Introduction
Ankyrin repeat-rich membrane spanning protein
(ARMS), also known as kinase D-interacting substrate of 220 kDa
(Kidins220), is an important molecule in the nerve growth factor
(NGF)/tyrosine kinase A (TrkA) receptor signaling pathway, as it
binds to NGF-TrkA, activating downstream enzymes (1). An association between TrkA and ARMS
was previously found to persist for several hours (2). During NGF-induced neuronal
differentiation, there is direct engagement of the TrkA receptor
with ARMS, leading to sustained mitogen-activated protein kinase
(MAPK) activation that regulates neurite outgrowth (3). Numerous inflammatory cytokines and
polypeptide growth factors elicit specific cell responses through
the activation of MAPK cascades (4,5). Among
the best characterized of the mammalian MAPK superfamily of
serine/threonine kinases are the p42- and p44-kDa extracellular
signal-regulated kinases (ERKs) ERK2 and ERK1 (collectively defined
as p42/p44 ERK), the p38 MAPK and the p46-p54-kDa c-Jun amino
(N)-terminal kinase (JNK) or stress-activated protein kinase
(6). A previous study demonstrated
that ERK activity in the lungs of asthmatic mice was significantly
higher compared to that in normal mice (7). We recently demonstrated that the
expression of ARMS in lung tissues was increased in asthmatic mice
compared to that in control mice (8,9).
Asthma is characterized by chronic airway
inflammation. Numerous cell types and cytokines contribute to this
inflammation (10). The
NGF-mediated TrkA pathway was reported to be involved in the
pathogenesis of asthma. In addition, interleukin (IL)-1β and tumor
necrosis factor (TNF)-α were shown to exert a prominent effect on
the development of airway responsiveness and airway inflammation in
bronchial asthma (11,12). IL-4 is a Th2 cytokine that plays
important roles in allergic inflammation and airway remodeling
(13,14). The total and phosphorylated
(activated) forms of ERK are present in dorsal root ganglion (DRG),
satellite and Schwann cells. The basal levels of activated ERK in
DRG cells are low, although they are selectively increased in
calcitonin gene-related peptide/TrkA cells with NGF treatment
(15). IL-1β activates p42/p44
ERK-dependent processes in human airway smooth muscle (ASM).
p42/p44 ERK affects the extent of IL-1β-stimulated cytokine release
(6,16). The synergistic action of the ERK and
p38 MAPK pathways is required for the optimal induction of cytokine
gene expression (17) and cytokine
release (18). NGF may be involved
in the pathogenesis of neurogenic inflammation in asthma through
the Ras-MAPK signal transduction pathway. NGF/TrkA and ARMS were
reported to participate in the pathogesis of asthma (8,9).
Kidins220/ARMS is a novel component of the uropod involved in the
regulation of T-cell motility, an essential process for the immune
response (19). The ERK signaling
pathway may modulate allergic airway inflammation (7,20).
However, whether the ARMS-mediated ERK signaling pathway is also
involved in the inflammation of asthma has not been elucidated.
Based on the above findings, we hypothesized that
Kidins220/ARMS-mediated ERK signaling pathway may be involved in
the inflammation of asthma. Our findings demonstrated that ERK and
ARMS were overexpressed in lung tissues following the allergen
challenge and the increased expression of p42/p44 ERK was decreased
with anti-NGF, anti-TrkA or anti-ARMS antibody treatment.
Materials and methods
Animals
A total of 62 female BALB/c mice, 6- to 8-weeks-old,
weighing 18–22 g, were obtained from the Beijing Laboratory Animal
Research Center (Beijing, China). The mice were maintained under
specific pathogen-free conditions. An ovalbumin (OVA)-free diet and
water were supplied ad libitum. The animal studies were
approved by the Ethics Committee for Animal Use and Care at the
Institute of Education of China Medical University.
Sensitization, challenge and
treatment
The BALB/c mice were randomized into five groups:
control (n=12), OVA (n=14), anti-NGF (n=12), anti-TrkA (n=12) and
anti-ARMS (n=12). Sensitization and challenge protocols were
conducted according to the methods of Elwood et al (21) and Vanacker et al (22), with certain modifications, as
described below. In the OVA group, mice were sensitized to
ovalbumin (20 μg per injection) absorbed in 2.0 mg per injection of
aluminium hydroxide administered intraperitoneally on day 1. On
days 8, 15 and 22, mice were again sensitized to ovalbumin (10 μg
per injection) absorbed in 1.0 mg per injection of aluminium
hydroxide administered intraperitoneally. Beginning on day 23, the
mice were administered inhaled aerosols of 4% ovalbumin in
phosphate-buffered saline (PBS) for 25–30 min (until the onset of
bronchial obstruction) daily for 7 consecutive days. The mice in
the anti-NGF, anti-TrkA and anti-ARMS groups were also subjected to
ovalbumin sensitization and asthma induction in the same manner.
Intranasal administration of 50 μl (1:50 dilution) of polyclonal
goat anti-mouse NGF antibody (biological activity: 1:4,000 dilution
blocks bioactivity of 5 ng/ml NGF) (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) dissolved in sterile PBS was performed 3 h
prior to each airway allergen challenge in the anti-NGF group.
Intranasal treatment with anti-NGF antibody was performed according
to the methods of Braun et al (23), Glaab et al (24) and Nagai et al (25), with minor modifications. The mice in
the anti-TrkA group received 0.2-mmol/l anti-TrkA antibody (200 ml
prepared with PBS) (Santa Cruz Biotechnology, Inc.) by intranasal
administration (26,27) 3 h prior to the induction of asthma.
Mice in the anti-ARMS group received intranasal goat polyclonal
anti-ARMS antibody (diluted 1:25 in PBS) (Santa Cruz Biotechnology,
Inc.). Mice in the control group were challenged with PBS alone,
administered by injection. All the animals were humanely sacrificed
within 24 h after the last ovalbumin or PBS exposure.
Pathological examination of bronchial and
lung tissues
The lungs of the BALB/c mice were perfused with 4%
paraformaldehyde to allow the pleura to extend and flatten prior to
fixation of the tissue in 4% paraformaldehyde. The tissues were
routinely embedded in paraffin and sectioned (5 μm) for hematoxylin
and eosin staining to assess the pathological changes in the lung
and bronchial tissues under a microscope.
Western blot analysis for ERK
Protein homogenates of lung tissue samples were
prepared by rapid homogenization in 10 volumes of ice-cold RIPA
lysis buffer (Beyotime, Shanghai, China). The protein
concentrations of the tissue lysates were determined using the
Enhanced BCA Protein Assay kit (Beyotime) and the supernatants were
boiled in sodium dodecyl sulfate sample buffer for 5 min. Equal
amounts of lysate proteins were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene difluoride membrane (Amersham Pharmacia Biotech,
Uppsala, Sweden). After blocking, the blots were incubated with
specific primary antibody overnight at 4°C and were then further
incubated for 1 h with horseradish peroxidase-conjugated secondary
antibody. The bound antibodies were detected using an enhanced
chemiluminescence kit with a Lumino-Image analyzer (Taitec Corp,
Tokyo, Japan). Integrated density values were analyzed using a
computerized image analysis system (Fluor Chen 2.0) and normalized
to those of β-actin.
ELISA for IL-1β, IL-4 and TNF-α levels in
lung tissues
Lung tissues (50 mg/500 μl) were homogenized in PBS
using a Polytron homogenizer (Kinematica, Littau, Switzerland),
then centrifuged at 800 x g for 10 min. The protein levels of
IL-1β, IL-4 and TNF-α in the lung tissue homogenates (50 μl) were
determined by ELISA, according to the manufacturer's instructions
(Bionewtrans Pharmaceutical Biotechnology Co., Ltd., Franklin, MA,
USA). The limit of detection for IL-1β, IL-4 and TNF-α was >1, 1
and 7.8 pg/ml, respectively.
Statistical analysis
The data were analyzed using SPSS statistical
software version 15.0 (SPSS Inc, Chicago, IL, USA) and were
expressed as mean ± standard deviation. Differences between groups
were analyzed, as appropriate, using the Student's t-test and
one-way analysis of variance followed by the Fisher's least
significant difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
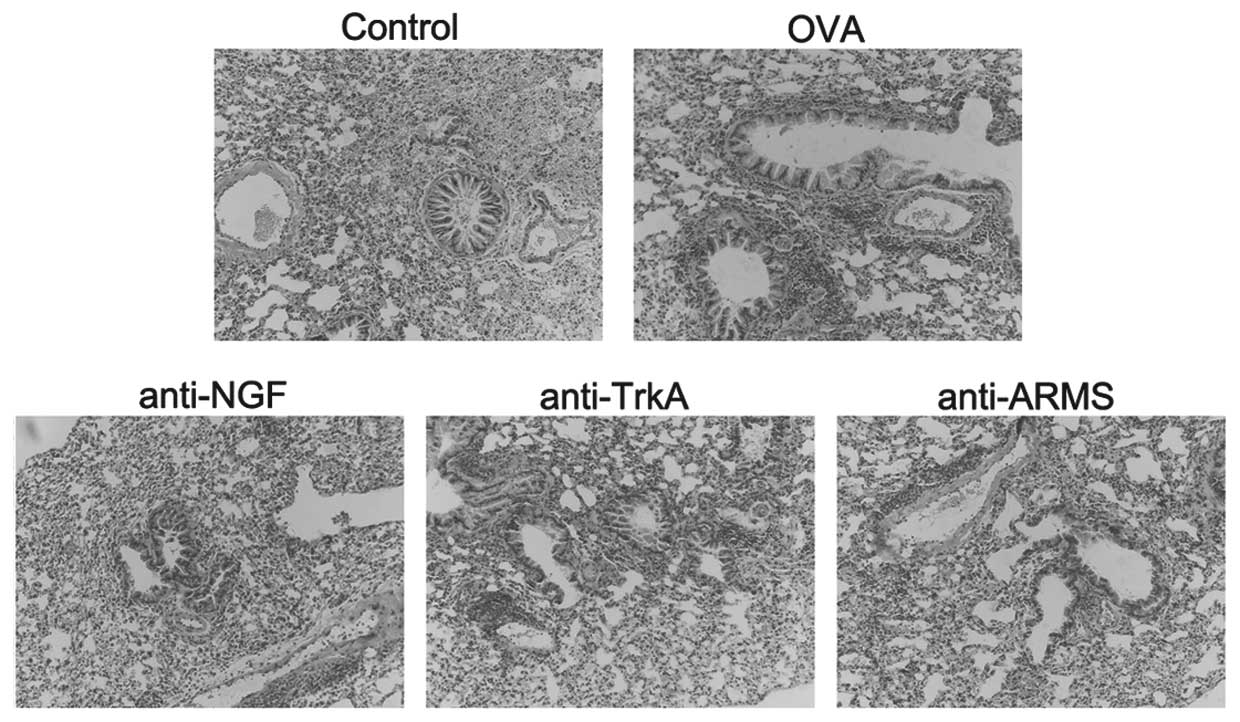
Pathological changes in the bronchial and
lung tissues
The mice in the control group exhibited normal
smooth bronchial and alveolar structures. There was no inflammatory
cell infiltration of the airway and lung tissues. The mice in the
OVA group exhibited an extensive inflammatory cell infiltration
around the bronchioles, blood vessels and alveoli. The airway
mucosa was edematous, with bronchiolar wall thickening causing
luminal stenosis, suggesting successful establishment of the
asthmatic model. In asthmatic mice treated with anti-NGF, anti-TrkA
and anti-ARMS antibodies, the extent of inflammation and cellular
infiltration in the airway was reduced and the pathological changes
of the bronchial and lung tissues were milder compared to those in
the asthma group (Fig. 1).
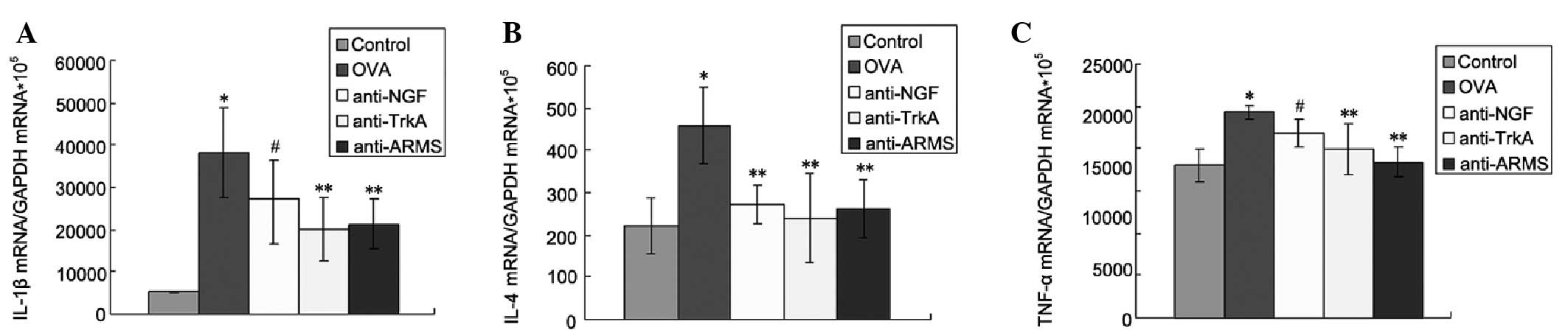
IL-1β, IL-4 and TNF-α concentration in
lung tissues
The concentration of IL-1β, IL-4 and TNF-α in the
lung tissues was significantly higher in the OVA compared to the
control group (P<0.01). The expression of IL-1β, IL-4 and TNF-α
was reduced following treatment with anti-NGF, anti-TrkA and
anti-ARMS antibodies, providing direct evidence that ARMS may
affect the expression of inflammatory cytokines in lung tissues and
suggesting the possible involvement of NGF/TrkA-ARMS signaling in
inflammatory reactions in asthma (Fig.
2).
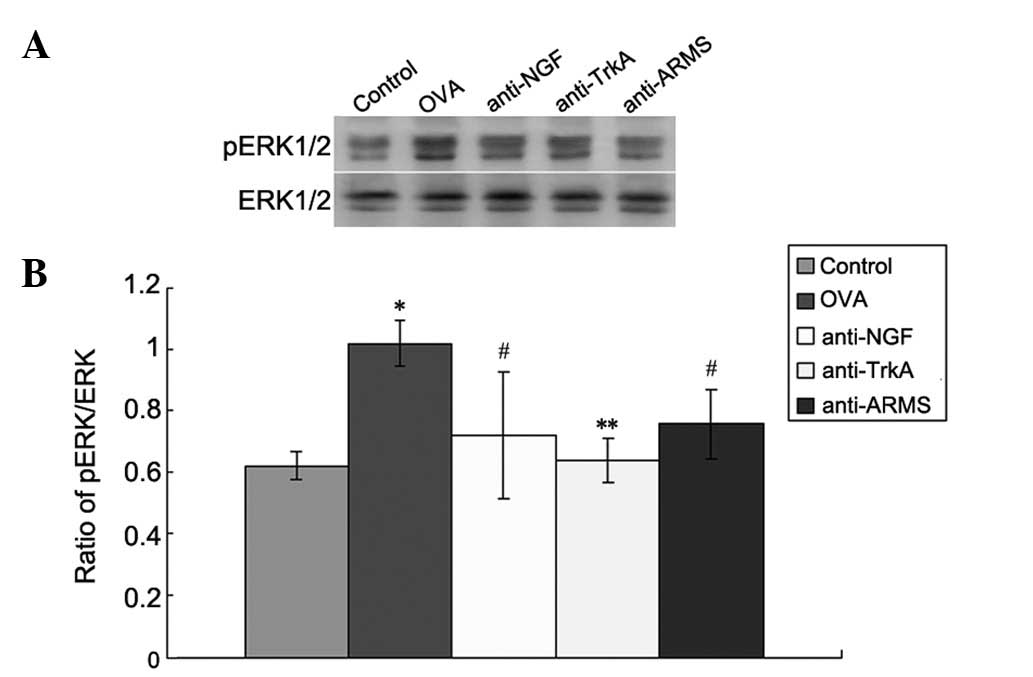
Expression of pERK
Western blot analysis was performed to measure the
levels of pERK/ERK in the lung tissues in each group (Fig. 3). The control group exhibited low
expression levels of pERK/ERK. However, the levels were
significantly increased in the OVA group (P<0.01). Furthermore,
the expression of pERK in the anti-NGF, anti-TrkA and anti-ARMS
groups was significantly lower compared to that in the OVA group
(P<0.01).
Discussion
In this study, we demonstrated that the expression
of pERK in lung tissues, as determined by western blot analysis,
was increased in the OVA group compared to that in the control
group. Intervention with anti-ARMS, anti-NGF and anti-TrkA
antibodies resulted in a significant reduction of pERK expression
in lung tissues in the OVA group. ARMS blockade was also associated
with reduced cytokine levels in the lung tissues of OVA-sensitized
mice. It was previously demonstrated that NGF/TrkA plays an
important role in bronchial hyperresponsiveness and inflammation
(28,29) in asthma. Kidins220/ARMS, a novel
transmembrane protein substrate of Trk receptors, is directly
involved in the downstream signaling events for neurotrophins. ARMS
was identified as an important molecule in the signaling pathway,
as it binds to NGF-TrkA and activates downstream enzymes (1). ARMS also serves as a substrate for
protein kinase D (30). The
interaction between Trk and ARMS was shown to result in an increase
in MAPK activation that persists for several hours.
The MAPK signaling cascade has been shown to be
important in the activation of various immune cells (31). Three major groups of MAPK are
encountered in mammalian cells, including extracellular
signal-regulated protein kinase (ERK), p38 MAPK and JNK. The
regulation of the ERK signaling pathway may modulate allergic
airway inflammation (20).
Kidins220/ARMS knockout mice exhibited developmental defects,
mainly in the nervous and cardiovascular systems, suggesting a
crucial role for this protein in the modulation of the crosstalk
between different signaling pathways (32). Therefore, it may be assumed that the
NGF-TrkA-ARMS signaling pathway is involved in the inflammation
associated with asthma by activating ERK.
TrkA, as a receptor tyrosine kinase, traditionally
signals through the activation of one of three major biochemical
pathways: phospholipase Cγ (PLCγ), p42/p44 ERK (ERK1/2) or
phosphatidylinositol-3-kinase (PI3K). MAPK activation is known to
play a crucial role in the proliferation of ASM cells induced by
various mitogens. Activation of ASM cells by IL-1β also leads to
the production of mediators and numerous cytokines and chemokines
(33). These effects are regulated
to some extent by MAPK activation (34). Allergic airway inflammation involves
multiple inflammatory cells and a wide array of mediators (35). It was previously demonstrated that
the activation of ARMS is mainly realized by the MAPK signaling
pathway (20). Specific cytokines
were associated with human asthma through examinations of blood,
exhaled breath condensates, bronchoalveolar lavage fluid (BALF)
cells or sputum cells (36).
Increased amounts of IL-1β and TNF-α were detected in BALF
(37) and in the culture
supernatants of alveolar macrophages from asthmatic patients
(11). Moreover, the level of the
pro-inflammatory cytokine IL-4 is increased in the lung tissues of
asthmatic human (38) and animal
subjects (39,40). The cytokines IL-1β, IL-4 and TNF-α
are key elements in the airway inflammation and hyperresponsiveness
observed in allergic asthma. Th1-associated IL-1β and TNF-α, as
well as Th2-associated IL-4, are considered as the key cytokines
involved in the induction of airway hyper-responsiveness,
infiltration of eosinophils into the lungs and mucus secretion
(41). The ERK signaling pathway
was shown to be involved in the cytokine production by a variety of
cell types (42–44). However, whether the ARMS-mediated
ERK signaling pathway is involved in asthma inflammation and its
underlying mechanisms has not yet been elucidated.
This study demonstrated that ARMS inhibition
effectively reduced OVA-induced cytokine production in a mouse
asthma model. The expression of IL-1β, IL-4 and TNF-α in the lungs
of OVA-sensitized mice was higher compared to that in control mice.
ERK activation was also observed in the lung tissues of
OVA-sensitized mice. However, treatment with anti-NGF, anti-TrkA
and anti-ARMS antibodies resulted in downregulation of the
expression of IL-1β, IL-4 and TNF-α in OVA-sensitized mice.
Moveover, intervention with anti-ARMS antibody resulted in a
significant reduction of NGF and TrkA and activation of ERK. These
results suggested that the NGF/TrkA-ARMS-ERK signaling pathway
activated by airway inflammation in allergic asthma may be involved
in the pathogenetic mechanism of asthma through the upregulation of
IL-1β, IL-4 and TNF-α expression. These results indicate that
ARMS-mediated ERK pathway plays a role in the inflammatory response
and that ARMS and pERK upregulation is involved in the
neuroimmunological pathogenesis of the allergen challenge in mice.
These findings may lead to the development of novel therapeutic
approaches to allergic diseases of the airways.
In conclusion, the present study has demonstrated
that NGF/TrkA-Kidins220/ARMS-ERK signaling contributes to airway
inflammation in OVA-sensitized mice. Intranasal administration of
anti-NGF, anti-TrkA and anti-ARMS antibodies effectively blocked
the inflammatory cascade. Our results support the hypothesis that
the NGF/TrkA-ARMS signaling pathway may be involved in the
inflammation of asthma via ERK activation. Identification of
NGF/TrkA-Kidins220/ARMS-ERK signaling may prove useful in the
treatment of allergic airway inflammation.
Acknowledgements
This study was supported by the
Education Department Project of Heilongjiang Province (contract no.
12511218).
References
|
1
|
Edwards MR, Bartlett NW, Clarke D, Birrell
M, Belvisi M and Johnston SL: Targeting the NF-κB pathway in asthma
and chronic obstructive pulmonary disease. Pharmacol Ther.
121:1–13. 2009.
|
|
2
|
Kong H, Boulter J, Weber JL, Lai C and
Chao MV: An evolutionarily conserved transmembrane protein that is
a novel downstream target of neurotrophin and ephrin receptors. J
Neurosci. 21:176–185. 2001.PubMed/NCBI
|
|
3
|
Arevalo JC, Yano H, Teng KK and Chao MV: A
unique pathway for sustained neurotrophin signaling through an
ankyrin-rich membrane-spanning protein. EMBO J. 23:2358–2368. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davids RJ: The mitogen-activated protein
kinase signal transduction pathway. J Biol Chem. 268:14553–14556.
1993.PubMed/NCBI
|
|
5
|
Robinson MJ and Cobb MH: Mitogen-activated
protein kinase pathways. Curr Opin Cell Biol. 9:180–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hallsworth MP, Moir LM, Lai D and Hirst
SJ: Inhibitors of mitogen-activated protein kinases differentially
regulate eosinophil-activating cytokine release from human airway
smooth muscle. Am J Respir Crit Care Med. 164:688–697. 2001.
View Article : Google Scholar
|
|
7
|
Kumar A, Lnu S, Malya R, Barron D, Moore
J, Corry DB and Boriek AM: Mechanical stretch activates nuclear
factor-κB, activator protein-1, and mitogen-activated protein
kinases in lung parenchyma: implications in asthma. FASEB J.
17:1800–1811. 2003.
|
|
8
|
Ni X, Li X, Fang X, Li N, Cui W and Zhang
B: NGF/TrkA-mediated Kidins220/ARMS signaling activated in the
allergic airway challenge in mice. Ann Allergy Asthma Immunol.
105:299–306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni X, Li X, Fang X, Li N, Cui W, Zhang B
and Liu Y: Kidins220/ARMS contributes to airway inflammation and
hyper-responsiveness in OVA-sensitized mice. Respir Physiol
Neurobiol. 175:97–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ying S, Zhang G, Gu S and Zhao J: How much
do we know about atopic asthma: where are we now? Cell Mol Immunol.
3:321–332. 2006.PubMed/NCBI
|
|
11
|
Gosset P, Tsicopoulos A, Wallaert B,
Vannimenus C, Joseph M, Tonnel AB and Capron A: Increased secretion
of tumor necrosis factor alpha and interleukin-6 by alveolar
macrophages consecutive to the development of the late asthmatic
reaction. J Allergy Clin Immunol. 88:561–571. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wills-Karp M, Uchida Y, Lee JY, Jinot J,
Hirata A and Hirata F: Organ culture with proinflammatory cytokines
reproduces impairment of the beta-adrenoceptor-mediated relaxation
in tracheas of a guinea pig antigen model. Am J Respir Cell Mol
Biol. 8:153–159. 1993. View Article : Google Scholar
|
|
13
|
Jie Z, Jin M, Cai Y, et al: The effects of
Th2 cytokines on the expression of ADAM33 in allergen-induced
chronic airway inflammation. Respir Physiol Neurobiol. 168:289–294.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshinaka T, Nishii K, Yamada K, et al:
Identification and characterization of novel mouse and human
ADAM33s with potential metalloprotease activity. Gene. 282:227–236.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Averill S, Delcroix JD, Michael GJ,
Tomlinson DR, Fernyhough P and Priestley JV: Nerve growth factor
modulates the activation status and fast axonal transport of ERK1/2
in adult nociceptive neurons. Mol Cell Neurosci. 18:183–196. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boehme SA, Sullivan SK, Crowe PD, Santos
M, Conlon PJ, Sriramarao P and Bacon KB: Activation of
mitogen-activated protein kinase regulates eotaxin-induced
eosinophil migration. J Immunol. 163:1611–1618. 1999.PubMed/NCBI
|
|
17
|
Hoffmeyer A, Grosse-Wilde A, Flory E,
Neufeld B, Kunz M, Rapp UR and Ludwig S: Different
mitogen-activated protein kinase signaling pathways cooperate to
regulate tumor necrosis factor alpha gene expression in T
lymphocytes. J Biol Chem. 274:4319–4327. 1999. View Article : Google Scholar
|
|
18
|
Maruoka S, Hashimoto S, Gon Y, Takeshita I
and Horie T: PAF-induced RANTES production by human airway smooth
muscle cells requires both p38 MAP kinase and Erk. Am J Respir Crit
Care Med. 161:922–929. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jean-Mairet RM, López-Menéndez C,
Sánchez-Ruiloba L, et al: The neuronal protein Kidins220/ARMS
associates with ICAM-3 and other uropod components and regulates
T-cell motility. Eur J Immunol. 41:1035–1046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duan W, Chan JH, Wong CH, Leung BP and
Wong WS: Anti-inflammatory effects of mitogen-activated protein
kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol.
172:7053–7059. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elwood W, Lotvall JO, Barnes PJ and Chung
KF: Characterization of allergen-induced bronchial
hyperresponsiveness and airway inflammation in actively sensitized
brown-Norway rats. J Allergy Clin Immunol. 88:951–960. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vanacker NJ, Palmans E, Kips JC and
Pauwels RA: Fluticasone inhibits but does not reverse
allergen-induced structural airway changes. Am J Respir Crit Care
Med. 163:674–679. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Braun A, Appel E, Baruch R, et al: Role of
nerve growth factor in a mouse model of allergic airway
inflammation and asthma. Eur J Immunol. 28:3240–3251. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Glaab T, Hoymann HG, Hecht M, et al:
Effect of anti-nerve growth factor on early and late airway
responses in allergic rats. Allergy. 58:900–904. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagai T, Arai Y, Emori M, Nunome SY, Yabe
T, Takeda T and Yamada H: Anti-allergic activity of a Kampo
(Japanese herbal) medicine ‘Sho-seiryu-to (Xiao-Qing-Long-Tang)’ on
airway inflammation in a mouse model. Int Immunopharmacol.
4:1353–1365. 2004.
|
|
26
|
de Vries A, Engels F, Henricks PA, et al:
Airway hyper-responsiveness in allergic asthma in guinea-pigs is
mediated by nerve growth factor via the induction of substance P: a
potential role for trkA. Clin Exp Allergy. 36:1192–1200.
2006.PubMed/NCBI
|
|
27
|
Li L, Kong L, Fang X, et al: SH2-Bβ
expression in alveolar macrophages in BAL fluid of asthmatic guinea
pigs and its role in NGF-TrkA-mediated asthma. Respirology.
14:60–68. 2009.
|
|
28
|
Frossard N, Naline E, Olgart Hoglund C,
Georges O and Advenier C: Nerve growth factor is released by
IL-1beta and induces hyperresponsiveness of the human isolated
bronchus. Eur Respir J. 26:15–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nockher WA and Renz H: Neurotrophins in
allergic diseases: from neuronal growth factors to intercellular
signaling molecules. J Allergy Clin Immunol. 117:583–589. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iglesias T, Cabrera-Poch N, Mitchell MP,
Naven TJ, Rozengurt E and Schiavo G: Identification and cloning of
Kidins220, a novel neuronal substrate of protein kinase D. J Biol
Chem. 275:40048–40056. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Neubrand VE, Cesca F, Benfenati F and
Schiavo G: Kidins220/ARMS as a functional mediator of multiple
receptor signalling pathways. J Cell Sci. 125(Pt 8): 1845–1854.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chung KF: Airway smooth muscle cells:
contributing to and regulating airway mucosal inflammation? Eur
Respir J. 15:961–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moore PE, Church TL, Chism DD, Panettieri
RA Jr and Shore SA: IL-13 and IL-4 cause eotaxin release in human
airway smooth muscle cells: a role for ERK. Am J Physiol Lung Cell
Mol Physiol. 282:L847–L853. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jorritsma PJ, Brogdon JL and Bottomly K:
Role of TCR-induced extracellular signal-regulated kinase
activation in the regulation of early IL-4 expression in naive
CD4+T cells. J Immunol. 170:2427–2434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Finkelman FD, Hogan SP, Hershey GK,
Rothenberg ME and Wills-Karp M: Importance of cytokines in murine
allergic airway disease and human asthma. J Immunol. 184:1663–1674.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tillie-Leblond I, Pugin J, Marquette CH,
et al: Balance between pro-inflammatory cytokines and their
inhibitors in bronchial lavage from patients with status
asthmaticus. Am J Respir Crit Care Med. 159:487–494. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zimmermann N, Hershey GK, Foster PS and
Rothenberg ME: Chemokines in asthma: cooperative interaction
between chemokines and IL-13. J Allergy Clin Immunol. 111:227–242.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Secor ER, Carson WF, Singh A, Pensa M,
Guernsey LA, Schramm CM and Thrall RS: Oral bromelain attenuates
inflammation in an ovalbum-induced murine model of asthma. Evid
Based Complement Alternat Med. 5:61–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yin H, Jin XB, Gong Q, Yang H, Hu LY, Gong
FL and Zhu JY: Fructose-1,6-diphosphate attenuates acute lung
injury induced by lipopolysaccharide in mice. Int Immunopharmacol.
8:1842–1847. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakae S, Lunderius C, Ho LH, Schafer B,
Tsai M and Galli SJ: TNF can contribute to multiple features of
ovalbumin-induced allergic inflammation of the airways in mice. J
Allergy Clin Immunol. 119:680–686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui CH, Adachi T, Oyamada H, et al: The
role of mitogen-activated protein kinases in eotaxin-induced
cytokine production from bronchial epithelial cells. Am J Respir
Cell Mol Biol. 27:329–335. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lorentz A, Klopp I, Gebhardt T, Manns MP
and Bischoff SC: Role of activator protein 1, nuclear factor-κB,
and nuclear factor of activated T cells in IgE receptor-mediated
cytokine expression in mature human mast cells. J Allergy Clin
Immunol. 111:1062–1068. 2003.
|
|
44
|
Langdon C, Kerr C, Tong L and Richards CD:
Oncostatin M regulates eotaxin expression in fibroblasts and
eosinophilic inflammation in C57BL/6 mice. J Immunol. 170:548–555.
2003. View Article : Google Scholar : PubMed/NCBI
|