Introduction
Simvastatin reportedly promotes osteoblastic
activity and inhibits osteoclastic activity (1). It was shown to increase bone formation
when injected subcutaneously over the calvaria in mice and has been
demonstrated to increase cancellous bone volume in rats following
oral administration (2). The
successful use of simvastatin to promote bone formation in
vivo reportedly depends upon the local concentration. There
have been continuous efforts to determine an appropriate treatment
protocol (1).
Fibroblast growth factor-2 (FGF-2), a member of the
FGF family, is expressed by cells of the osteoblastic lineage.
FGF-2 promotes osteoblast proliferation and is secreted during the
healing process of fractures or at bone surgery sites (3). It was previously demonstrated that
FGF-2 stimulates bone formation and osteoblast differentiation
(4). However, the results of a
previous study demonstrated that cultures grown in the presence of
FGF-2 show an increased value for
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay
and a decreased value for alkaline phosphatase (ALP) activity
(5). Similarly, FGF-2 was shown to
inhibit bone morphogenetic protein-9 (BMP-9)-induced osteogenic
differentiation by blocking BMP-9-induced Smads signaling (6).
In this study, the combined effects of simvastatin
and FGF-2 on the proliferation and differentiation of
preosteoblasts were investigated. The dose-dependent effect of
simvastatin and FGF-2 on the proliferation of preosteoblasts was
also evaluated. An ALP test was performed to assess differentiation
and the expression of proteins associated with bone formation.
Specifically, estrogen receptor-α (ER-α) and estrogen receptor-β
(ER-β) were measured using western blot analysis to evaluate the
underlying mechanism. To the best of the author’s knowledge, this
study is the first to elucidate the combined effects of simvastatin
and FGF-2 on the expression of ER-α in preosteoblasts.
Materials and methods
Cell culture
Mouse calvarial preosteoblasts (MC3T3-E1) were
plated and maintained in an α-Minimum Essential Medium (αMEM;
Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (Invitrogen), antibiotics (penicillin 100 U/ml and
streptomycin 100 μg/ml; Invitrogen), 10 mM β-glycerophosphate
(Sigma, St. Louis, MO, USA) and 50 μg/ml ascorbic acid (Sigma). The
cells were stimulated with simvastatin and FGF-2 at final
concentrations of 0.1 μM (S1) to 1 μM (S2) for simvastatin and 2
ng/ml (F1) to 20 ng/ml (F2) for FGF-2. The cultures were maintained
in a humidified atmosphere with 5% CO2 and 95% air at
37°C.
Protein measurement
Mouse cells were incubated in αMEM in the presence
of ascorbic acid and β-glycerophosphate for 2 days. The protein
content was determined based on the Bradford method, using the
Coomassie protein assay reagent with a series of bovine serum
albumins used as internal standards (7). The absorbance was measured at 595 nm
using a microplate spectrophotometer (BioTek, Winooski, VT, USA).
The results are presented as the percentage of control values.
ALP activity assay
An ALP assay for osteoblast differentiation was
performed after 2 days. The mouse preosteoblasts were lysed with a
buffer containing 10 mM Tris-HCl (pH 7.4) and 0.2% Triton X-100.
The samples were then sonicated for 20 sec at 4°C and incubated
with 10 mM p-nitrophenylphosphate as a substrate in 100 mM glycine
buffer (pH 10.5) containing 1 mM MgCl2 in a water bath
at 37°C. The absorbance was measured at 405 nm using a microplate
reader (BioTek). In addition, ALP activity was normalized with
respect to the total protein content (8,9).
Western blot analysis
The preosteoblasts were washed twice with ice-cold
phosphate-buffered saline (PBS) and solubilized with a lysis
buffer. The lysates were centrifuged at 16,000 × g for 20 min at
4°C to remove the nuclear pellet. The supernatants were boiled in a
sodium dodecyl sulfate sample buffer containing β-mercaptoethanol.
Equal amounts of cell extracts were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride microporous membranes (Immobilon-P;
Millipore Corporation, Billerica, MA, USA). The membranes were then
blocked for at least 1 h in 0.1% (v/v) PBS and Tween-20 containing
5% (w/v) powdered milk. Each membrane was probed with the desired
antibodies, which were diluted in the same buffer at the
recommended concentrations. Each membrane was incubated with
horseradish peroxidase-conjugated secondary antibody. Subsequently,
the washed blot was developed with enhanced chemiluminescence
detection kits (5,10). The mouse anti-ER-α and anti-ER-β
antibodies and the secondary antibodies conjugated with horseradish
peroxidase were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA), Abcam (Cambridge, MA, USA), and Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Results
Protein measurement
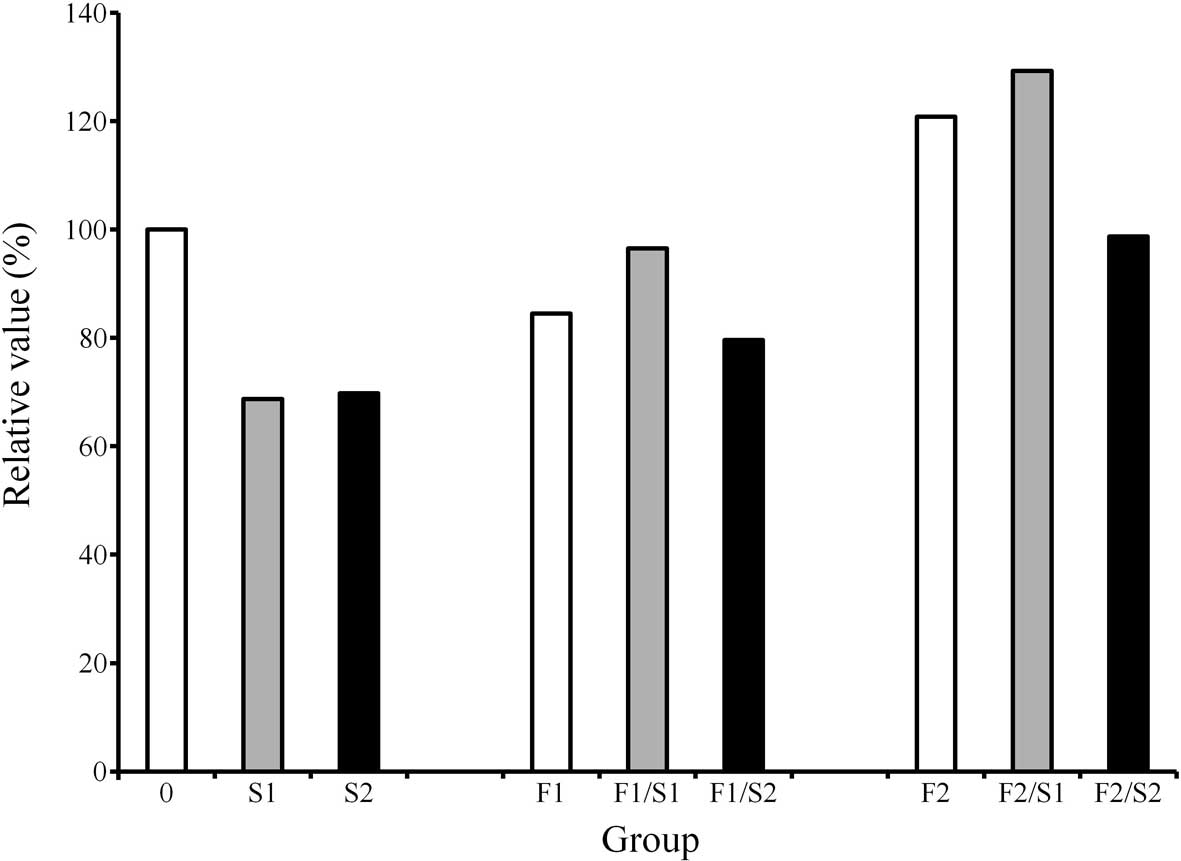
The protein content in each culture plate was
evaluated (Fig. 1). The results
demonstrated that the protein content of the cultures grown in the
osteogenic differentiation media in the presence of FGF-2 at 20
ng/ml was higher compared to that of the control cultures. However,
the addition of 1.0 μM simvastatin to the cultures uniformly led to
a decrease in protein content compared to the simvastatin-unloaded
group.
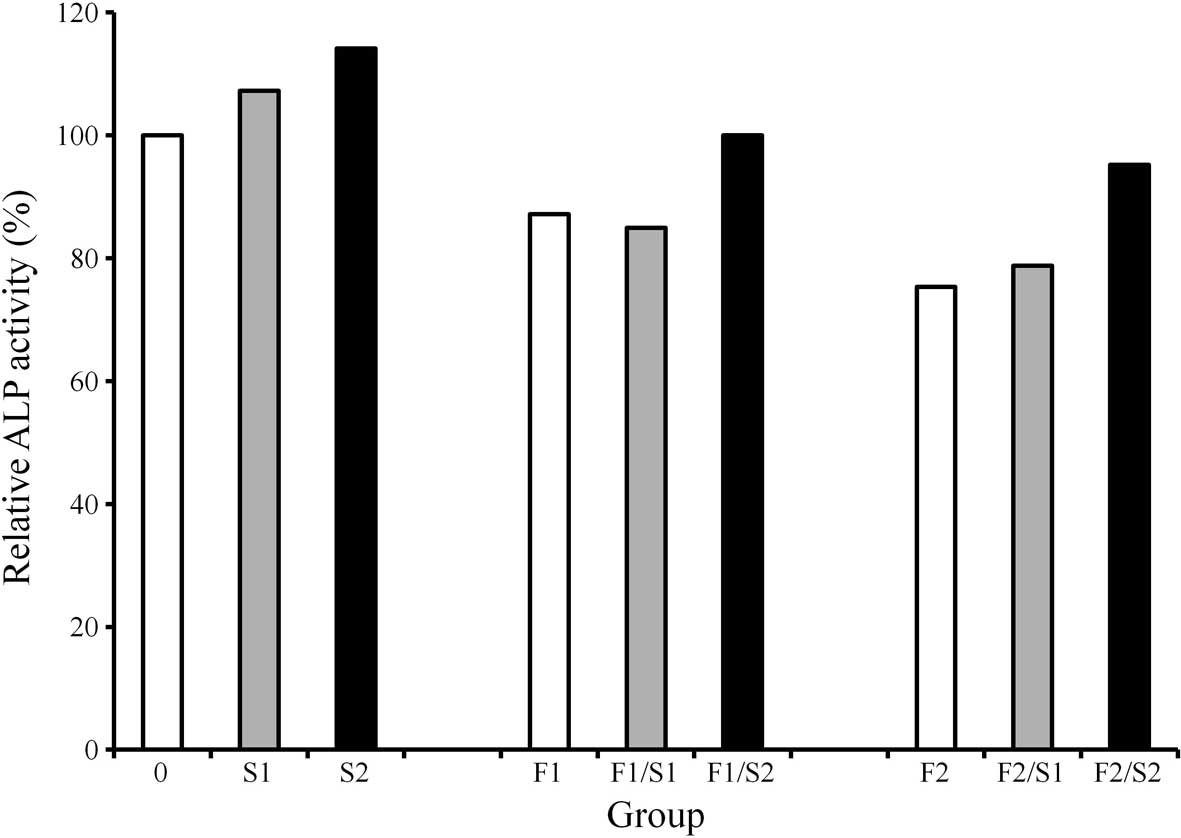
ALP activity assay
ALP activity decreased when cells were treated with
FGF-2 (2 and 20 ng/ml) (Fig. 2).
The cultures grown in the presence of simvastatin (0.1 and 1.0 μM)
exhibited an increase in ALP activity compared to the control
cultures. Similarly, cultures grown in the presence of 1 μM
simvastatin and 2 ng/ml FGF-2 exhibited an increased value of ALP
activity when compared to that of the 2 ng/ml FGF-2-only group. The
addition of 1 μM simvastatin to 20 ng/ml of FGF-2 resulted in an
increase in ALP activity compared to that of the 20 ng/ml FGF-2
group.
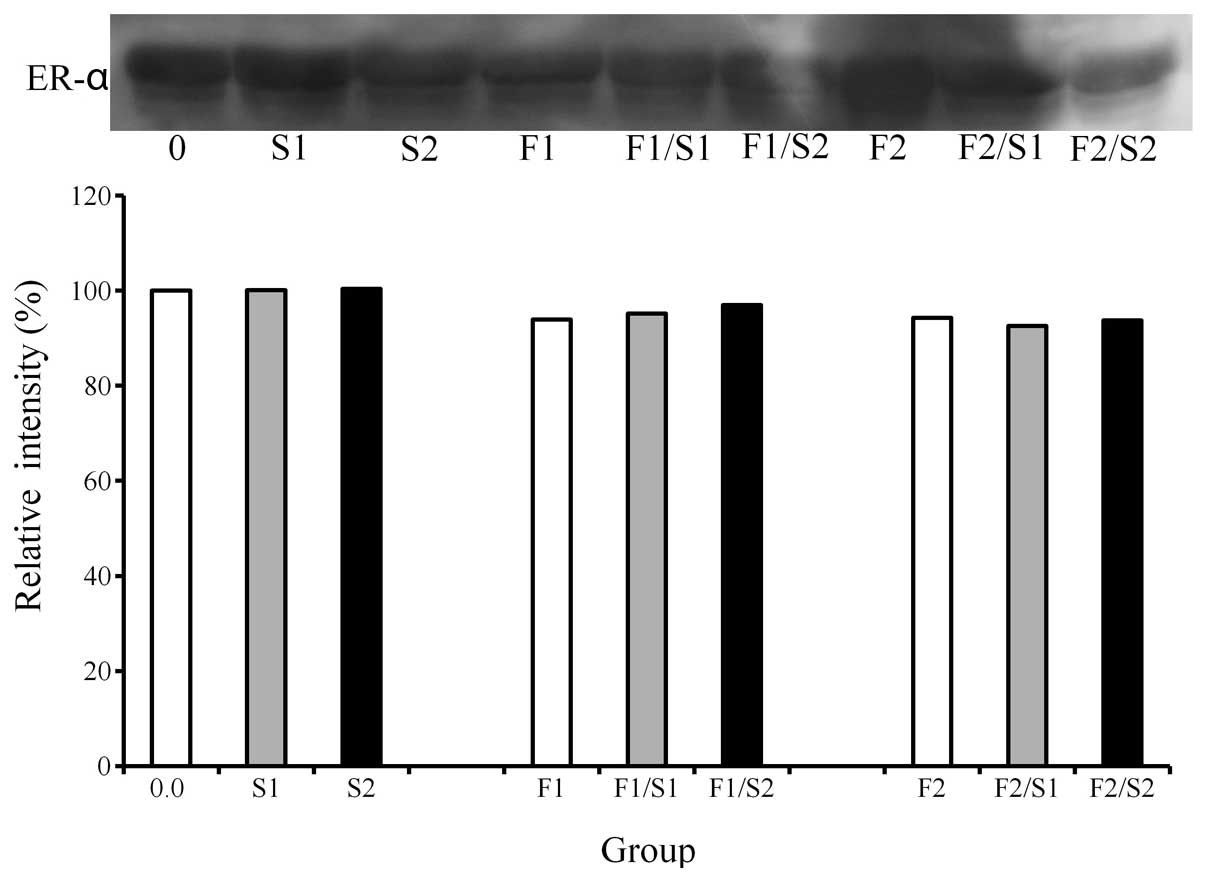
Western blot analysis
A western blot analysis was performed to detect the
protein expression following treatment with simvastatin and FGF-2
(Fig. 3). The results demonstrated
that the addition of simvastatin (0.1 and 1.0 μM) appeared to
increase the expression of ER-α. Similarly, the combination of 1.0
μM simvastatin and 2 ng/ml FGF-2 led to a higher ER-α expression
compared to that of the 2 ng/ml FGF-2-only group.
Discussion
In this study, the combined effects of simvastatin
and FGF-2 on the protein content, differentiation and protein
expression of preosteoblasts under predetermined concentrations
(0.1 and 1.0 μM simvastatin; 2 and 20 ng/ml FGF-2) were
investigated. The mechanism of action of simvastatin and FGF-2 on
the regulation of protein expression in mouse preosteoblasts was
also investigated. In addition, evaluations were performed to
determine whether the combination of simvastatin and FGF-2 produced
effects additively, synergistically, or competitively.
An increase in protein content was achieved in the
20 ng/ml FGF-2 group under osteogenic differentiation media. This
finding was consistent with the results of a previous study
demonstrating that FGF-2 affected the proliferation of osteoblasts
(5). The treatment of mouse
preosteoblasts with simvastatin increased the level of ALP
activity. Similarly, previous results demonstrated that cultures
grown in the presence of simvastatin exhibited an increased value
of ALP activity and mineralization (11). However, treatment with FGF-2 yielded
a decreased value of ALP activity. This result was consistent with
that of a previous study demonstrating that FGF-2 affected the
differentiation of the cells under investigation (5). This study has demonstrated that the
addition of 1.0 μM simvastatin to 20 ng/ml FGF-2 led to an increase
in ALP activity compared to that of the 20 ng/ml FGF-2 group.
However, the value of ALP activity with the combination of 1.0 μM
simvastatin and 20 ng/ml FGF-2 did not reach the value of the
untreated control group.
Western blot analysis was performed to detect the
protein expression of ER-α and ER-β to in order to provide
information on potential additional mechanisms. The results
demonstrated that the addition of simvastatin (0.1 and 1.0 μM)
appeared to increase the expression of ER-α. Similarly, the
combination of 1.0 μM simvastatin and 2 ng/ml FGF-2 led to a higher
ER-α expression compared to that of the 2 ng/ml FGF-2-only
group.
Estrogens reportedly play a key role in bone
formation and bind to estrogen receptors to exert their
tissue-specific effects (7,12). The findings of this study suggest
that a combination of simvastatin and FGF-2 may partially exert
effects on preosteoblasts through the expression of ER-α (11). The results regarding the effect of
the combination of simvastatin and FGF-2 on osteoblastic
proliferation and differentiation may be controversial due to the
different culture conditions, type of cells, maturation stages of
the cells under investigation and differences among species
(13,14).
Within the limits of this study, simvastatin
enhanced osteoblast differentiation. However, the combined
treatment with simvastatin and FGF-2 did not exert synergistic
effects on osteoblast differentiation under the current
experimental conditions. Therefore, future studies are required to
evaluate divergent conditions and determine the selective timing
and the optimal dosage for the delivery of these agents.
Acknowledgements
This study was supported by the Seoul St. Mary’s
Hospital Clinical Medicine Research Program year of 2013 through
the Catholic University of Korea.
References
|
1
|
Park JB: The use of simvastatin in bone
regeneration. Med Oral Patol Oral Cir Bucal. 14:e485–e488.
2009.PubMed/NCBI
|
|
2
|
Mundy G, Garrett R, Harris S, et al:
Stimulation of bone formation in vitro and in rodents by statins.
Science. 286:1946–1949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Biver E, Soubrier AS, Thouverey C, et al:
Fibroblast growth factor 2 inhibits up-regulation of bone
morphogenic proteins and their receptors during osteoblastic
differentiation of human mesenchymal stem cells. Biochem Biophys
Res Commun. 427:737–742. 2012. View Article : Google Scholar
|
|
4
|
Zhou L and Ogata Y: Transcriptional
regulation of the human bone sialoprotein gene by fibroblast growth
factor 2. J Oral Sci. 55:63–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JB: Effects of fibroblast growth
factor 2 on osteoblastic proliferation and differentiation by
regulating bone morphogenetic protein receptor expression. J
Craniofac Surg. 22:1880–1882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song T, Wang W, Xu J, et al: Fibroblast
growth factor 2 inhibits bone morphogenetic protein 9-induced
osteogenic differentiation of mesenchymal stem cells by repressing
Smads signaling and subsequently reducing Smads dependent
up-regulation of ALK1 and ALK2. Int J Biochem Cell Biol.
45:1639–1646. 2013. View Article : Google Scholar
|
|
7
|
Park JB: Low dose of doxycyline promotes
early differentiation of preosteoblasts by partially regulating the
expression of estrogen receptors. J Surg Res. 178:737–742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JB: The effects of dexamethasone,
ascorbic acid, and β-glycerophosphate on osteoblastic
differentiation by regulating estrogen receptor and osteopontin
expression. J Surg Res. 173:99–104. 2012.
|
|
9
|
Park JB: Combination of simvastatin and
bone morphogenetic protein-2 enhances differentiation of
osteoblastic cells by regulating the expression of
phospho-Smad1/5/8. Exp Ther Med. 4:303–306. 2012.PubMed/NCBI
|
|
10
|
Park JB: Effects of doxycycline,
minocycline, and tetracycline on cell proliferation,
differentiation, and protein expression in osteoprecursor cells. J
Craniofac Surg. 22:1839–1842. 2011. View Article : Google Scholar
|
|
11
|
Park JB, Zhang H, Lin CY, et al:
Simvastatin maintains osteoblastic viability while promoting
differentiation by partially regulating the expressions of estrogen
receptors α. J Surg Res. 174:278–283. 2012.PubMed/NCBI
|
|
12
|
Pinzone JJ, Stevenson H, Strobl JS and
Berg PE: Molecular and cellular determinants of estrogen receptor α
expression. Mol Cell Biol. 24:4605–4612. 2004.
|
|
13
|
Kim CH, Cheng SL and Kim GS: Effects of
dexamethasone on proliferation, activity, and cytokine secretion of
normal human bone marrow stromal cells: possible mechanisms of
glucocorticoid-induced bone loss. J Endocrinol. 162:371–379. 1999.
View Article : Google Scholar
|
|
14
|
Ishida Y and Heersche JN:
Glucocorticoid-induced osteoporosis: both in vivo and in vitro
concentrations of glucocorticoids higher than physiological levels
attenuate osteoblast differentiation. J Bone Miner Res.
13:1822–1826. 1998. View Article : Google Scholar
|