Introduction
Ranpirnase (Onconase) was isolated from oocytes or
early embryos of the northern leopard frog (Rana pipiens)
and is a polypeptide with 104 amino acid residues and four
disulfide bonds (Fig. 1).
Ranpirnase is the smallest member of the ribonuclease (RNase A)
superfamily, and appears to be a promising drug with broad clinical
application in tumor treatment due to its moderate cytotoxicity,
unique synergy, low immunogenicity and few side effects (1–10).
Between 1996 and 2004, Tamir Biotechnology, Inc. (formerly Alfacell
Corporation) successively conducted clinical investigations
regarding the effects of ranpirnase on breast cancer, pancreatic
cancer, renal cell carcinoma, non-small cell lung cancer and
malignant mesothelioma, for which the therapeutic effect was the
most significant with few side effects. Ranpirnase is currently
used as a drug for malignant mesothelioma in a phase IIIb clinical
trial and for non-small cell lung cancer in a phase II clinical
trial.
Materials and methods
Materials
The oligonucleotide primers were chemically
synthesized at BioSune (Shanghai, China). Aminopeptidase and Papain
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Agilent
reverse-phase columns (analytical column, Zorbax 300SB-C18, 4.6×250
mm; semi-preparative column, Zorbax 300SB-C18, 9.4×250 mm) were
used in the experiments. The peptide was eluted from the columns
with an acetonitrile gradient composed of solvent A and B. Solvent
A was 0.1% aqueous TFA, and solvent B was acetonitrile containing
0.1% TFA. The elution gradient was as follows: 0 min, 10% solvent
B; 3 min, 10% solvent B; 53 min, 60% solvent B; 55 min, 100%
solvent B; 56 min, 100% solvent B, and 57 min, 10% solvent B. The
flow rate for the analytical column was 0.5 ml/min, and that for
the semi-preparative column was 1.0 ml/min. The eluted peptide was
detected by UV absorbance at 280 and 214 nm.
Gene construction, recombinant
expression, and purification of 6xHis-Ranpirnase
The gene of 6xHis-Ranpirnase was constructed from
two chemically synthesized DNA primers according to a previous
study (11). Briefly, we designed
two primers with the following sequences: P1 5′-CCATCACCATC
ATATGCAGGATTGGCTGACCTTTCAAAAAAAACATA TTACGAACACTCGTGAT-3′; and P2
5′-TGAATTCTTAACA AGAGCCAACGCCCACGAAGTGGACCGGTGCCTGGTT
TTCGCACGTAACACAAAATTTATTGGTGCT-3′; and four DNA fragments with the
following sequences: F1 5′-CTGT
TTCACTGCAAAGATAAAAATACCTTCATCTATTCTCG
CCCGGAACCGGTTAAAGCGATTTGCAAAGGCATT ATC-3′; F2
5′-TTTACCTGAGCGACTGTAACGTAACCTCG
CGCCCGTGCAAATATAAACTGAAAAAGAGCACCAAT AAATTTTGTGTT-3′; F3
5′-GGTTACGTTACAGTCGCT CAGGTAAAACTCGCTCGTGGTCAGGACATTTTTTGG
AGGCGATAATGCCTTTGCAAATCGC-3′; and F4 5′-GGT
GTTTTTATCTTTGCAGTGAAACAGGTTAGTGCTCATG
ATGTTATCACAGTCCACATCACGAGTGTTCGTAAT ATG-3′. These DNA fragments
were linked by T4 DNA polymerase and dNTP, and constructed the
Onconase gene. After annealing, elongation by T4 DNA polymerase,
cleavage by restriction enzyme NdeI and EcoRI, the
DNA fragment was ligated into a pET28a vector pretreated with the
same restriction enzymes. Its sequence was confirmed by DNA
sequencing. Thereafter, the expression construct
pET28a/6xHis-Ranpirnase was transformed into Escherichia
coli (E. coli) strain BL21 (DE3) star, and the
transformed cells were cultured in liquid TB medium (with 100 μg/ml
ampicillin) to OD600 nm=2.5 at 37°C with vigorous shaking (7 × g).
After being induced by 0.8 mM of isopropyl thio-β-D-galactoside
(IPTG) at 37°C for 6–8 h, the E. coli cells were harvested
by centrifugation (5,000 × g, 10 min), re-suspended in lysis buffer
(50 mM Tris-HCl, pH 8.5, 0.5 M NaCl), and lysed by sonication.
After centrifugation (10,000 × g, 15 min), the inclusion body
pellet was re-suspended in solubilizing buffer (50 mM Tris-HCl, 6 M
guanidine chloride, pH 8.5) and S-sulfonated by the addition of
solid sodium sulfite and sodium tetrathionate to a final
concentration of 200 and 150 mM, respectively. The S-sulfonation
reaction was carried out at 4°C with gentle agitation for 2–3 h.
After centrifugation (10,000 × g, 15 min), the supernatant was
loaded onto a Ni2+ column that was pre-equilibrated with
the washing buffer (50 mM Tris-HCl, 3 M guanidine chloride, pH
8.5). The S-sulfonated precursor was eluted from the column by
step-wise increase of imidazole concentration in the washing
buffer. The eluted S-sulfonated 6xHis-Ranpirnase was subjected to
dialysis in water at 4°C overnight in order to remove imidazole.
After centrifugation (6,000 × g, 10 min), the pellet was
re-suspended in solubilization buffer (50 mM Tris-HCl, 2.4 M
guanidine chloride, pH 8.5).
Aminopeptidase cleavage of the
S-sulfonated ranpirnase precursors, cyclization and in vitro
refolding
The S-sulfonated ranpirnase precursors
(6xHis-Ranpirnase) were digested by aminopeptidase (peptide enzyme
molar ratio 2,000:1) in the digestion buffer (2.4 M guanidine
chloride, 50 mM Tris-HCl, 0.1 mM Zncl2, pH 8.5) at 37°C
overnight, and the N-terminal of the digestion products was
directly cyclized at 30°C overnight by cyclotransferase purified
from Papain (crude powder from Papaya Latex, Sigma) according to
the procedures mentioned in Zerhouni et al(12). The cyclization mixture was initially
treated with 50 mM dithiothreitol (DTT) at room temperature for 15
min. Subsequently, the treated mixture was 50-fold diluted into the
pre-incubated refolding buffer (0.5 M L-arginine, 1.0 mM EDTA, 3.0
mM oxidized glutathione, pH 8.5). The refolding reaction was
carried out at 4°C for 6–8 h. The refolding mixture was then
acidified to pH 3.0 by trifluoroacetic acid and subjected to C18
reverse-phase high-performance liquid chromatography (HPLC). The
eluted refolded ranpirnase fraction was manually collected,
lyophilized and analyzed by mass spectrometry.
The effect of the folded ranpirnase on
human glioma cell line SHG-44
Human glioma cell line SHG-44 was obtained from the
cell library of the Shanghai Institute and cultured in 96-well
plates at a density of 1×104/ml Dulbecco’s modified
Eagle’s medium, supplemented with glutamine, penicillin,
streptomycin and 10% fetal calf serum. The folded ranpirnase was
added to the cells after plating overnight. The cells were then
examined using MTT assay following treatment with the folded
ranpirnase in CO2 incubator for 36 h, allowing ≥3–4
rounds of application at different concentrations of the folded
ranpirnase.
Results
Expression, and purification of
ranpirnase precursors
The recombinant plasmid pET-Ranpirnase was
constructed, and 6xHis-Ranpirnase was recombinantly expressed in
the E. coli strain BL21(DE3)star under IPTG induction. As
analyzed by tricine SDS-PAGE, a band with a molecular weight of ~12
kDa was significantly increased following IPTG induction (Fig. 2A). After the E. coli cells
were lysed by sonication, the precursor was present in the pellet
(Fig. 2B), suggesting that
6xHis-Ranpirnase formed inclusion bodies. The inclusion bodies were
solubilized by 6 M guanidine chloride and then treated with sodium
sulfite and sodium tetrathionate to obtain an S-sulfonated
precursor. The S-sulfonated precursor was then subjected to
immobilized metal-ion affinity chromatography (Ni2+
column) (Fig. 2C). The S-sulfonated
precursor (indicated by a star) was eluted by 250 mM imidazole from
the Ni2+ column, and the eluted S-sulfonated precursor
was subjected to dialysis to remove imidazole. Following
centrifugation, the pellet was re-suspended in the solubilization
buffer.
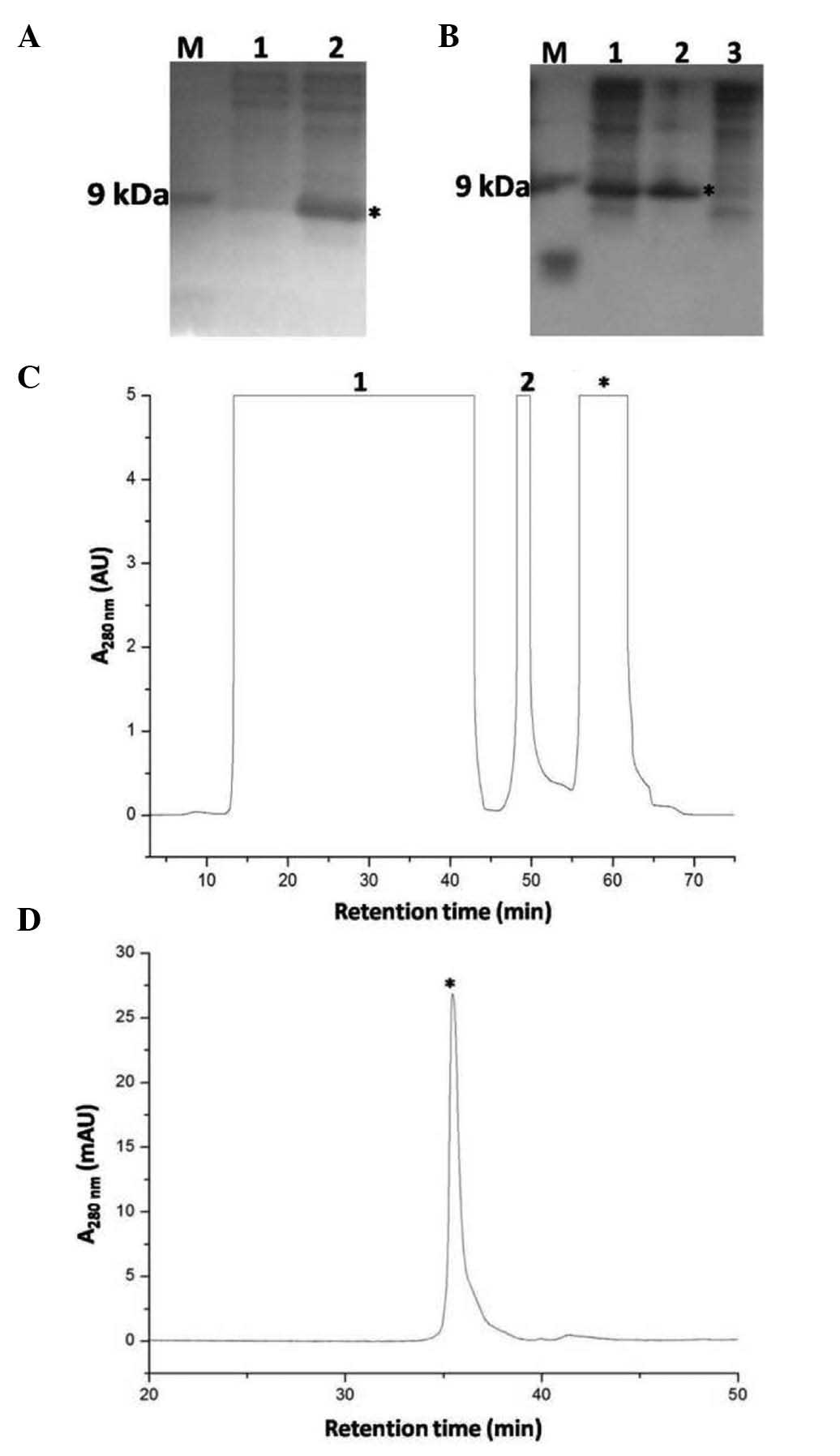 | Figure 2(A) SDS-PAGE analysis of
6xHis-ranpirnase expression. M, Marker; lane 1, before IPTG
induction; lane 2, after IPTG induction. The band of
6xHis-ranpirnase was indicated in lane 2 after induction by IPTG
(indicated by a star). (B) SDS-PAGE analyses of the
6xHis-ranpirnase precursor at the primary purification stage. M,
Marker; lane 1, total lysate; lane 2, P, pellet; lane 3,
supernatant. The band of 6xHis-ranpirnase was indicated by a star.
(C) FPLC profile of the S-sulfonated 6xHis-ranpirnase purified with
an immobilized metal-ion affinity column (Ni2+ column).
Lane 1, flow-through; lane 2, eluted fraction by 30 mM imidazole;
eluted S-sulfonated 6xHis-ranpirnase precursor by 250 mM imidazole
was indicated by a star. (D) HPLC profile of the S-sulfonated
6xHis-ranpirnase precursor eluted from the Ni2+ column.
The peak of S-sulfonated 6xHis-ranpirnase precursor was indicated
by a star. |
Aminopeptidase cleavage of ranpirnase
precursors, cyclization and in vitro refolding of S-sulfonated
ranpirnase
The dialysised S-sulfonated 6xHis-Ranpirnase was
analyzed by C18 reverse-phase HPLC (Fig. 2D). The measured molecular mass of
the eluted peak (indicated by a star) was 13,566, which was similar
to the expected value (13,561.8) of the S-sulfonated precursor, and
it was confirmed in later studies that the peak was the expected
S-sulfonated 6xHis-Ranpirnase. The dialysised S-sulfonated
precursors were digested by aminopeptidase, and the N-terminal of
the digestion mixture was directly cyclized by cyclotransferase.
The digested and cyclized S-sulfonated ranpirnase was analyzed by
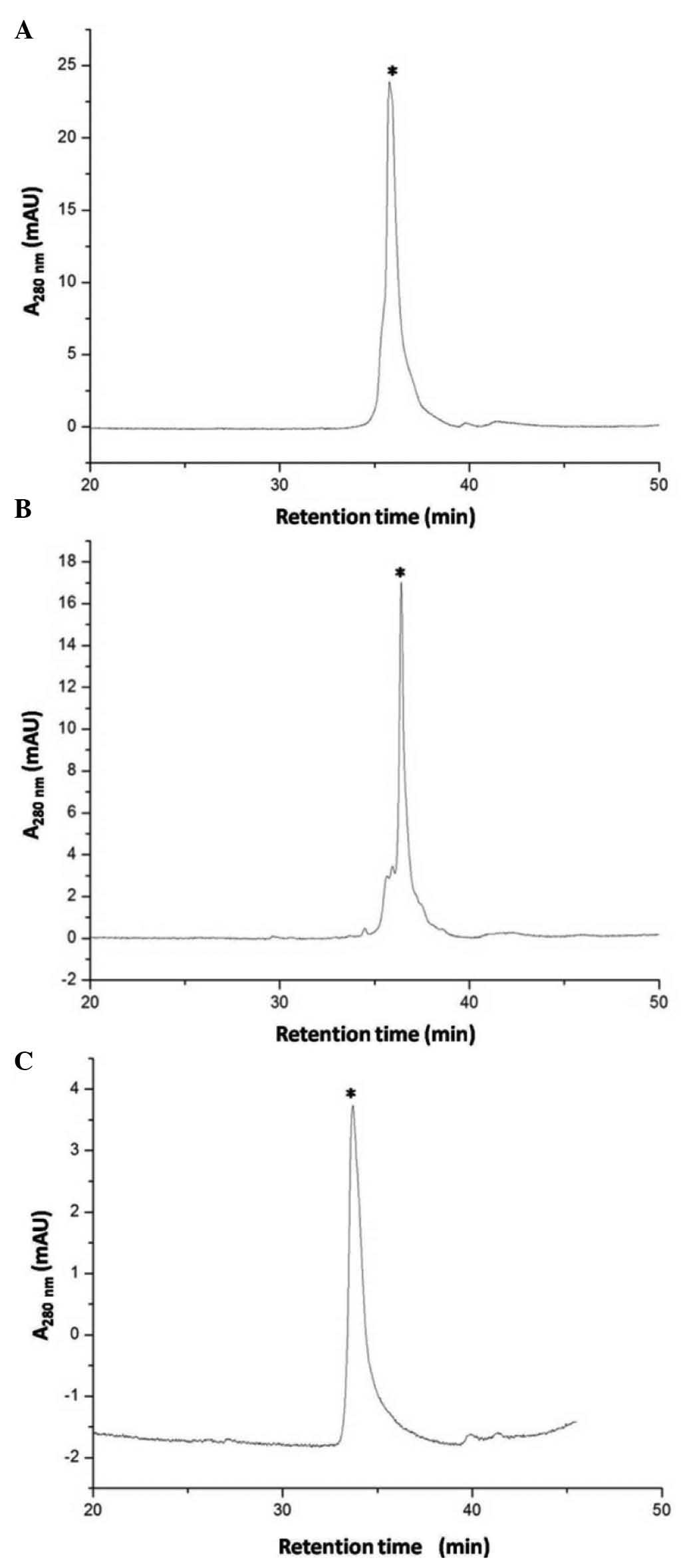
C18 reverse-phase HPLC, as shown in Fig. 3A and B. The measured molecular mass
of the eluted peak (indicated by a star) was 12,485 and 12,466,
respectively, similar to the expected values (12,484 and 12,467) of
the digested and cyclized S-sulfonated ranpirnase, and it was
confirmed in later studies that the peaks were the expected
digested and cyclized S-sulfonated ranpirnase. As shown in Fig. 3C, in in vitro refolding the
measured molecular mass of the eluted peak (indicated by a star)
was 11,819, consistent with the expected value 11,819.5 of the
refolded ranpirnase.
The effect of the folded ranpirnase on
human glioma cell line SHG-44
To determine the cytotoxic effect of ranpirnase on
the SHG-44 cell line, MTT assay was performed after incubation with
ranpirnase. The ranpirnase concentration required for 50% cell
survival (the surviving fraction is 50%, SF0.5) was determined as
shown in Fig. 4. Following
co-incubation with the SHG-44 cell line for 36 h, the ranpirnase
concentration for SF0.5 was ~15 μM.
Discussion
Ranpirnase is a disulfide-rich peptide with 104
amino acids and four disulfide bonds. Although the recombinant
expression procedure of ranpirnase has been previously reported by
Notomista et al(2), we found
that ranpirnase was autocatalytically cyclized at 30°C overnight
with secondary reaction products after digestion with
aminopeptidase. Thus, we utilized cyclotransferase to catalyze
ranpirnase cyclization after digestion with the aminopeptidase to
reduce the secondary reaction products. Additionally, the plant
cyclotransferase, which is resistant to chemical denaturation,
simplified the purification procedure following digestion with the
aminopeptidase because it could be used in the buffer containing
guanidine chloride at a high concentration level, which made the
ranpirnase precursors soluble at a high concentration for the high
digestive efficiency with aminopeptidase.
The folded 6xHis-Ranpirnase could not be efficiently
digested by aminopeptidase probably due to steric hindrances. Thus,
we employed an S-sulfonation approach, by which the eight cysteine
residues of the ranpirnase precursors were reversibly modified by
sulfonate moieties, in order to improve digestion. This also
reduced the crosslink between two ranpirnase molecules, rendering
ranpirnase precursors highly soluble in the enzyme digestion buffer
for efficient cleavage with aminopeptidase. After removal of the
N-terminal 6xHis tag, the S-sulfonated ranpirnase was efficiently
refolded in vitro with ~70% yield under optimized condition,
and the final yield of mature ranpirnase was ~50–60 mg per liter
cultures. In addition, ranpirnase inhibited the growth of human
glioma cells SHG-44 in a dose-dependent manner. Thus, the present
study has provided an efficient approach for the preparation of
active ranpirnase and its analogues for future studies.
Acknowledgements
This study was supported by the Chinese Major
Scientific and Technological Special Project for ‘Major New Drugs
Creation’ (2009ZX09103-656).
References
|
1
|
Darzynkiewicz Z, Carter SP, Mikulski SM,
Ardelt WJ and Shogen K: Cytostatic and cytotoxic effects of Pannon
(P-30 Protein), a novel anticancer agent. Cell Tissue Kinet.
21:169–182. 1988.PubMed/NCBI
|
|
2
|
Notomista E, Cafaro V, Fusiello R, Bracale
A, D’Alessio G and Di Donato A: Effective expression and
purification of recombinant onconase, an antitumor protein. FEBS
Lett. 463:211–215. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newton DL, Hansen HJ, Mikulski SM,
Goldenberg DM and Rybak SM: Potent and specific antitumor effects
of an anti-CD22-targeted cytotoxic ribonuclease: potential for the
treatment of non-Hodgkin lymphoma. Blood. 97:528–535. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavlakis N and Vogelzang NJ: Ranpirnase -
an antitumour ribonuclease: its potential role in malignant
mesothelioma. Expert Opin Biol Ther. 6:391–399. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee I, Kalota A, Gewirtz AM and Shogen K:
Antitumor efficacy of the cytotoxic RNase, ranpirnase, on A549
human lung cancer xenografts of nude mice. Anticancer Res.
27:299–307. 2007.PubMed/NCBI
|
|
6
|
Chang CH, Gupta P, Michel R, Loo M, Wang
Y, Cardillo TM and Goldenberg DM: Ranpirnase (frog RNase) targeted
with a humanized, internalizing, anti-Trop-2 antibody has potent
cytotoxicity against diverse epithelial cancer cells. Mol Cancer
Ther. 9:2276–2286. 2010. View Article : Google Scholar
|
|
7
|
Nasu M, Carbone M, Gaudino G, et al:
Ranpirnase interferes with NF-κB pathway and MMP9 activity,
inhibiting malignant mesothelioma cell invasiveness and xenograft
growth. Genes Cancer. 2:576–584. 2011.PubMed/NCBI
|
|
8
|
Qiao M, Zu LD, He XH, Shen RL, Wang QC and
Liu MF: Onconase downregulates microRNA expression through
targeting microRNA precursors. Cell Res. 22:1199–1202. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao HL, Xue C, Du JL, Ren M, Xia S, Cheng
YG and Liu ZM: Sustained and cancer cell targeted cytosolic
delivery of Onconase results in potent antitumor effects. J Control
Release. 159:346–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Westekemper H, Freistuehler M, Bornfeld N,
Steuhl KP, Scheulen M and Hilger RA: Chemosensitivity of
conjunctival melanoma cell lines to target-specific
chemotherapeutic agents. Graefes Arch Clin Exp Ophthalmol.
251:279–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ardelt W, Mikulski SM and Shogen K: Amino
acid sequence of an anti-tumor protein from Rana pipiens
oocytes and early embryos. Homology to pancreatic ribonucleases. J
Biol Chem. 266:245–251. 1991.PubMed/NCBI
|
|
12
|
Zerhouni S, Amrani A, Nijs M, Smolders N,
Azarkan M, Vincentelli J and Looze Y: Purification and
characterization of papaya glutamine cyclotransferase, a plant
enzyme highly resistant to chemical, acid and thermal denaturation.
Biochim Biophys Acta. 1387:275–290. 1998. View Article : Google Scholar : PubMed/NCBI
|