Introduction
Osteoarthritis (OA) is a slow progressing
degenerative disease characterized by cartilage damage, synovial
fibrosis and osteophyte formation which may involve several joints,
including the temporomandibular joint (TMJ) (1,2). Once
the TMJ is involved, severe handicap and significant pain generally
occur. Clinically, temporomandibular joint osteoarthritis (TMJ OA)
is characterized by joint pain, crepitus, restricted motion and
eventually loss of joint function (1–3).
Recent advances in imaging, particularly magnetic
resonance imaging (MRI) and arthroscopy have contributed greatly to
the understanding of the intra-articular lesions of the disc and
condyle (4,5). Pathological changes, such as disc
displacement (DD) may be involved in the development of TMJ OA. In
their studies, Macher et al (6) and Ali et al (7) demonstrated that the surgical induction
of anterior DD in rabbits might lead to OA. Long et al
(8) showed that the degree of
anterior DD of TMJ is correlated with OA in rabbits. However, a
correlation between the change and detailed pathogenesis remains to
be adequately elucidated.
Transforming growth factor-β1 (TGF-β1) is an
important inducer of cartilage extracellular matrix (ECM)
production and is suggested to be a potential tool to enhance
cartilage repair upon damage in OA (9–11). By
contrast, matrix metalloproteinases (MMPs) are a large group of
matrix degrading enzymes that contribute to joint destruction in
OA. High activities of diverse MMPs, especially of MMP-3 are
believed to be highly involved in matrix breakdown (12–14).
These cytokines can infiltrate the synovium, which may alter the
synovial fluid (SF) viscosity and lead to impairment in the
lubrication and nutrition of articular cartilage and disc.
The aim of the present study was to investigate the
levels of TGF-β1 and MMP-3 in SF to evaluate the initiation and
progression of TMJ OA combined with DD and provide a scientific
basis for the assessment of treatment effectiveness for these
patients.
Patients and methods
Patients
Consecutive patients referred to our department and
who had been selected for TMJ surgical treatment were studied.
Patients with inflammation or arthritic diseases other than
osteoarthritis and patients who had had traumatic events were
excluded. The criteria for surgical treatment were unsuccessful
non-surgical treatment and a clinical diagnosis of TMJ OA. The
surgical technique has been thoroughly described elsewhere
(15–17). The inclusion criteria for TMJ OA
combined DD staging were a painful impaired TMJ mobility,
radiographic signs on the preoperative tomograms and surgical
findings at operation (Table I,
Fig. 1). The mean age for these
patients was 36.75 years (range, 18–58). There were 15 females and
1 male with a mean duration of symptom of 1.6 years (range, 4
months–5 years) (Table II). The
present study was approved by the ethics committee of Shanghai
Ninth People’s Hospital affiliated to Shanghai JiaoTong University,
School of Medicine. Informed consent and written agreement was
obtained from each patient.
 | Table IStaging of TMJ OA combined with
DD. |
Table I
Staging of TMJ OA combined with
DD.
| Staging (TMJ OA
combined with DD) | Imaging findings | Surgical
findings |
|---|
| Early | Anterior disc
displacement, disc deformed moderate to marked disc thickening,
abnormal bone contours | Disc deformed disc
displaced anteriorly, variable adhesion |
| Intermediate | Anterior disc
displacement, Disc deformed, Marked disc thickening, Abnormal bone
contours | Degenerative
remodeling of condylar cartilage surface, Adhesion, deformed disc
without perforation |
| Late | Anterior disc
displacement with disc perforation, Disc deformed badly,
Degenerative osseous changes | Gross degenerative
changes of disc and hard tissue, Disc perforation, Multiple
adhesion |
 | Table IIPatient characteristics. |
Table II
Patient characteristics.
| No. | Gender | Age (years) | Staging | Surgical
treatment |
|---|
| 1 | Female | 20 | Intermediate | Open disc
repositioning |
| 2 | Female | 50 | Late | Shave the osteophyte
combined with open disc repositioning |
| 3 | Female | 53 | Intermediate | Open disc
repositioning |
| 4 | Female | 21 | Early | Open disc
repositioning |
| 5 | Male | 53 | Late | Condylectomy and
reconstruction with costochondral graft |
| 6 | Female | 58 | Early | Open disc
repositioning |
| 7 | Female | 52 | Late | Shave the osteophyte
combined with open disc repositioning |
| 8 | Female | 18 | Late | Condylectomy and
reconstruction with costochondral graft |
| 9 | Female | 28 | Intermediate | Open disc
repositioning |
| 10 | Female | 22 | Late | Open disc
repositioning |
| 11 | Female | 25 | Intermediate | Open disc
repositioning |
| 12 | Female | 43 | Late | Condylectomy and
reconstruction with costochondral graft |
| 13 | Female | 23 | Early | Open disc
repositioning |
| 14 | Female | 51 | Intermediate | Open disc
repositioning |
| 15 | Female | 51 | Intermediate | Open disc
repositioning |
| 16 | Female | 20 | Early | Open disc
repositioning |
SF samples from TMJ
The experimental SFs were collected from >16
patients during surgery. The control SFs were collected from 10
healthy check-up examinees with no clinical and radiological
evidence of TMJ OA. The TMJ was punctured with a standard
disposable needle (diameter of 0.65 mm) inserted into the posterior
part of the upper joint compartment. TMJ SF samples were obtained
by washing the joint cavity with saline using the push and pull
technique. SFs were then centrifuged for 20 min at 2,000 x g using
serum monovettes (Sarstedt, Nümbrecht, Germany) and stored at −80°C
until use.
Enzyme-linked immunosorbent assay
(ELISA)
Commercially available human DuoSet ELISAs (R&D
Systems, Inc., Minneapolis, MN, USA) were used to estimate the
concentrations of TGF-β1 and MMP-3 in appropriately diluted SFs.
Ninety-six-well flat plates were coated overnight with the primary
antibody at room temperature (HCl 1 N) for 10 min, following
reneutralization (NaOH 2.7 N/HEPES 1 M). Samples and controls of
known concentration were added in wells for 2 h and were incubated
with the secondary antibody for an additional 2 h. Conjugation with
horseradish peroxidase and addition of tetramethylbenzidine and
H2O2 produced light emission at 450 nm.
Results were corrected by subtraction of light emission at an
aborbance value of 540 nm.
Statistical analysis
Data were presented as the mean ± standard error of
the mean. The Statistical Package for Social Science (SPSS)
software version 11.5 (SPSS, Inc, Chicago, IL, USA) was used for
statistical processing. The non-parametric Mann-Whitney U Test was
used. P<0.05 was considered to indicate a statistically
significant difference.
Results
TGF-β1 levels in SF
The median value of TGF-β1 in SF was three times
higher when comparing patients with TMJ OA combined with DD to the
healthy volunteers. (391.0205 pg/ml vs. 121.6628 pg/ml, P=0.000,
z=−4.217) (Table III).
 | Table IIITGF-β1 levels in synovial fluid of TMJ
OA combined with DD in patients and healthy volunteers. |
Table III
TGF-β1 levels in synovial fluid of TMJ
OA combined with DD in patients and healthy volunteers.
| Group | Cases | TGF-β1 (pg/ml) |
|---|
| Control | 10 |
121.6628±15.20046 |
| TMJ OA combined
DDa | 16 |
391.0205±130.6354 |
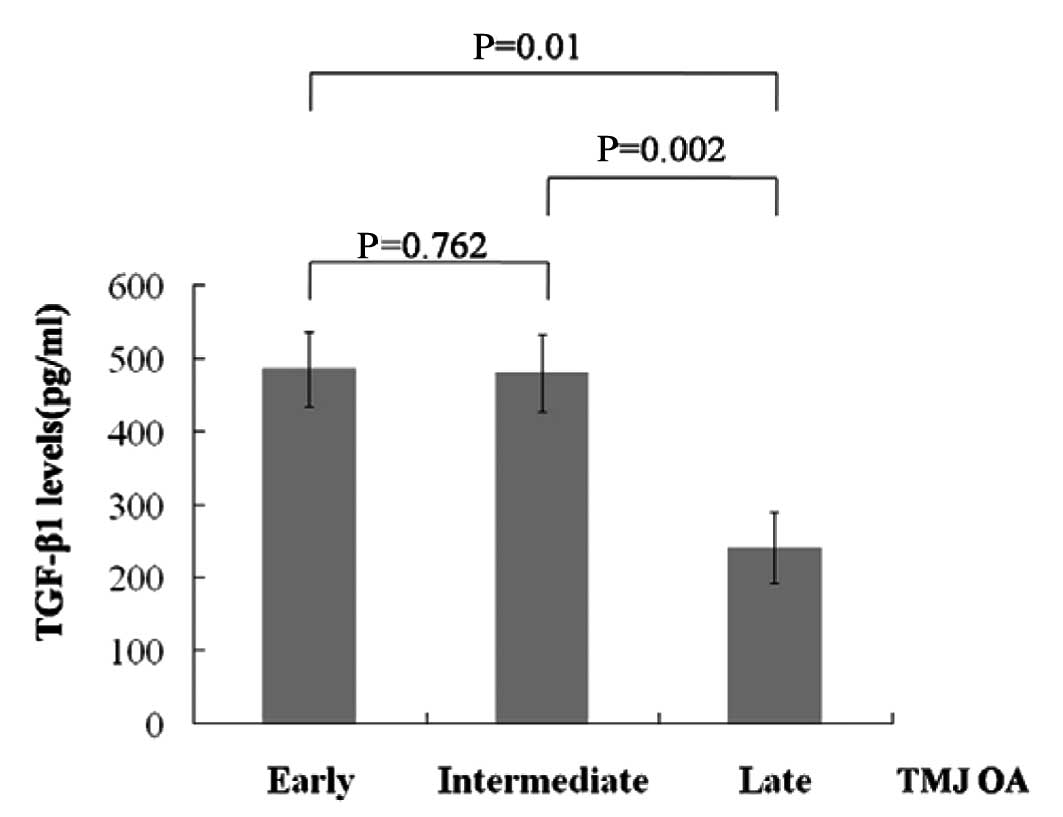
Of the patients, 4 had TMJ OA combined with DD of
early staging, 6 had TMJ OA combined with DD of intermediate
staging and 6 had TMJ OA combined with DD of late staging (Table II). Notably, the SF from patients
with TMJ OA combined with DD of late staging had lower TGF-β1
levels compared with patients of early or intermediate staging
(Fig. 2).
MMP-3 levels in SF
The median value of MMP-3 in SF was six times higher
when comparing patients with TMJ OA combined with DD to the healthy
volunteers (4047.418 pg/ml vs. 660.3411 pg/ml, P=0.000, z=−4.216)
(Table IV).
 | Table IVMMP-3 levels in synovial fluids of
TMJ OA combined with DD in patients and healthy volunteers. |
Table IV
MMP-3 levels in synovial fluids of
TMJ OA combined with DD in patients and healthy volunteers.
| Group | Cases | MMP-3 (pg/ml) |
|---|
| Control | 10 |
660.3411±110.9955 |
| TMJ OA combined
DDa | 16 |
4074.418±933.0046 |
Moreover, SF from patients with TMJ OA combined with
DD of early staging had lower MMP-3 levels compared with patients
of intermediate or late staging (Fig.
3).
Discussion
SF produced by TMJ synovium normally functions as a
biological lubricant as well as a biochemical pool through which
nutrients and regulatory cytokines traverse (18,19).
The cytokines are secreted by chondrocytes in articular cartilage
and synoviocytes in synovium and concentrated in the synovial space
by the semi-permeable synovial lining (20,21).
Synovial membrane permeability can be altered by inflammation,
which produces diseased SF because of alterations to biochemical
mediators. Therefore, measuring the change of cytokines in TMJ may
contribute to understanding the disease process of TMJ OA combined
with DD.
The present study has shown that TGF-β1 and MMP-3 in
SF are potential markers in assessing microenviroment inflammation
or cartilage change in TMJ OA combined with DD. TGF-β1 is mainly
released by articular chondrocytes in TMJ and secreted as an
inactive complex comprising a TGF-β1 dimer, its propeptide
latency-associated peptide (22).
Active TGF-β1 stimulates chondrocytes responding with ECM synthesis
from cartilage (23,24) and stabilizes the phenotype of the
prehypertrophic chondrocytes (25,26).
MMP-3 is secreted from synoviocytes and articular chondrocytes in a
latent form, and binds with the tissue inhibitor of the
metalloproteinase. MMP-3 is reported to crucial in matrix breakdown
since it is capable of degrading a number of cartilage components,
including proteoglycan, fibronection and collagens (12–14).
Thus, in normal TMJ, small quantities of active TGF-β1 and MMP-3
are well-known in the development and homeostasis of cartilage and
bone tissues (27–29).
At the initial stage of TMJ OA combined DD, TGF-β1
and MMP-3 levels in SF were increased while the destruction of bone
and cartilage was not evident, which manifested as a reparative
response at an early stage. Previous studies have shown TGF-β1 to
be an important anabolic with proven beneficial effects on
cartilage repair in the initiation of OA (30–32).
In their study, van der Kraan et al (10) have demonstrated that an upregulated
expression of TGF-β1 in early OA is found to be accompanied by
increased synthetic activity. Blaney et al (31) have shown that TGF-β stimulates ECM,
and counteracts the main catabolic roles at the early stage of
murine knee joints OA. Takahashi et al (32) show that TGF-β1 counteracts the
interleukin-1 (IL-1) upregulation of MMPs.
In the process of TMJ OA combined with DD, MMP-3
concentrations in SF were higher while TGF-β1 concentrations in SF
showed a gradual decrease. MMP gene expression can be enhanced by
pro-inflammatory cytokines, such as IL-1 and tumor necrosis
factor-α (TNF-α) (33). Previous
studies have shown a high prevalence of synovial inflammation in
TMJ OA (34,35). Therefore, with the progression of
TMJ OA combined DD, MMP-3 may be activated, while an excessive
amount of active MMP-3 directly degrades the cartilage matrix. In
addition, MMP-3 is able to activate other MMPs, such as MMP-1,
MMP-7 and MMP-9. Thus, a significant increase of MMP-3 was not
counteracted by insufficient amounts of TGF-β1, which aggravates
the destruction of cartilage that results in the release of
osteoclasia of cartilage and TMJ disc perforation.
Therefore, TMJ OA combined with DD is not considered
to be a simple and unavoidable result of wear and tear, and
sustaining or even enhancing the initial process may be an option.
In our study, DD on MRI was observed in the 12 patients. This
obvious change in TMJ anatomical structure may affect the
homeostasis of joint cartilage. Thus, TMJ open disc repositioning
procedures were performed on the 10 patients in the early and
intermediate stages of TMJ OA, as well as another 2 patients in the
late stage of TMJ OA combined DD, who had small disc perforation.
Postoperative MRIs after three or six months showed that: i) the
displaced disc was placed in its normal anatomical location; ii)
preoperative degenerative changes did not exhibit any evident
further development. Moreover, physiological remodeling, such as
new bone formation was observed. These findings suggest that the
displaced disc should be repositioned as early as possible in
patients with TMJ OA combined with DD, in order that the
aggravation of OA might be terminated and good condylar remodeling
be achieved after disc repositioning, since sufficient amounts of
TGF-β1 may be able to counteract the destructive effects of MMP-3
at an early stage.
To elucidate the cause of TMJ OA combined with DD,
further investigation regarding the regulatory mechanism of TGF-β1
and MMP-3 in pre- and postoperative periods is required in animals
and humans.
Acknowledgements
The present study was supported by a
grant from the Natural Science Foundation of China (Grant no.
81070848).
References
|
1
|
Hawker GA, Mian S, Bednis K and Stanaitis
I: Osteoarthritis year 2010 in review: pathomechnisms.
Osteoarthritis Cartilage. 19:366–374. 2011.PubMed/NCBI
|
|
2
|
Loeser SF: Age-related changes in the
musculoskeletal system and the development of osteoarthritis. Clin
Geriatr Med. 26:371–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rando C and Waldron T: TMJ osteoarthritis:
a new approach to diagnosis. Am J Phys Anthropol. 148:45–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishimaru JI, Oguma Y and Goss AN: Matrix
metalloproteinase and tissue inhibitor of metalloproteinase in
serum and lavage synovial fluid of patients with temporomandibular
joint disorders. Br J Oral Maxillofac Surg. 38:354–359. 2000.
View Article : Google Scholar
|
|
5
|
Ogura I, Kaneda T, Mori S, Sakayanagi M
and Kato M: Magnetic resonance characteristics of temporomandibular
joint disc displacement in elderly patients. Dentomaxillofac
Radiol. 41:122–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macher DJ, Westesson PL, Broks SL, Hicks
DG and Tallents RH: Temporomandibular joint: surgically created
disk displacement causes arthrosis in the rabbit. Oral Surg Oral
Med Oral Pathol. 73:645–649. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ali AM and Sharawy M: Enlargement of the
rabbit mandibular condyle after experimental induction of anterior
disc displacement: a histomorphometric study. J Oral Maxillofac
Surg. 53:544–560. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long X, Li JR and Chen X: Evaluation in
the relationship of disc displacement of temporomandibular joint to
osteoarthrosis in the rabbit. Chin J Stomatol. 33:264–266. 1998.(In
Chinese).
|
|
9
|
Tanimoto K, Suzuki A, Ohno S, Honda K,
Tanaka N, Doi T, et al: Effects of TGF-beta on hyaluronan anabolism
in fibroblasts derived from the synovial membrane of the rabbit
temporomandibular joint. J Dent Res. 83:40–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van der Kraan PM, Blaney Davidson EN and
van den Berg WB: A role for age-related changes in TGF beta
signaling in aberrant chondrocyte differentiation and
osteoarthritis. Arthritis Res Ther. 12:201–209. 2010.PubMed/NCBI
|
|
11
|
Baugé C, Girard N, Leclercq S, Galéra P
and Boumédiene K: Regulatory mechanism of transforming growth
factor beta receptor type II degradation by interleukin-1 in
primary chondrocytes. Biochim Biophys Acta. 1823:983–986.
2012.PubMed/NCBI
|
|
12
|
Hegemann N, Wondimu A, Ullrich K and
Schmidt MF: Synovial MMP-3 and TIMP-1 levels and their correlation
with cytokine expression in canine rheumatoid arthritis. Vet
Immunol Immunopathol. 91:199–204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar
|
|
14
|
Kubota E, Kubota T, Matsumoto J, Shibata T
and Murakami KI: Synovial fluid cytokines and proteinases as
markers of temporomandibular joint disease. J Oral Maxillofac Surg.
56:192–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Liu X, Yang X, Yang C, Chen M,
Haddad MS and Chen Z: Temporomandibular joint disc repositioning
using bone anchors: an immediate post surgical evaluation by
magnetic resonance imaging. BMC Musculoskelet Disord. 11:262–268.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He D, Yang C, Chen M, Yang X, Li L and
Jiang Q: Surgical treatment of traumatic temporomandibular joint
ankylosis with medially displaced residual condyle: surgical
methods and long-term results. J Oral Maxillofac Surg.
69:2412–2418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang B, Chen M, Zhang S and Yang C: Disc
replacement with temporalis myofascial flap pedicled on the middle
temporal artery and vein. China J Oral and Maxillofac Surg.
6:491–494. 2009.
|
|
18
|
Kim YK, Kim SG, Kim BS, Lee JY, Yun PY,
Bae JH, et al: Analysis of the cytokine profiles of the synovial
fluid in a normal temporomandibular joint: preliminary study. J
Craniomaxillofac Surg. Mar 15–2012.(Epub ahead of print).
|
|
19
|
Blewis ME, Nugent-Derfus GE, Schmidt TA,
Schumacher BL and Sah RL: A model of synovial fluid lubricant
composition in normal and injured joints. Eur Cell Mater. 13:26–39.
2007.PubMed/NCBI
|
|
20
|
Briston L, Dudhia J and Lees P:
Age-related differences in prostaglandin E2 synthesis by equine
cartilage explants and synoviocytes. J Vet Pharmacol Ther.
33:268–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roman-Blas JA, Contreras-Blasco MA, Largo
R, Alvarez-Soria MA, Castañeda S and Herrero-Beaumont G:
Differential effects of the antioxidant n-acetylcysteine on the
production of catabolic mediators in IL-1beta-stimulated human
osteoarthritic synoviocytes and chondrocytes. Eur J Pharmacol.
623:125–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee MC, Goomer RS, Takahashi K, Harwood
FL, Amiel M and Amiel D: Transforming growth factor beta one
(TGF-beta 1) enhancement of the chondrocytic phenotype in aged
perichondrial cells: an in vitro study. Iowa Orthop J. 20:11–16.
2000.PubMed/NCBI
|
|
23
|
Grimaud E, Heymann D and Rédini F: Recent
advances in TGF-beta effects on chondrocyte metabolism. Potential
therapeutic roles of TGF-beta in cartilage disorders. Cytokine
Growth Factor Rev. 13:241–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ulrich-Vinther M, Stengaard C, Schwarz EM,
Goldring MB and Soballe K: Adeno-associated vector mediated gene
transfer of transforming growth factor-beta1 to normal and
osteoarthritic human chondrocytes stimulates cartilage anabolism.
Eur Cell Mater. 10:40–50. 2005.
|
|
25
|
Song JJ, Aswad R, Kanaan RA, Rico MC, Owen
TA, Barbe MF, et al: Connective tissue growth factor (CTGF) acts as
a downstream mediator of TGF-beta1 to induce mesenchymal cell
condensation. J Cell Physiol. 210:398–410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van der Kraan PM, Blaney Davidson EN, Blom
A and van den Berg WB: TGF-beta signaling in chondrocyte terminal
differentiation and osteoarthritis: modulation and integration of
signaling pathways through receptor-Smads. Osteoarthritis
Cartilage. 17:1539–1545. 2009.
|
|
27
|
Serra R, Johnson M, Filvaroff EH, LaBorde
J, Sheehan DM, Derynck R and Moses HL: Expression of a truncated,
kinase-defective TGF-beta type II receptor in mouse skeletal tissue
promotes terminal chondrocyte differentiation and osteoarthritis. J
Cell Biol. 139:541–552. 1997. View Article : Google Scholar
|
|
28
|
Pombo-Suarez M, Castaño-Oreja MT, Calaza
M, Gomez-Reino J and Gonzalez A: Differential upregulation of the
three transforming growth factor beta isoforms in human
osteoarthritic cartilage. Ann Rheum Dis. 68:568–571. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niikura T and Reddi AH: Differential
regulation of lubricin/superficial zone protein by transforming
growth factor beta/bone morphogenetic protein superfamily members
in articular chondrocytes and synoviocytes. Arthritis Rheum.
56:2312–2321. 2007. View Article : Google Scholar
|
|
30
|
Boumediene K, Vivien D, Macro M,
Bogdanowicz P, Lebrun E and Pujol JP: Modulation of rabbit
articular chondrocyte (RAC) proliferation by TGF-beta isoforms.
Cell Prolif. 28:221–234. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blaney Davidson EN, van der Kraan PM and
van den Berg WB: TGF-beta and osteoarthritis. Osteoarthritis
Cartilage. 15:597–604. 2007.
|
|
32
|
Takahashi N, Rieneck K, van der Kraan PM,
van Beuningen HM, Vitters EL, Bendtzen K and van den Berg WB:
Elucidation of IL-1/TGF-beta interactions in mouse chondrocyte cell
line by genome-wide gene expression. Osteoarthritis Cartilage.
13:426–438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liacini A, Sylvester J, Li WQ and
Zafarullah M: Mithramycin downregulates proinflammatory
cytokine-induced matrix metal-loproteinase gene expression in
articular chondrocytes. Arthritis Res Ther. 7:R777–R783. 2005.
View Article : Google Scholar
|
|
34
|
Vernal R, Velásquez E, Gamonal J,
Garcia-Sanz JA, Silva A and Sanz M: Expression of proinflammatory
cytokines in osteoarthritis of the temporomandibular joint. Arch
Oral Biol. 53:910–915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kacena MA, Merrel GA, Konda SR, Wilson KM,
Xi Y and Horowitz MC: Inflammation and bony changes at the
temporomandibular joint. Cells Tissues Organs. 169:257–264. 2001.
View Article : Google Scholar : PubMed/NCBI
|