact) colonic mucosa,
without the intervening step of an adenoma, has been a matter of
discussion for decades (5–11). By definition, de novo lesions
are characterized by the lack of any adenomatous remnant (i.e., the
only indisputable evidence of the origin of adenoma) (7,9).
However, the application of this criterion is hampered by the
obliteration of adenomatous elements during tumor growth.
Therefore, their detection is confined to a minority of CRC
(∼15–20%) (6,7), mostly those diagnosed in early stages
and thought to retain their initial structural characteristics
(7,9,10).
This objective difficulty may explain the wide variation in the
reported frequencies of de novo tumors in the literature
(range, 1–80%) (6–13). It also necessitates the
implementation of additional criteria, potentially associated with
these lesions, including small size (<1 or 2 cm), the limited
invasion of the bowel wall (T1 lesions) and, mostly, the
nonpolypoid growth pattern (presenting in various forms: flat,
depressed and infiltrative) (6–11).
Despite the lack of absolute specificity for any of these criteria
(9), their application may be
helpful in the identification of de novo tumors (6,7,10,13).
At the genetic level, de novo carcinogenesis
has been associated with specific molecular characteristics, such
as a reduced proportion of Ki-Ras mutation (9,13–15) [a
key genetic event of the adenoma-carcinoma sequence (3)]. Moreover, de novo lesions
exhibited a predilection for proximal tumor location (7,16), an
association strongly suggesting genetic disparity, given the
considerable predominance of the microsatellite instability (MSI)
and CpG island methylator phenotype (CIMP) tumorigenic pathways
among proximal tumors (4). Other
genetic and epigenetic alterations correlated with de novo
tumors have also been detected (15,17–19),
although inconsistently (9,11).
Clinically, de novo tumors may represent a
more aggressive (fast-growing at an early stage) subtype of CRC
(14,15,20).
Consequently, their identification may be important in the planning
of treatment and follow-up (10,12,14).
In this study, we investigated the presence of de
novo tumors, particularly among cases referred for surgery
[being the large majority of CRC (1)], using a combination of
histomorphological criteria for their identification (see Materials
and methods). We also examined their association with particular
clinicopathological parameters affecting CRC prognosis (i.e.,
stage, grade and site).
Materials and methods
Study population
The sample initially examined included 147 CRC
cases, surgically treated between 2000 and 2003 in the Second
Surgical Department of Tzaneio Hospital in Piraeus (Piraeus,
Greece). Subsequent to excluding cases with recurrences, hereditary
cancer, synchronous tumors of double location and unclear pathology
reports, 119 patients were finally deemed eligible for the
conducted retrospective investigation. None of the 119 patients had
undergone neoadjuvant therapy, not performed during the study
period.
De novo determination
Tumor specimens were examined for de novo
origin based on the following criteria: i) lack of adenomatous
remnants; ii) absence of coexisting polyps in the surgical specimen
over a 10 cm distance from the tumor (potentially implying ‘field
cancerization’, i.e., carcinogenic molecular alterations of the
intestinal epithelium close to the tumor site). Similar genetic
alterations in cancers of other anatomic areas (head and neck) have
been detected within this distance (15). The existence of polyps beyond this
limit was considered coincidental (i.e., not correlated with the
primary lesion) and therefore was not recorded; and iii) apparently
nonpolypoid growth pattern, based on morphological and histological
appearance, in particular infiltrative lesions (without overhanging
edge and showing massive infiltration of tumor cells) (6), including even those with a relatively
limited ulceration (7,20) but always excluding protruding
exophytic tumors (with overhanging edge) (6). The latter type was also identified on
the basis of tumor thickness (at least 2-fold greater than that of
the adjacent normal mucosa) (20).
Tumor size and extent of invasion were not included
in the examining criteria since only three cases of lesions were
<2 cm or presented with T1 invasion, characteristics also
thought to facilitate de novo investigation. Moreover, flat
and depressed lesions were not identified in our sample. These
tumors are usually found in early CRC (not invading beyond the
submucosa), since in an advanced disease their characteristics are
frequently altered, being virtually indistinguishable from those
with polypoid origin (21).
Tumors fulfilling all the aforementioned criteria
were considered nonpolypoid (possibly de novo). By contrast,
cases exhibiting either remnants or an apparently exophytic growth
pattern were designated as ‘polypoid’. Lesions with coexisting
polyps in their vicinity were also classified as ‘polypoid’,
provided they were not infiltrative. Otherwise, they were
classified as cases of ‘unclear origin’, a category comprising
tumors without remnants but with a mixed or unclear gross
configuration (mostly due to extended ulceration).
Therefore, our stratification is based on the tumor
growth pattern [according to the Japanese classification for CRC
(22), slightly modified by
integrating infiltrative with ulceroinfiltrative and ulcerated with
unclassified lesions] and in combination with the presence/absence
of remnants and coexisting polyps.
Clinicopathological classification
Tumors were classified as stage I, II, III, IV and
grade G1, G2, G3 (well, moderate and poor), following the TNM and
World Health Organization (WHO) classifications, respectively. We
also divided cases into proximal (right-sided) and distal
(left-sided), with regard to the splenic flexure (7,16).
Moreover, in order to examine the combined effect of stage and
grade on de novo distribution, we stratified cases into
three additional subsets: indolent (stage I or G1), unfavorable
(stage IV or G3) and intermediate (stages II–III, G2). The absence
of tumors with entirely conflicting characteristics (stage I/G3 or
stage IV/G1) rendered any exclusion unnecessary.
Statistical analysis
The distribution of the particular criteria
suggesting de novo origin among the various
clinicopathological categories (stage, grade and site) was analyzed
using the χ2 test (with Yates’s correction when
necessary) and Fisher’s exact test, depending on the dataset. These
tests were also used for the subsequent analysis of the
distribution of tumors considered de novo (according to the
aforementioned criteria) among the same clinicopathological
categories. The tests were two-sided and P≤0.05 was considered to
indicate a statistically significant difference.
Results
Prevalence of the examining variables and
classification according to tumor origin
Table I shows the
clinicopathological characteristics of the sample as well as the
detection rates of the parameters investigated in this study.
Adenomatous remnants were detected in 9 (7.5%), while coexisting
polyps were observed in 11 cases (9.2%). The recorded frequencies
of the exophytic and infiltrative (or ulceroinfiltrative) growth
patterns were 20 and 32%, respectively. The remaining 48% of tumors
exhibited a mixed or unclear growth pattern. Representative
histologic images of lesions with remnants and of the various
patterns are shown in Fig. 1.
 | Table I.Clinicopathological
characteristics. |
Table I.
Clinicopathological
characteristics.
|
Characteristics | No. of cases
(n=119) | % |
|---|
| Age (years) | | |
| <70 | 56 | 47 |
| >70 | 63 | 53 |
| Gender | | |
| Male | 69 | 38 |
| Female | 50 | 42 |
| Site | | |
| Proximal | 36 | 30 |
| Distal | 83 | 70 |
| TNM stage | | |
| I | 12 | 10 |
| II | 50 | 42 |
| III | 44 | 37 |
| IV | 13 | 11 |
| Grade | | |
| Well | 7 | 6 |
| Moderate | 103 | 86.5 |
| Poor | 9 | 7.5 |
| Combined
stage-grade | | |
| Indolent (stage
I, G1) | 16 | 13.5 |
| Intermediate
(stage II–III, G2) | 82 | 69 |
| Unfavorable
(stage IV, G3) | 21 | 17.5 |
| Growth pattern | | |
| Exophytic | 23 | 20 |
| Infiltrative | 38 | 32 |
| Ulcerated or
mixed | 58 | 48 |
| Remnants | 9 | 7.5 |
| Coexisting
polyps | 11 | 9.0 |
| Combined polypoid
characteristicsa | 8 | 7.0 |
Based on these results and according to the
aforementioned criteria we classified 27% of the cases as
‘apparently polypoid’ (those with remnants, coexisting polyps
and/or exophytic pattern), 28.5% as potentially de novo
(infiltrative, always without remnants or coexisting polyps) and
44.5% as ‘of unclear origin’ (Fig.
2).
Associations of the examining variables
with clinicopathological characteristics
As shown in Table
II, remnants were more frequently detected among stage I tumors
compared to the other stages in combination (II–IV, 33 vs. 5.5%,
P=0.003) or individually (II, III and IV, P=0.01, 0.049 and 0.04,
respectively). The remnants also exhibited a trend towards
well-differentiation (22 vs. 6%, P=0.09). These were observed in a
markedly higher proportion in exophytic lesions compared to other
growth patterns (22 vs. 4%, P=0.015). Notably, this predilection
remained significant in the particular comparison of exophytic
tumors with infiltrative lesions (P=0.048), however, not with those
exhibiting an unclear pattern (P=0.07). The presence of coexisting
polyps was also more commonly recorded in stage I tumors. However,
this trend was not statistically significant. In addition, no
statistically significant difference was observed in the incidence
of polyps in the other clinicopathological categories.
 | Table II.Distribution of remnants and
co-existing polyps in various clinicopathological categories. |
Table II.
Distribution of remnants and
co-existing polyps in various clinicopathological categories.
| Remnants
| Polyps
|
|---|
| Category
(n)a | No. | Percentage | P-value | No. | Percentage | P-value |
|---|
| Growth pattern | | | | | | |
| Exophytic
(23) | 5 | 22 | 0.02b | 3 | 13 | NSb |
| Infiltrative
(38) | 1 | 2.6 | | 3 | 8 | |
| Ulcerated
(58) | 3 | 5 | | 5 | 8 | |
| Stage | | | | | | |
| I (12) | 4 | 33 | 0.003b | 3 | 25 | NS (0.14b) |
| II (50) | 2 | 4 | | 3 | 6 | |
| III (44) | 3 | 7 | | 2 | 4.5 | |
| IV (13) | - | 0 | | 3 | 23 | |
| Grade | | | | | | |
| Well (G1)
(7) | 2 | 29 | NS (0.09)b | - | 0 | NSb |
| Moderate (G2)
(103) | 6 | 6 | | 11 | 11 | |
| Poor (G3)
(9) | 1 | 11 | | - | 0 | |
| Site | | | | | | |
| Proximal
(36) | 3 | 8.5 | NSb | 2 | 5.5 | NSb |
| Distal (83) | 6 | 7.2 | | 9 | 11 | |
| Total (119) | 9 | 7.5 | | 11 | 9.2 | |
Moreover, remnants and polyps (considered together)
displayed clear predilection, mostly for stage I disease (58 vs.
12%, P<0.001) and, to a lesser extent, for exophytic pattern (35
vs. 12,5%, P=0.025). Also, while half of stage I tumors were
exophytic, the incidence of this growth pattern among stages II–IV
was only 16% (P=0.014). Overall, polypoid characteristics
(remnants, polyps and exophytic lesions) were detected in a
markedly higher proportion in stage I compared to stages II–IV (66
vs. 22%, P=0.003). In addition, tumors combining these
characteristics (exophytic lesions with remnants or polyps) were
predominantly found in stage I (42 vs. 2.8%, P<0.0001) (Fig. 3).
The distribution of growth pattern by stage, grade
and site is demonstrated in detail in Table III. Apart from the abovementioned
findings regarding stage, exophytic lesions exhibited a trend for
well-differentiation and a total lack of poor grade. However, the
two findings were not statistically significant. Infiltrative
lesions exhibited a tendency for proximal location (P=0.052)
becoming significant for tumors finally classified as de
novo (P=0.04). As shown in Fig.
4, the pattern of segmental distribution in nonpolypoid cases
was prominently different compared to polypoid and cases of unclear
origin.
 | Table III.Distribution of growth pattern by
stage, grade and site. |
Table III.
Distribution of growth pattern by
stage, grade and site.
| Exophytic
| Growth pattern
Infiltrative/ulceroinfiltrative
| Ulcerated and/or
mixed
|
|---|
| Category
(n)a | No. | Percentage | P-value | No. | Percentage | P-value | No. | Percentage | P-value |
|---|
| Stage | | | | | | | | | |
| I (12) | 6 | 50.0 | 0.014b | 3 | 25.0 | NSb | 3 | 25.0 | NSb |
| II (50) | 9 | 18.0 | | 14 | 28.0 | | 27 | 54.0 | |
| III (44) | 6 | 13.5 | | 16 | 36.5 | | 22 | 50.0 | |
| IV (13) | 2 | 15.5 | | 5 | 38.5 | | 6 | 46.0 | |
| Grade | | | | | | | | | |
| Well (7) | 3 | 43.0 | 0.1b | 2 | 28.5 | | 2 | 28.5 | NSb |
| Moderate
(103) | 20 | 19.5 | | 32 | 31.5 | | 51 | 50.0 | |
| Poor (9) | - | 0.0 | 0.13c | 4 | 44.5 | NSc | 5 | 55.5 | NSc |
| Site | | | | | | | | | |
| Proximal
(36) | 5 | 14.0 | NSb | 16 | 44.5 | 0.052b | 15 | 41.5 | NSb |
| Distal (83) | 18 | 21.5 | | 22 | 26.5 | | 43 | 52.0 | |
| Total (119) | 23 | 20.0 | | 38 | 32.0 | | 58 | 48.0 | |
Clinicopathological distribution of de
novo tumors
Table IV shows the
distribution of lesions classified as de novo by stage,
grade and site, exhibiting the aforementioned predilection for
proximal location as well as a higher (albeit not significantly)
detection rate in cases with poor grade or unfavorable
characteristics (stage IV or G3). However, the observed total
pattern of distribution of these lesions varying between 12.5%
(indolent cases) and 33% (unfavorable cases) clearly differed from
that observed for polypoid tumors, ranging from 62.5 to 19% in the
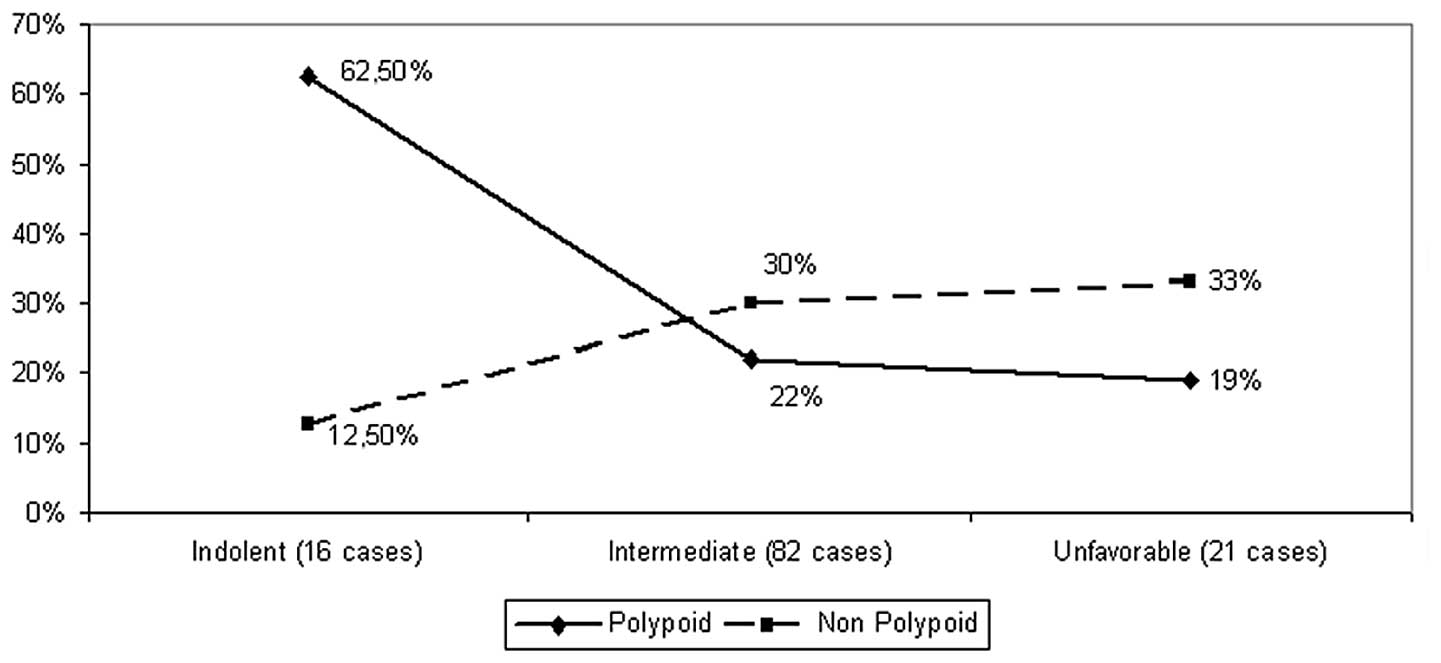
same categories (P=0.03, Fig. 5).
This disparity was more evident in the indolent subset
(P=0.008).
 | Table IV.Distribution of de novo tumors
in various clinicopathological categories. |
Table IV.
Distribution of de novo tumors
in various clinicopathological categories.
| De novo
lesions
|
|---|
| Category
(n)a | No. | Percentage | P-valueb |
|---|
| Stage | | | |
| I (12) | 2 | 17 | NSc |
| II (50) | 14 | 28 | |
| III (44) | 15 | 34 | |
| IV (13) | 3 | 23 | |
| Grade | | | |
| Well (G1)
(7) | 2 | 29 | NSd |
| Moderate (G2)
(103) | 28 | 27 | |
| Poor (G3)
(9) | 4 | 44 | |
| Site | | | |
| Proximal
(36) | 15 | 42 | 0.04e |
| Distal (83) | 19 | 23 | |
| Combined
stage-grade | | | |
| Indolent
(16) | 2 | 12.5 | NS (0.12)f |
| Intermediate
(82) | 25 | 30 | |
| Unfavorable
(21) | 7 | 33 | |
| Total (119) | 34 | 28.5 | |
Discussion
The aim of this study was to identify de novo
tumors on the basis of certain histomorphological criteria (lack of
remnants or coexisting polyps, nonpolypoid configuration). We also
examined their correlation with particular clinicopathological
characteristics (stage, grade and site). The study was conducted in
a sample mainly comprising ‘advanced’ CRC (i.e., tumors invading
beyond the submucosa), consistent with certain previous studies
(7,12,13,20,23),
although in contrast to most Japanese (5,6,10,18,19)
and certain Western studies (8,11)
focusing on early (T1) lesions.
Consistent with the findings reported by Chen et
al, the ascertained incidence of potentially de novo
tumors in our series was 28.5% (12). However, the incidence of such
lesions is widely varying (even within a given study) according to
the criteria occasionally used for their detection. In the
large-scale study by Bedenne et al(7)de novo tumors (defined as those
lacking remnants) were found in 40% of the cases, ranging from 17%
in small T1 lesions to almost 100% in tumors with an endophytic
(nonpolypoid) growth pattern. Largely diverging frequencies were
also reported by Shimoda et al(6) (25% in early and 80% in advanced
lesions). Moreover, in the study by Goto et al(10), the proportion of de novo
lesions was 23% in early (T1) CRC, increasing to 32% when small
advanced lesions were additionally included in the analyzed sample.
These discrepancies might be attributed in part to the difficulty
in the colonoscopic detection of early de novo lesions and
their potentially rapid growth, leading to their underestimation in
early CRC and, concurrently to a diagnostic delay (14,21)
resulting in their overrepresentation in advanced CRC.
The presence of infiltrative growth pattern seems to
be suggestive of de novo origin, as indicated by the rarity
in the appearance of remnants among infiltrative lesions (2.5%),
consistent with previous results (6,7).
However, a number of those may actually arise from adenomas, with
their gross configuration being altered into nonpolypoid during
their evolution (10,23), although this has been disputed
(6,13). Nevertheless, the effect of this bias
(if any) may be counteracted by the potentially simultaneous
presence of certain tumors with nonpolypoid route among cases
classified as of unclear origin (due to their extended
ulceration).
The low proportion (27%) of lesions with apparently
polypoid characteristics in our sample is probably not
representative of the actual incidence of CRC of adenoma origin,
although similar results have been occasionally reported (6,24).
Tumors of ‘unclear origin’, accounting for 44.5% of our cohort,
likely arise from polyps (for the most part) (23), as possibly suggested by their
predilection for distal tumor site (similar to polypoid and unlike
de novo lesions). Supportive of this assumption was also the
recorded high incidence of polypoid lesions in stage I (the
earliest disease cases in our sample), 67% consistent with findings
of a previous study in a similar subset (T2 lesions) (25). Moreover, this tendency towards
earlier stage (considerably stronger for cases combining polypoid
characteristics) is possibly indicative of more favorable behavior
and/or easier colonoscopic detection of polypoid lesions and should
be investigated in larger samples.
Consistent with results of previous studies
(7,16), we found lesions possibly developing
de novo, in a significantly higher proportion among proximal
tumors. This trend was particularly associated with the
infiltrative growth pattern, more frequently observed proximally.
Given the known clinicopathological and molecular differences of
proximal CRC (4,16,26,27),
the recorded link between nonpolypoid lesions and proximal location
supports the assumption of a different tumorigenic mechanism for
de novo CRC. Moreover, this association suggests a higher
malignant potential of de novo tumors, anticipated to the
corresponding clinicopathological pattern reported for proximal
tumors [higher stage and grade, worse outcome (16,27,28)].
The recorded rarity of de novo lesions in the indolent
subset is also supportive of this state and consistent with
previous findings (20,29). It is also potentially correlated
with the proximal predilection of these tumors, considering the
milder clinical manifestations and the lower efficacy of
colonoscopy observed in proximal CRC, resulting in later stage
presentation (16,28).
From the clinical aspect, these results require more
awareness and persistence in the colonoscopic detection of
nonpolypoid lesions (particularly in the proximal segment), more
intensive surveillance of the colonoscopically treated cases and
surgical treatment for selected patients. These suggestions are
also applicable for cancers alternatively evolving through a
corresponding nonpolypoid adenoma, instead of direct de novo
development (14). The clinical
significance of nonpolypoid lesions, emphasized in recent studies
(21,30), is expected to be further elucidated
by the ongoing prospective Japan Polyp Study (31), evaluating CRC surveillance
strategies after initial colonoscopic removal of the detected
neoplasia.
To the best of our knowledge, the current study is
the first using the presence of coexisting polyps as a
discriminative criterion between polypoid and nonpolypoid CRC. This
application was based on the ‘field cancerization theory’,
involving the dissemination of genetic changes characteristic of
the primary tumor over a wide distance from the edges of the lesion
(15,32,33).
Therefore, coexisting polyps, likely originating from the same
changes, are indicative of a polypoid origin of the primary tumor.
Additional investigation of the molecular alterations potentially
occurring in the seemingly healthy colonic mucosa near the tumor is
necessary to determine the exact distance required for the definite
characterization of a given polyp as actually correlated with the
primary lesion.
A limitation to our study was the relatively small
sample size [although comparable to other relevant studies
(10,11,13,20,25)],
potentially affecting results, particularly in subset analysis.
Another limitation could be the almost complete lack of small
and/or T1 lesions in our samples, potentially hampering de
novo identification. However, such investigation in tumors
invading beyond the submucosa (as we did), was also conducted by
other scientists (7,12,13,20,25),
reporting frequencies of de novo lesions ranging from 11 to
40%. Our findings (28.5%) were within this range. Additionally,
consistent with our findings, the incidence of nonpolypoid small T1
tumors has been found to be markedly low (<5%) in previous
studies (9,11,16,34),
suggesting a rather limited utility of the particular criteria for
de novo identification (particularly among CRC referred for
surgery).
In conclusion, our study was suggestive of a
potential de novo origin for a considerable proportion of
CRC, concurrently indicating the predilection of these lesions for
proximal tumor location and introducing an additional approach
(that of coexisting polyps) potentially facilitating de novo
identification. The clinical impact of our findings should be
examined, especially with regard to possible associations between
de novo lesions and important prognostic variables. The
tendency for a worse behavior of nonpolypoid lesions, as suggested
by their increasing incidence with disease progression and
aggressiveness may necessitate adequate readjustments in the
diagnosis, treatment and surveillance of these cases.
Acknowledgements
The authors would like to thank Dr F.
Georgiadis for his help, Mrs. N. Vathi and Mr. V. Anthoulis for
their valuable assistance in the preparation of the manuscript.
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics. CA Cancer J Clin.
58:71–96. 2008.
|
|
2.
|
Morson B: President’s address. The
polyp-cancer sequence in the large bowel. Proc R Soc Med.
67:451–457. 1974.
|
|
3.
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 6:759–767. 1990.
View Article : Google Scholar
|
|
4.
|
Jass JR: Classification of colorectal
cancer based on correlation of clinical, morphological and
molecular features. Histopathology. 50:113–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kuramoto S and Oohara T: Minute cancers
arising de novo in the human large intestine. Cancer. 61:829–834.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shimoda T, Ikegami M, Fujisaki J, Matsui
T, Aizawa S and Ishikawa E: Early colorectal carcinoma with special
reference to its development de novo. Cancer. 64:1138–1146. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bedenne L, Faivre J, Boutron MC, Piard F,
Cauvin MJ and Hillon P: Adenoma-carcinoma sequence or de
novo carcinogenesis? A study of adenomatous remnants in
population-based series of large bowel cancers. Cancer. 69:883–888.
1992.PubMed/NCBI
|
|
8.
|
Stolte M and Bethke B: Colorectal mini-de
novo carcinoma: a reality in Germany too. Endoscopy. 27:286–290.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mueller JD, Bethke B and Stolte M:
Colorectal de novo carcinoma: a review of its diagnosis,
histopathology, molecular biology, and clinical relevance. Virchows
Arch. 440:453–460. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Goto H, Oda Y, Murakami Y, et al:
Proportion of de novo cancers among colorectal cancers in Japan.
Gastroenterology. 131:40–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hornic J, Farraye F and Odze R:
Clinicopathologic and immunohistochemical study of small apparently
‘de novo’ colorectal adenocarcinomas. Am J Surgh Pathol.
31:207–215. 2007.
|
|
12.
|
Chen CD, Yen MF, Wang WM, Wong JM and Chen
TH: A case-cohort study for the disease natural history of
adenoma-carcinoma and de novo carcinoma and surveillance of colon
and rectum after polypectomy: implication for efficacy of
colonoscopy. Br J Cancer. 88:1866–1873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kaneko K, Kurahashi T, Makino R, et al:
Pathological features and genetic alterations in colorectal
carcinomas with characteristics of nonpolypoid growth. Br J Cancer.
91:312–318. 2004.PubMed/NCBI
|
|
14.
|
Kashida H and Kudo S: Early colorectal
cancer: concept, diagnosis, and management. Int J Clin Oncol.
11:1–8. 2006. View Article : Google Scholar
|
|
15.
|
Tanaka T: Colorectal carcinogenesis:
review of human and experimental animal studies. J Carcinog.
8:52009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nawa T, Kato J, Kawamoto H, et al:
Differences between right-and left-sided colon cancer in patient
characteristics, cancer morphology and histology. J Gastroenderol
Hepatol. 23:418–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ogawa T, Yoshida T, Tsuruta T, Saigenji K
and Okayasu I: Genetic instability on chromosome 17 in the
epithelium of non-polypoid colorectal carcinomas compared to
polypoid lesions. Cancer Sci. 97:1335–1342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nosho K, Yamamoto H, Takahashi T, et al:
Genetic and epigenetic profiling in early colorectal tumors and
prediction of invasive potential in pT1 (early invasive) colorectal
cancers. Carcinogenesis. 28:1364–1370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yasugi A, Yashima K, Hara A, et al: Fhit,
Mlh1, p53 and phenotypic expression in the early stage of
colorectal neoplasms. Oncol Rep. 19:41–47. 2008.PubMed/NCBI
|
|
20.
|
Nasir A, Boulware D, Kaiser H, et al: Flat
and polypoid adenocarcinomas of the colorectum: a comparative
histomorphologic analysis of 47 cases. Hum Pathol. 35:604–611.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Soetikno R, Kaltenbach T, Rouse R, et al:
Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms
in asymptomatic and symptomatic adults. JAMA. 299:1027–1035. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yasutomi M, Baba S and Hojo K: Japanese
classification of colorectal cancinoma. Kanehara & Co, Ltd;
Tokyo: 1997
|
|
23.
|
Matsui T, Yao T and Iwashita A: Natural
history of early colorectal cancer. World J Surg. 24:1022–1028.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Nakamura K: De novo cancer and
adenoma-carcinoma sequence of the colorectum-clinicopathological
differences between de novo carcinoma and carcinoma with the
sequence. Nihon Geka Gakkai Zaasshi. 100:766–775. 1999.(In
Japanese).
|
|
25.
|
Goi T, Kawasaki M, Hirono Y, Katayama K
and Yamaguchi A: Clinicopathological analysis of invading
muscularis propria (T2) cancers ≤20 mm in diameter. Int Surg.
93:1–5. 2008.
|
|
26.
|
Sugai T, Habano W, Jiao YF, Tsukahara M,
Takeda Y, Otsuka K and Nakamura S: Analysis of molecular
alterations in left- and right-sided colorectal carcinomas reveals
distinct pathways of carcinogenesis. J Mol Diagn. 8:193–201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Papagiorgis PC, Zizi AE, Tseleni S, et al:
Site impact on colorectal cancer biological behavior in terms of
clinicopathological and molecular features. J BUON. 16:84–92.
2011.PubMed/NCBI
|
|
28.
|
Wong R: Proximal tumors are associated
with greater mortality in colon cancer. J Gen Intern Med.
25:1157–1163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kurisu Y, Shimoda T, Ochiai A, Nakanishi
Y, Hirata I and Katsu KI: Histologic and immunohistochemical
analysis of early submucosal invasive carcinoma of the colon and
rectum. Pathol Int. 49:608–616. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Matsuda T, Saito Y, Hotta K, Sano Y and
Fujii T: Prevalence and clinicopathological features of nonpolypoid
colorectal neoplasms: should we pay more attention to indentifying
flat and depressed lesions? Dig Endoscopy. 22:S57–S62. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Sano Y, Fujii T, Oda Y, et al: A
multicenter randomized controlled trial designed to evaluate
follow-up surveillance strategies for colorectal cancer: the Japan
Polyp Study. Dig Endoscop. 16:376–378. 2004. View Article : Google Scholar
|
|
32.
|
Dakubo GD, Jakupciak JP, Birch-Machin MA
and Parr RL: Clinical implications and utility of field
cancerization. Cancer Cell Int. 7:22007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Aivado M, Gynes M, Golerov V, Schmidt WU,
Röher HD and Goretzki PE: ‘Field cancerization’ - an additional
phenomenon in development of colon tumors? K-ras codon mutations in
normal colonic mucosa of patients with colorectal neoplasms.
Chirurg. 71:1230–1234. 2000.(In German).
|
|
34.
|
Weil R, Ohana G, Haplern M, Estlein D,
Anvi A and Wolloch Y: Small nonpolypoid colorectal carcinoma. World
J Surg. 26:503–508. 2002. View Article : Google Scholar : PubMed/NCBI
|