Introduction
Metabolic syndrome (MS), an extremely frequent
condition characterized by dyslipidemia, insulin resistance,
abdominal obesity and hypertension, is associated with an elevated
risk of cardiovascular events. Of the risk factors for MS, high
blood pressure is the most significant, since hypertension is
closely associated with insulin resistance and dyslipidemia.
Angiotensin (Ang) type I receptor (AT1R) blockers (ARBs) have been
widely used in the treatment of hypertension and
hypertension-related cardiovascular end-organ damage (1,2), since
treatment with ARBs is known to improve the clinical manifestations
of MS. However, new antihypertensive drugs should be designed to
affect cell and biochemical mechanisms contributing to increased
blood pressure and to also address disordered lipid metabolism in a
more favorable manner.
To this end, peroxisome proliferator-activated
receptor-γ (PPARγ) is in the center of interest, since ligands for
PPARγ improve insulin sensitivity, reduce triglyceride levels and
decrease the risk for atherosclerosis (3–5).
Notably, among the approved ARBs, irbesartan and telmisartan were
demonstrated to constitute a unique subset of ARBs that are also
capable of activating PPARγ (2,6).
Findings by Schupp et al(2)
demonstrated that irbesartan and telmisartan could be considered
partial and selective PPAR modulators (SPPARMs) (2,7). The
SPPARM approach has also been suggested to be a method to avoid
unwanted complications of PPARγ ligands, such as obesity and edema
(7). In patients with MS,
irbesartan markedly reduced blood pressure, and was associated with
a reduction in cardiovascular risk factors, such as high-density
lipoprotein (HDL) cholesterol, serum triglyceride, fasting blood
glucose and waist circumference (8). Beneficial therapeutic effects of
irbesartan in hypertensive patients with MS might be mediated via
the AT1 receptor antagonistic and partial PPARγ agonistic actions.
Based upon these observations, ARBs with partial PPARγ agonistic
activity, such as irbesartan are now termed ‘metabosartans’.
Hepatocyte growth factor (HGF) was previously shown to be a
downstream effector of PPARγ agonists (9). Thus, in this study, we investigated
the effects of a metabosartan, irbesartan, on fatty liver and the
hypertrophy of adipocytes in apolipoprotein E (ApoE) knockout (KO)
mice.
Materials and methods
Animals, diets and drug treatment
ApoE KO mice were used as a murine model exhibiting
fatty liver and hyperlipidemia, which are typical phenotypes of MS.
The experiments carried out in animals were performed in accordance
with guidelines laid down by the Ethics Committee for Animal
Experiments of the Osaka University Graduate School of Medicine
(Osaka, Japan). Male ApoE KO mice with a C57/BL6 background were
obtained from the Jackson Laboratory (Bar Harbor, ME, USA).
Irbesartan was donated by Shionogi Pharma, Inc. (Osaka, Japan). HGF
neutralizing antibody was purchased from Kringle Pharma, Inc.
(Osaka, Japan). GW9662 was purchased from Cayman Chemical Company
(Ann Arbor, MI, USA).
Ten-week old mice (n=6 per group) were fed a
high-fat diet (HFD) (MF plus 0.5% (wt/wt) cholesterol and 10% yashi
oil; Oriental Yeast Co., Ltd., Tokyo, Japan). Mice were divided
into four groups: i) the control group, ApoE KO mice with HFD only;
ii) the irbesartan group, ApoE KO mice with HFD and 5 mg/kg/day
irbesartan; iii) the HGF antibody group, ApoE KO mice with HFD, 5
mg/kg/day irbesartan and 200 μg/week HGF neutralizing antibody and
iv) the GW9662 group, ApoE KO mice with HFD, 5 mg/kg/day irbesartan
and 0.5 mg/kg/day GW9662 (Fig. 1).
Drugs were dissolved in water and administered ad libitum.
There were 6 mice/group, which were housed in the animal facilities
of the Osaka University (Osaka, Japan). The mice had free access to
water and food during the experimental period. Following three
months of drug and HFD treatment, the mice were sacrificed. Samples
of epididymal adipose tissue and liver were evaluated.
Measurement of HGF and adiponectin
HGF concentration was measured by an enzyme-linked
immunosorbent assay (ELISA), using an IMMUNIS mouse HGF ELISA kit
(Institute of Immunology Co., Ltd., Tokyo, Japan). Mouse liver
samples were disintegrated with IMMUNIS HGF extraction buffer,
using a Multi-beads shocker (Yasui Kikai, Osaka, Japan) at 2000 × g
for 15 sec. Homogenates were centrifuged at 14,000 × g for 30 min.
The supernatant was used for the HGF assay, according to the
manufacturer’s instructions. Serum adiponectin level was also
measured using an ELISA kit (Otsuka Pharmaceuticals, Co., Ltd.,
Tokyo, Japan).
Immunohistochemical staining
Tissues fixed with 4% formalin and embedded in
paraffin were subjected to immunohistochemical staining, as
described previously (10).
Immunostaining of F4/80 was performed using anti-mouse F4/80
antibody (ab6640; Abcam, Cambridge, UK). Immunostained images were
quantified using the NIH Image J software (http://rsb.info.nih.gov/ij/) and then analyzed
visually under a light microscope by two investigators blinded to
treatment.
Statistical analysis
Data were expressed as the mean ± standard error of
the mean (SEM). Comparisons were made using analysis of variance
(ANOVA) followed by Tukey’s simultaneous multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of irbesartan on adipose tissue
in ApoE KO mice
Administration of irbesartan at 5 mg/kg/day for 12
weeks lowered the epididymal adipose tissue weight. Histological
examination showed a smaller adipocyte size and crown-like
structures (formed by the gathering of macrophages), as detected by
F4/80 (a macrophage marker)-positive areas in the
irbesartan-treated group (Fig. 2A).
Consistent with the histo-logical findings, adipocyte diameter was
markedly decreased by irbesartan (Fig.
2B) and the F4/80-positive areas were also markedly decreased
by irbesartan (Fig. 2C). These
beneficial effects of irbesartan were attenuated by treatment with
anti-HGF antibody or an inhibitor of PPARγ, GW9662.
 | Figure 2.Effects of irbesartan, GW9662 and
hepatocyte growth factor neutralizing antibody (HGF-Ab) on F4/80
staining and adipocyte diameter in adipose tissue. (A) Typical
micrographs of immunostaining for F4/80 in periodic cross-sections
of epididymal adipose tissue after 12 weeks of high-fat diet (HFD).
Brown-stained area shows F4/80 protein-positive area; (a) control
(HFD only), (b) HFD + irbesartan (5 mg/kg/day), (c) HFD +
irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day) and (d) HFD
+ irbesartan + neutralizing antibody against HGF (200 μg/week);
bar, 100 μm. (B) Adipocyte diameter (μm). (C) Quantitative data of
immunohistochemical staining for macrophages (F4/80) (%). Control,
HFD only; (−), HFD + irbesartan (5 mg/kg/day); GW9662, HFD +
irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day); HGF-Ab,
HFD + irbesartan + neutralizing antibody against HGF (200 μg/week).
*P<0.01 vs. control, †P<0.05 vs.
irbesartan only. Data are shown as the mean ± standard error of the
mean. PPARγ; peroxisome proliferators activated receptor-γ. |
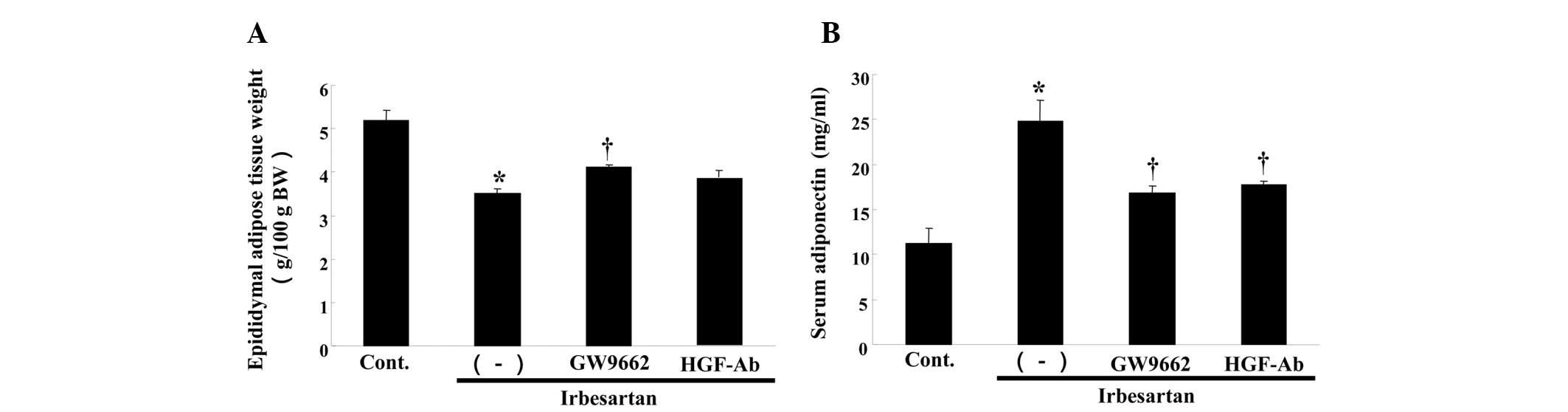
Irbesartan also reduced the weight of epididymal
adipose tissue (Fig. 3A). This
effect was also attenuated by anti-HGF antibody or GW9662 treatment
(Fig. 3A). Notably, irbesartan
significantly increased the serum adiponectin level, while anti-HGF
antibody or GW9662 treatment decreased the serum adiponectin level
(Fig. 3B).
Effect of irbesartan on fatty liver in
ApoE KO mice
In this model, the control group demonstrated
findings of severe fatty liver, such as marked deposition of lipid,
as assessed by oil-red O staining (Fig.
4A). Notably, irbesartan markedly reduced lipid accumulation,
while anti-HGF antibody or GW9662 treatment reversed the beneficial
effect of irbesartan (Fig. 4A and
B). Irbesartan also decreased serum aspartate transaminase
(AST) as compared to the control (Table
I), whereas anti-HGF antibody or GW9662 treatment attenuated
the changes induced by irbesartan. Although no significant changes
were detected in the low-density lipoprotein (LDL) cholesterol and
total cholesterol levels, the free fatty acid (FFA) level was
markedly decreased and HDL cholesterol level was markedly increased
by irbesartan (Table I). The
changes were also reversed by anti-HGF antibody or GW9662
treatment. No statistically significant changes were observed in
body weight (BW), blood pressure or food intake in the groups
(Table II). Therefore, this
beneficial effect of irbesartan was not due to blood pressure
lowering or a decrease in food intake.
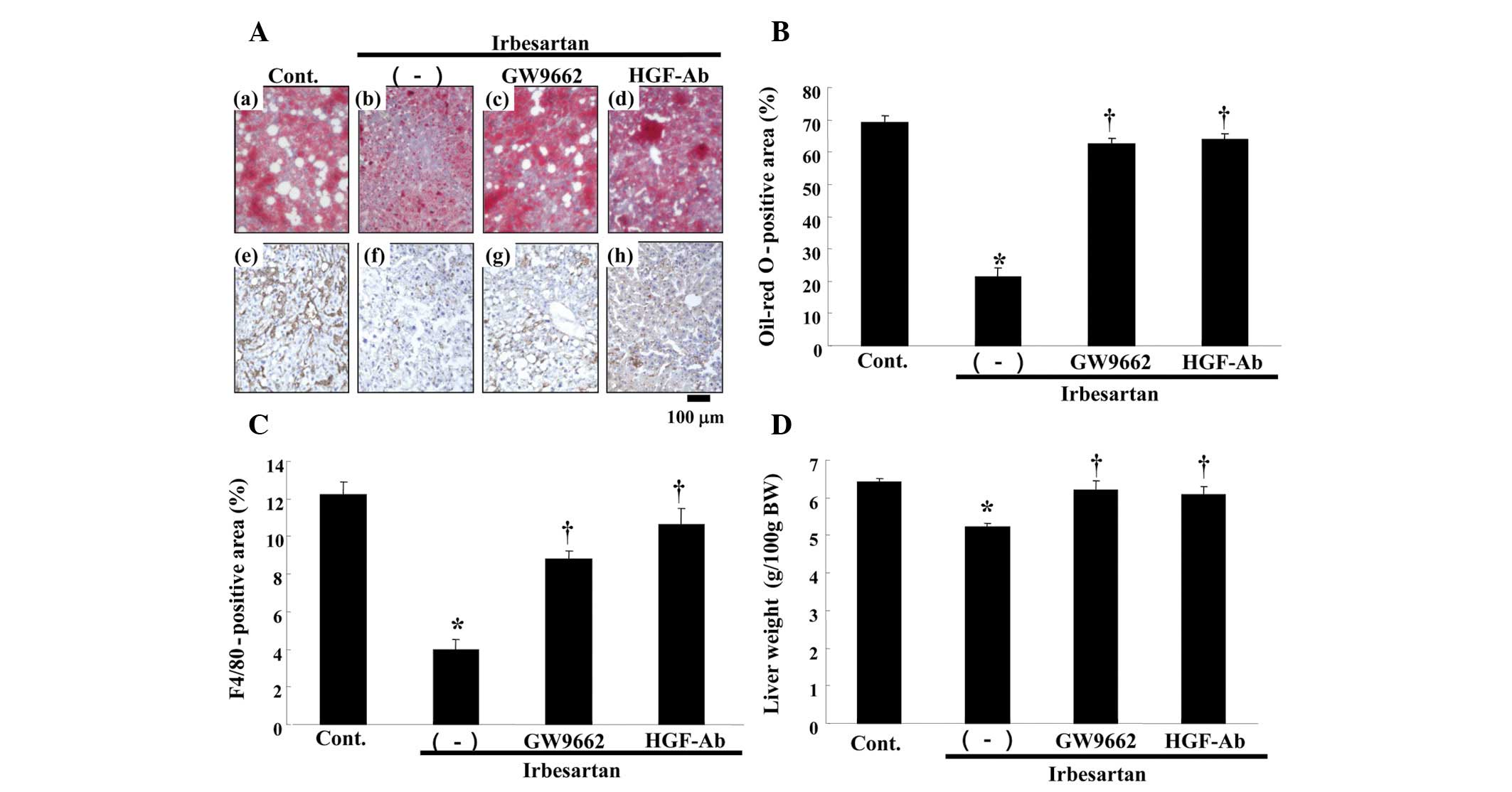 | Figure 4.Effects of irbesartan, GW9662 and
hepatocyte growth factor neutralizing antibody (HGF-Ab) on liver.
(A) Upper panels: typical micro-graphs of periodic cross-sections
of liver with oil-red O staining after 12 weeks of high-fat diet
(HFD). (a) Control (HFD only), (b) HFD + irbesartan (5 mg/kg/day),
(c) HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day)
and (d) HFD + irbesartan + neutralizing antibody against HGF (200
μg/week); bar, 100 μm. Lower panels: typical micrographs of
immunostaining for macrophages (F4/80) in periodic cross-sections
of liver tissue after 12 weeks of HFD. Brown-stained area shows
F4/80 protein-positive area; (e) control (HFD only), (f) HFD +
irbesartan (5 mg/kg/day), (g) HFD + irbesartan + GW9662, a PPARγ
antagonist (0.5 mg/kg/day) and (h) HFD + irbesartan + neutralizing
antibody against HGF (200 μg/week); bar, 100 μm. (B) Quantitative
data for percentage of oil deposits (oil-red O-positive area) in
liver. (C) Quantitative data of immunohistochemical staining for
macrophages (F4/80) in liver (%). (D) Liver weight [g/100g of body
weight (BW)]. Control, HFD only; (−), HFD + irbesartan (5
mg/kg/day); GW9662, HFD + irbesartan + GW9662, a PPARγ antagonist
(0.5 mg/kg/day); HGF-Ab, HFD + irbesartan + neutralizing antibody
against HGF (200 μg/week). *P<0.01 vs. control,
†P<0.05 vs. irbesartan only. PPARγ; peroxisome
proliferators activated receptor-γ. |
 | Table I.Serum parameters in each group. |
Table I.
Serum parameters in each group.
| Parameter | Irbesartan
|
|---|
| Control | (−) | GW9662 | HGF-Ab |
|---|
| AST (U/l) | 182.0±10.3 | 133.3±5.7a | 168.8±5.7b | 161.5±4.8b |
| ALT (U/l) | 30.5±2.3 | 29.6±1.7 | 30.9±2.5 | 31.4±2.9 |
| LDL-chol (mg/dl) | 1034±56 | 925±16 | 991±73 | 1006±29 |
| T-chol (mg/dl) | 1248±21 | 1069±48 | 1095±89 | 1194±47 |
| FFA (mEq/l) | 1.58±0.19 | 1.13±0.02a | 1.29±0.05 | 1.43±0.08b |
| HDL-chol (mg/dl) | 62.2±3.7 | 88.2±4.4a | 58.0±5.7a | 66.3±6.6 |
 | Table II.Body weight, blood pressure and food
intake in each group. |
Table II.
Body weight, blood pressure and food
intake in each group.
| Irbesartan
|
|---|
| Control | (−) | GW9662 | HGF-Ab |
|---|
| Body weight (g) | | | | |
| PreHFD | 27.6±0.9 | 27.8±0.9 | 27.4±0.6 | 27.6±0.7 |
| PostHFD | 35.7±1.6 | 31.1±0.8 | 33.3±1.6 | 33.2±1.3 |
| Systolic BP
(mmHg) | | | | |
| PreHFD | 103.8±2.0 | 104.3±2.2 | 108.2±0.9 | 105.5±3.4 |
| PostHFD | 104.7±2.1 | 101.3±2.3 | 103.5±2.1 | 107.7±3.1 |
| Diastolic BP
(mmHg) | | | | |
| PreHFD | 70.3±1.6 | 68.5±1.9 | 69.8±2.0 | 68.7±3.3 |
| PostHFD | 67.3±2.9 | 65.0±2.3 | 67.5±1.5 | 66.5±2.1 |
| Food intake
(g/day) | 2.06±0.11 | 2.05±0.10 | 2.09±0.11 | 2.08±0.09 |
As resident macrophages are crucially involved in
the progression of fatty liver 11, immunostaining with F4/80 was
performed. As shown in Fig. 4C,
irbesartan markedly reduced the infiltration of macrophages in the
liver, as detected by F4/80 staining. Irbesartan also reduced the
liver weight (Fig. 4D).
Consistently, these effects of irbesartan were also reversed by
anti-HGF antibody or GW9662 treatment (Fig. 4C and D).
Effect of irbesartan on HGF protein
levels in serum and liver
To confirm the role of HGF in the reduction of fatty
liver by irbesartan, we measured the tissue HGF protein level in
the liver. As shown in Fig. 5A,
irbesartan markedly increased hepatic HGF protein as compared to
the control, while GW9662 treatment markedly inhibited the increase
in the HGF level induced by irbesartan. Similar changes were
observed in the serum HGF level. As shown in Fig. 5B, the serum HGF level was markedly
increased by irbesartan, while GW9662 treatment reduced the
increase.
Discussion
The present study has demonstrated that irbesartan
significantly reduced fatty liver and chronic inflammation, such as
macrophage infiltration through the PPARγ-HGF pathway, beyond its
blood pressure-lowering effect. In general, adiponectin is a
well-known downstream effector of PPARγ (12,13).
The present study demonstrated that irbesartan markedly increased
adiponectin expression. However, another downstream effector of
PPARγ, HGF, is also likely to act as a ‘guardian’ in MS, in
addition to adiponectin. Previous studies suggested that HGF
mediates multiple various biological effects in various cells
including anti-fibrotic, anti-inflammatory and anti-apoptotic
activities (14). The present study
clearly demonstrated that the anti-metabolic effects of irbesartan
were largely mediated by the PPARγ-HGF pathway, with the exception
of Ang II blockade.
Recent data have suggested that the presence of
NAFLD in type 2 diabetes mellitus is linked to an increased risk of
cardiovascular disease (CVD) independent of MS. In addition, the
prevalence of carotid plaques has been reported to be higher in
patients with NAFLD compared to the normal controls, regardless of
classical cardiovascular risk factors (15). Therefore, NAFLD should be considered
an independent risk factor for CVD. However, currently there are
limited therapeutic options to treat NAFLD. To this end, the
present findings suggesting that irbesartan reduced fatty liver and
adipocyte hypertrophy may provide a new therapeutic option to treat
NAFLD.
The manner in which PPARγ activates local HGF has
yet to be elucidated. PPARγ has been reported to bind to the
putative peroxisome proliferator response element (PPRE) in the
promoter region of the HGF gene, resulting in an increase in HGF
gene transcription, mRNA expression and protein secretion (9). Our group of investigators have
reported that telmisartan (another PPARγ agonistic ARB), but not
losartan (a classical ARB), improved endothelial dysfunction and
fatty liver, due to an increased tissue HGF level (16). More directly, using AT1R KO mice,
telmisartan, but not losartan, exhibited renal protective effects
in a unilateral ureteral obstruction model (10). The partial PPARγ agonistic effect of
irbesartan might provide an additional advantage as a strategy for
the prevention and treatment of CVD, beyond its blood
pressure-lowering effect through Ang II blockade. Notably, although
irbesartan as well as telmisartan selectively induced PPARγ target
genes, distinctive gene expression profiles have also been reported
in 3T3-L1 adipocytes (2). The X-ray
crystal structure exhibited various binding modes to PPARγ between
irbesartan and telmisartan (6). In
addition, irbesartan is also known to activate PPARα. Irbesartan
has been reported to improve hepatic steatosis and hepatic fibrosis
by activating PPARα, in NASH model FLS ob/ob mice (17). Another study also demonstrated that
irbesartan upregulated PPARα in obese Koletsky
(fak/fak) rats and improved their
metabolic disorders (18). In our
model, PPARα activation may also act to improve the pathological
characteristics of the MS.
Overall, irbesartan, an ARB with partial PPARγ
agonistic activity (metabosartan), demonstrated a reduction in
fatty liver and chronic inflammation, such as macrophage
infiltration, beyond its blood pressure-lowering effect (Fig. 6). These favorable characteristics of
irbesartan might be due to local HGF activation through partial
PPARγ agonistic activity, in addition to Ang II blockade.
Upregulation of local HGF by irbesartan might provide a novel
advantage in a strategy for the prevention and treatment of
CVDs.
Acknowledgements
This study was partially supported by
a Grant-in-Aid from the Organization for Pharmaceutical Safety and
Research, a Grant-in-Aid from the Ministry of Public Health and
Welfare, a Grant-in-Aid from the Japan Promotion of Science and by
special coordination funds of the Ministry of Education, Culture,
Sports, Science and Technology of the Japanese Government.
References
|
1.
|
de Gasparo M, Catt KJ, Inagami T, Wright
JW and Unger T: International union of pharmacology. XXIII. The
angiotensin II receptors. Pharmacol Rev. 52:415–472.
2000.PubMed/NCBI
|
|
2.
|
Schupp M, Janke J, Clasen R, Unger T and
Kintscher U: Angiotensin type 1 receptor blockers induce peroxisome
proliferator-activated receptor-gamma activity. Circulation.
109:2054–2057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lehmann JM, Moore LB, Smith-Oliver TA,
Wilkison WO, Willson TM and Kliewer SA: An antidiabetic
thiazolidinedione is a high affinity ligand for peroxisome
proliferator-activated receptor gamma (PPAR gamma). J Biol Chem.
270:12953–12956. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Rosen ED and Spiegelman BM: PPARgamma: a
nuclear regulator of metabolism, differentiation, and cell growth.
J Biol Chem. 276:37731–37734. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Picard F and Auwerx J: PPAR(gamma) and
glucose homeostasis. Annu Rev Nutr. 22:167–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Benson SC, Pershadsingh HA, Ho CI,
Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA and
Kurtz TW: Identification of telmisartan as a unique angiotensin II
receptor antagonist with selective PPARgamma-modulating activity.
Hypertension. 43:993–1002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Schupp M, Clemenz M, Gineste R, Witt H,
Janke J, Helleboid S, Hennuyer N, Ruiz P, Unger T, Staels B and
Kintscher U: Molecular characterization of new selective peroxisome
proliferator-activated receptor gamma modulators with angiotensin
receptor blocking activity. Diabetes. 54:3442–3452. 2005.
View Article : Google Scholar
|
|
8.
|
Parhofer KG, Münzel F and Krekler M:
Effect of the angiotensin receptor blocker irbesartan on metabolic
parameters in clinical practice: the DO-IT prospective
observational study. Cardiovasc Diabetol. 6:362007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Li Y, Wen X, Spataro BC, Hu K, Dai C and
Liu Y: Hepatocyte growth factor is a downstream effector that
mediates the antifibrotic action of peroxisome
proliferator-activated receptor-gamma agonists. J Am Soc Nephrol.
17:54–65. 2006. View Article : Google Scholar
|
|
10.
|
Kusunoki H, Taniyama Y, Azuma J, Iekushi
K, Sanada F, Otsu R, Iwabayashi M, Okayama K, Rakugi H and
Morishita R: Telmisartan exerts renoprotective actions via
peroxisome proliferator-activated receptor-γ/hepatocyte growth
factor pathway independent of angiotensin II type 1 receptor
blockade. Hypertension. 59:308–316. 2012.PubMed/NCBI
|
|
11.
|
Bieghs V, Rensen PC, Hofker MH and
Shiri-Sverdlov R: NASH and atherosclerosis are two aspects of a
shared disease: central role for macrophages. Atherosclerosis.
220:287–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Janke J, Schupp M, Engeli S, Gorzelniak K,
Boschmann M, Sauma L, Nystrom FH, Jordan J, Luft FC and Sharma AM:
Angiotensin type 1 receptor antagonists induce human in-vitro
adipogenesis through peroxisome proliferator-activated
receptor-gamma activation. J Hypertens. 24:1809–1816. 2006.
View Article : Google Scholar
|
|
13.
|
Clasen R, Schupp M, Foryst-Ludwig A,
Sprang C, Clemenz M, Krikov M, Thöne-Reineke C, Unger T and
Kintscher U: PPARgamma-activating angiotensin type-1 receptor
blockers induce adiponectin. Hypertension. 46:137–143. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nakamura T and Mizuno S: The discovery of
hepatocyte growth factor (HGF) and its significance for cell
biology, life sciences and clinical medicine. Proc Jpn Acad Ser B
Phys Biol Sci. 86:588–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Choi SY, Kim D, Kang JH, Park MJ, Kim YS,
Lim SH, Kim CH and Lee HS: Nonalcoholic fatty liver disease as a
risk factor of cardiovascular disease: relation of non-alcoholic
fatty liver disease to carotid atherosclerosis. Korean J Hepatol.
14:77–88. 2008.(In Korean).
|
|
16.
|
Nakagami H, Osako MK, Takami Y, Hanayama
R, Koriyama H, Mori M, Hayashi H, Shimizu H and Morishita R:
Differential response of vascular hepatocyte growth factor
concentration and lipid accumulation between telmisartan and
losartan in ApoE-deficient mice. Mol Med Rep. 1:657–661. 2008.
|
|
17.
|
Kato J, Koda M, Kishina M, Tokunaga S,
Matono T, Sugihara T, Ueki M and Murawaki Y: Therapeutic effects of
angiotensin II type 1 receptor blocker, irbesartan, on
non-alcoholic steatohepatitis using FLS-ob/ob male mice. Int J Mol
Med. 30:107–113. 2012.PubMed/NCBI
|
|
18.
|
Rong X, Li Y, Ebihara K, Zhao M, Kusakabe
T, Tomita T, Murray M and Nakao K: Irbesartan treatment
up-regulates hepatic expression of PPARalpha and its target genes
in obese Koletsky (fa(k)/fa(k)) rats: a link to amelioration of
hypertriglyceridaemia. Br J Pharmacol. 160:1796–1807. 2010.
View Article : Google Scholar : PubMed/NCBI
|