Introduction
Cystatin C (Cys C) is a non-glycosylated cationic
13.3-kDa protein belonging to the cystatin superfamily of cysteine
protease inhibitors (1–3). Cys C is produced by nucleated cells
and is secreted into the blood at a constant rate (1–3). It is
freely filtered through the glomerular membrane, completely
re-absorbed and then catabolized in the proximal tubular cells
(1–3). Thus, similarly to creatinine, the
biological fate of Cys C is a good endogenous marker of the
glomerular filtration rate (GFR).
In patients with esophageal cancer, cisplatin (CDDP)
is used as a neoadjuvant or as a post-operative adjuvant
chemotherapy in combination with continuous infusion of
5-fluorouracil (5-FU) (4,5). When CDDP-based chemotherapy is
administered, antiemetic drugs, such as dexamethasone (DEX),
5-HT3 serotonin receptor antagonists or aprepitant are
administered to prevent treatment-associated nausea and vomiting
(6). A transient elevation was
previously reported in serum Cys C concentration during the
perioperative chemotherapy period in patients with esophageal
cancer. We suggested that renal function estimates determined on
the basis of serum Cys C levels during this treatment period might
be misleading (7).
To understand the effect of DEX and other drugs in
detail, it is crucial to investigate the renal effects associated
with serum Cys C concentration. The aim of this study was to
investigate the ability of DEX to induce Cys C secretion in human
cancer cell lines, as well as the effect of CDDP, 5-FU and
mifepristone (RU-486) on Cys C secretion.
Materials and methods
Materials
DEX, 5-FU and CDDP were purchased from Wako Pure
Chemical Industries, Ltd. (Osaka, Japan). RU-486 was purchased from
Sigma-Aldrich (St. Louis, MO, USA), while
2-(4-iodophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, mono-sodium
salt (WST-1) and 1-methoxy-5-methylphenazinium methyl sulfate were
purchased from Dojindo Laboratories (Kumamoto, Japan). The
remaining reagents were of the highest grade commercially available
for biochemical use.
Cell lines and cell culture
The KYSE150 human esophageal squamous cell
carcinoma, A549 human non-small cell lung cancer and the Caki-2
human renal carcinoma cell lines were used in this study. KYSE150
and A549 cells were obtained from the Health Science Research
Resources Bank (Osaka, Japan), and Caki-2 cells were obtained from
Summit Pharmaceuticals International (Tokyo, Japan). KYSE150, and
Caki-2 cells were maintained in RPMI-1640 medium (Invitrogen,
Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (Invitrogen),
100 U/ml penicillin G and 100 μg/ml streptomycin (Invitrogen). A549
cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM;
Invitrogen) supplemented with 10% heat-inactivated FBS, 100 U/ml
penicillin G and 100 μg/ml streptomycin. The cells were seeded in
culture flasks, cultured in a humidified atmosphere of 5%
CO2-95% air at 37°C, and subcultured with 0.05%
trypsin-0.02% EDTA (Invitrogen).
Enzyme-linked immunosorbent (ELISA) assay
for Cys C
For the quantification of Cys C protein released
from the cells into the culture medium, KYSE150, A549 and Caki-2
cells were seeded in 60-mm dishes at 1×106 cells/dish
and incubated overnight prior to treatment with each drug for the
indicated periods. The final concentrations of the drugs during
exposure were 100 nM, 10, 2 and 1 μM for DEX, CDDP, 5-FU and
RU-486, respectively. The concentrations of DEX, CDDP and 5-FU were
set to mimic clinical conditions (7–9). To
examine the inhibitory effects on DEX-induced Cys C secretion,
CDDP, 5-FU and RU-486 were added to the culture medium containing
DEX at the abovementioned concentrations for each drug. Control
cells were incubated with the culture medium without drugs in each
experiment. The culture medium was collected and analyzed with the
Quantikine® Human Cystatin C Immunoassay kit (R&D
Systems, Inc., Minneapolis, MN, USA), according to the
manufacturer’s instructions. The cells were rinsed twice with
phosphate-buffered saline (PBS) and harvested with lysis buffer
(Sigma-Aldrich). Cell lysates were then vortexed at room
temperature for 15 min, and centrifuged at 13,000 × g at room
temperature for 15 min. Cell lysates were assayed for total protein
levels by using the bicinchoninic acid (BCA) Protein assay kit
(Sigma-Aldrich) to adjust Cys C levels. The culture media and cell
lysates were stored at −20°C until the ELISA and BCA assays were
performed.
WST-1 colorimetric assay
The WST-1 assay was used to evaluate the effect of
DEX, CDDP and 5-FU on KYSE150 cell viability (10). The cells were seeded in 96-well
plates and pre-cultured for 24 h. The medium was exchanged with one
containing each drug at various concentrations. Cells were then
incubated for 72 h at 37°C. The culture medium was replaced with a
medium containing a WST-1 reagent, and 3 h later the absorbance in
the well was determined at 450 nm with a reference wavelength of
630 nm using a microplate reader (SpectraFluor™; Tecan, Maennedorf,
Switzerland).
Statistical analysis
The data for Cys C protein release in samples
treated with the indicated drugs were expressed as a percentage of
the data obtained from the control. Data are presented as the means
± standard error (SE) of the results of at least three independent
experiments. Statistical analyses were performed using the
Tukey-Kramer test. P<0.05 (two-tailed) was considered to
indicate a statistically significant difference.
Results
Effect of DEX on Cys C release into
culture medium
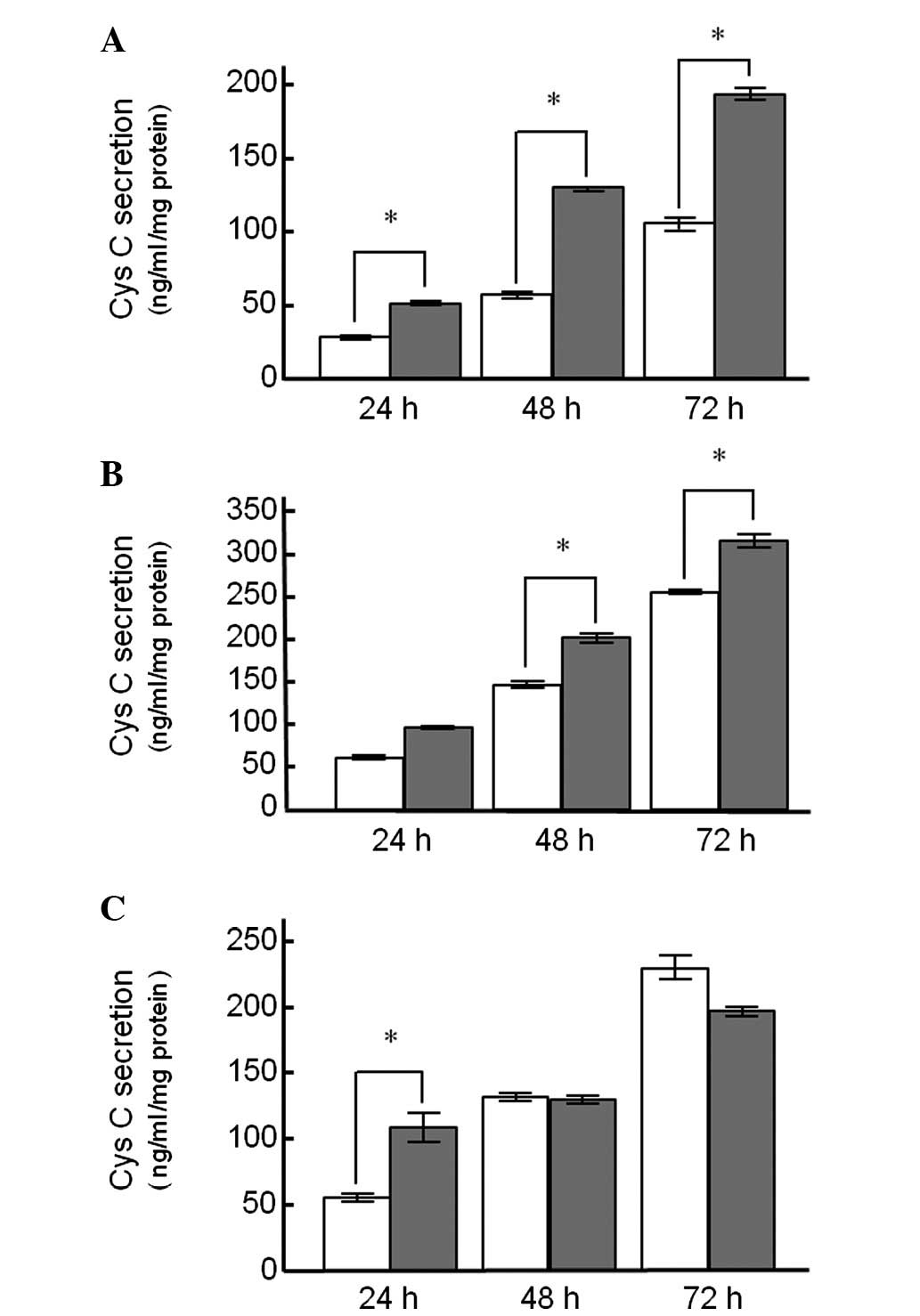
Fig. 1 shows the Cys
C protein release from the KYSE150, A549 and Caki-2 cell lines
treated with DEX. In the cell lines treated with DEX alone, there
was a time-dependent increase in Cys C release into the medium
(Fig. 1). Cys C release from
KYSE150 cells treated with DEX for 24 h was significantly higher
compared to the control cells (50.3±2.5 and 27.8±1.4 ng/ml/mg
protein, respectively), and a statistically significant difference
between DEX-treated and untreated control cells was observed up to
72 h after treatment (Fig. 1A).
Similar findings were also observed in A549 cells (Fig. 1B). However, Cys C release from
Caki-2 cells following treatment with DEX for 24 h was
significantly different from the control group, whereas this
difference was not observed for the 48- and 72-h treatment groups
(Fig. 1C).
Effects of concurrent drug treatment on
DEX-induced Cys C release
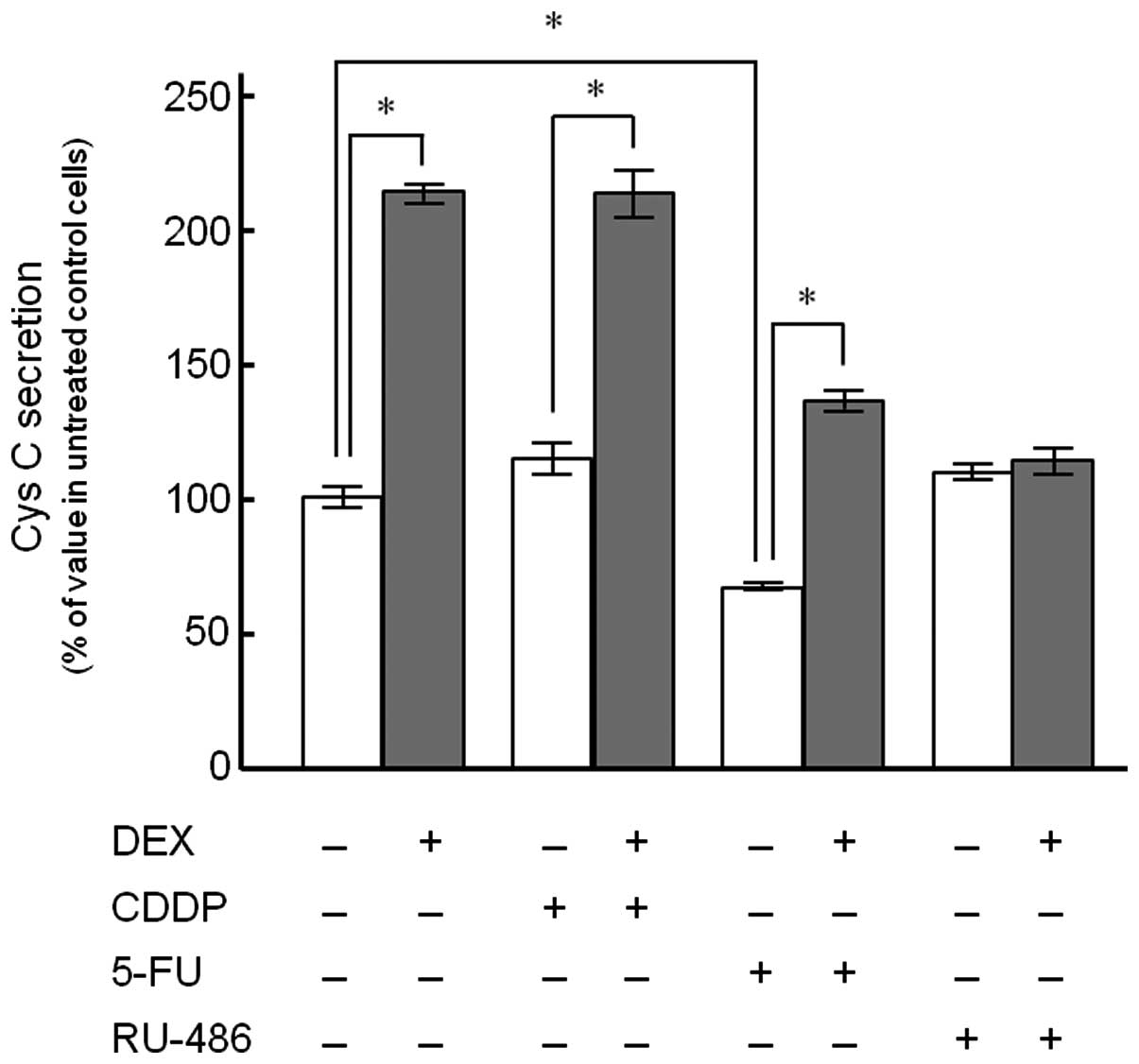
Cys C release from KYSE150 cells following treatment
with CDDP, 5-FU and RU-486 alone or in combination with DEX was
detected (Fig. 2). The cells
treated with 100 nM DEX for 72 h demonstrated a 2.1-fold increase
in Cys C release compared to the control. DEX significantly
enhanced Cys C release up to 1.9- and 2.0-fold in the presence of
10 μM CDDP and 2 μM 5-FU, respectively, whereas no such effect was
observed in the presence of RU-486 (1 μM). Treatment with 5-FU
alone significantly decreased Cys C release (66.5±4.0%) compared to
the control, although CDDP and RU-486 had no significant inductive
or suppressive effects when administered alone.
Cell viability analysis
The cytotoxic effect of DEX, CDDP and 5-FU in
KYSE150 cells was examined using the WST-1 assay. No cytotoxicity
was observed (Fig. 3A) following
incubation of KYSE150 cells with DEX at the concentration used in
the present experiments (100 nM) for 72 h. KYSE150 cells were also
exposed to CDDP or 5-FU (Fig. 3B and
C). Each drug reduced cell viability in a
concentration-dependent manner, and the number of viable cells at 2
μM 5-FU and 10 μM CDDP was ∼60% of the control cells.
Discussion
Treatment with DEX induced Cys C release into the
culture medium in the cell lines used in this study (Fig. 1), a fact suggesting that DEX
treatment partly contributes to the elevation in serum Cys C
concentration observed during chemotherapy in esophageal cancer
patients (7). Co-treatment of DEX
with CDDP or 5-FU demonstrated higher extracellular secretion of
Cys C, compared to the values observed in the cells treated with
the anticancer drug alone, while a synergistic effect between the
drugs was not observed (Fig. 2).
Regarding the effect of DEX on Cys C production, Bjarnadóttir et
al(11) reported that Cys C
expression and secretion from HeLa cells into tissue culture medium
increased following treatment with dexamethasone and suggested an
association with the Cys C promoter in transcription of the Cys C
gene (11). In this study, we
examined the effect of RU-486, a glucocorticoid receptor
antagonist, on the enhanced Cys C release from KYSE150 cells
induced by DEX. The results showed that RU-486 almost completely
suppressed Cys C release from the cells treated with DEX, probably
due to the inhibition of the transcriptional regulation mediated by
steroid receptors (Fig. 2).
Additionally, CDDP and 5-FU induced apoptosis, whereas the
inhibition of apoptosis by DEX promoted proliferation in various
established and primary cancer cells (12). The enhanced secretion of Cys C
induced by co-treatment with DEX might be correlated with the
inhibition of apoptosis as well as the abovementioned
transcriptional regulation. Corticosteroids are widely used in
cancer as well as immunotherapy, while the potential to
underestimate normal renal function during these therapies is of
marked importance.
Notably, the secretion of Cys C was significantly
decreased following 5-FU treatment alone compared to the control,
whereas no such effect was observed in CDDP treatment alone
(Fig. 2). The difference in Cys C
secretion is potentially due to a difference in cytotoxicity
between CDDP and 5-FU. When assessing the cytotoxic effects of CDDP
and 5-FU in KYSE150 cells using the WST-1 assay, treatment with
either drug at the concentrations used in the present study reduced
cell viability by ∼60% of their respective control values (Fig. 3B and C). The decreased cell
viability was not specific to the 5-FU treatment. However, the
correlation between cytotoxicity and reduced Cys C secretion was
not fully elucidated.
According to the available literature, the Cys C
housekeeping gene is constantly expressed by most nucleated cell
types (13), while extracellular
cystatins are broadly distributed and detected in most body fluids
(14). Cys C is a cysteine protease
inhibitor that targets cathepsins (15). Exposure to 5-FU has been reported to
result in cleavage of cathepsin B and caspases in human colon
carcinoma cell lines, while cathepsin B activation has been
reported to contribute to 5-FU-induced apoptosis (16). Additionally, autophagy is believed
to be crucially involved in the suppression of tumorigenesis
(17), with 5-FU activating
autophagic survival as well as apoptotic cell death (16). Although Cys C has been demonstrated
to affect basal autophagy in neuronal cells under normal culture
conditions and its deficiency suppresses autophagy (18), the reduced secretion of Cys C by
5-FU treatment observed in the present study may contribute to the
acceleration of apoptotic cell death. Furthermore, necrosis is a
key pathway in non-apoptotic cell death (17). The balance of apoptotic and
non-apoptotic cell death varies among types of esophageal and
colorectal cancer cell lines (16,19).
Additionally, when treated with CDDP and 5-FU, certain cell lines
show predominantly apoptotic cell death morphology, while others
exhibit predominantly non-apoptotic morphology (19). Although it remains unclear to what
extent apoptotic and non-apoptotic cell death were induced in the
cells used in the present study, the extent of cytotoxicity might
be correlated with the difference in the extracellular secretion of
Cys C between treatments and cell types. Flow cytometry is required
to address these issues in the future.
Circadian variations in physiological and behavioral
processes are affected by several endogenous and exogenous factors.
DEX has been reported to induce transient changes in the phase of
circadian gene expression in peripheral tissues (20), while 5-FU has been demonstrated to
have the ability to inhibit oscillation in the expression of clock
genes (21). However, to what
extent DEX-induced circadian gene expression affects extracellular
Cys C secretion in esophageal cancer patients remains unclear.
Moreover, is it not clear to what degree the in vitro
reduction in Cys C secretion induced by treatment with 5-FU
contributes to changes in systemic Cys C concentration. Further
investigation concerning the effects of DEX and 5-FU on the cycle
of extracellular Cys C secretion is required to clarify the
molecular mechanisms underlying the transient elevation of serum
Cys C concentrations observed in our previous clinical study.
Acknowledgements
This study was supported in part by a
Grant-in-Aid for Young Scientists (B) and a Grant-in-Aid for
Encouragement of Scientists from the Japan Society for the
Promotion of Science.
References
|
1.
|
Filler G, Bökenkamp A, Hofmann W, Le
Bricon T, Martinez-Brü C and Grubb A: Cystatin C as a marker of GFR
- history, indications, and future research. Clin Biochem. 38:1–8.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Newman DJ: Cystatin C. Ann Clin Biochem.
39:89–104. 2002. View Article : Google Scholar
|
|
3.
|
Chew JS, Saleem M, Florkowski CM and
George PM: Cystatin C - a paradigm of evidence based laboratory
medicine. Clin Biochem Rev. 29:47–62. 2008.PubMed/NCBI
|
|
4.
|
Ando N, Iizuka T, Ide H, et al: Surgery
plus chemotherapy compared with surgery alone for localized
squamous cell carcinoma of the thoracic esophagus: a Japan Clinical
Oncology Group Study-JCOG9204. J Clin Oncol. 21:4592–4596. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ando N, Kato H, Igaki H, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar
|
|
6.
|
American Society of Clinical Oncology;
Kris MG, Hesketh PJ, Somerfield MR, et al: American Society of
Clinical Oncology guideline for antiemetics in oncology: update
2006. J Clin Oncol. 24:2932–2947. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kume M, Yasui H, Yoshikawa Y, et al:
Transient elevation of serum cystatin C concentrations during
perioperative cisplatin-based chemotherapy in esophageal cancer
patients. Cancer Chemother Pharmacol. 69:1537–1544. 2012.
View Article : Google Scholar
|
|
8.
|
Miki I, Tamura T, Nakamura T, et al:
Circadian variability of pharmacokinetics of 5-fluorouracil and
CLOCK T3111C genetic polymorphism in patients with esophageal
carcinoma. Ther Drug Monit. 27:369–374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nakade S, Ohno T, Kitagawa J, et al:
Population pharmacokinetics of aprepitant and dexamethasone in the
prevention of chemotherapy-induced nausea and vomiting. Cancer
Chemother Pharmacol. 63:75–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Takara K, Fujita M, Minegaki T, et al:
Treatment schedule-dependent effect of 5-fluorouracil and platinum
derivatives in colorectal cancer cells. Eur J Pharm Sci.
45:272–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bjarnadóttir M, Grubb A and Olafsson I:
Promoter-mediated, dexamethasone-induced increase in cystatin C
production by HeLa cells. Scand J Clin Lab Invest. 55:617–623.
1995.PubMed/NCBI
|
|
12.
|
Zhang C, Beckermann B, Kallifatidis G, et
al: Corticosteroids induce chemotherapy resistance in the majority
of tumour cells from bone, brain, breast, cervix, melanoma and
neuroblastoma. Int J Oncol. 29:1295–1301. 2006.
|
|
13.
|
Abrahamson M, Olafsson I, Palsdottir A, et
al: Structure and expression of the human cystatin C gene. Biochem
J. 268:287–294. 1990.PubMed/NCBI
|
|
14.
|
Abrahamson M, Barrett AJ, Salvesen G and
Grubb A: Isolation of six cysteine proteinase inhibitors from human
urine. Their physicochemical and enzyme kinetic properties and
concentrations in biological fluids. J Biol Chem. 261:11282–11289.
1986.
|
|
15.
|
Grzonka Z, Jankowska E, Kasprzykowski F,
et al: Structural studies of cysteine proteases and their
inhibitors. Acta Biochim Pol. 48:1–20. 2001.PubMed/NCBI
|
|
16.
|
Bijnsdorp IV, Peters GJ, Temmink OH,
Fukushima M and Kruyt FA: Differential activation of cell death and
autophagy results in an increased cytotoxic potential for
trifluorothymidine compared to 5-fluorouracil in colon cancer
cells. Int J Cancer. 126:2457–2468. 2010.
|
|
17.
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar
|
|
18.
|
Tizon B, Sahoo S, Yu H, et al: Induction
of autophagy by cystatin C: a mechanism that protects murine
primary cortical neurons and neuronal cell lines. PLoS One.
5:e98192010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
O’Donovan TR, O’Sullivan GC and McKenna
SL: Induction of autophagy by drug-resistant esophageal cancer
cells promotes their survival and recovery following treatment with
chemotherapeutics. Autophagy. 7:509–524. 2011.
|
|
20.
|
Balsalobre A, Brown SA, Marcacci L, et al:
Resetting of circadian time in peripheral tissues by glucocorticoid
signaling. Science. 289:2344–2347. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Terazono H, Hamdan A, Matsunaga N, et al:
Modulatory effects of 5-fluorouracil on the rhythmic expression of
circadian clock genes: a possible mechanism of chemotherapy-induced
circadian rhythm disturbances. Biochem Pharmacol. 75:1616–1622.
2008. View Article : Google Scholar
|