Introduction
Influenza is an acute respiratory disease, with high
morbidity and mortality rates in humans and animals (1–2). It
has become one of the major diseases worldwide, with a significant
impact on the health of the general population and the national
economy. The influenza A virus is prone to antigenic drift or
antigenic conversion and reorganization of the genome, which leads
to the emergence of new subtypes and lack of immunity in the
majority of the population. The currently available western
medicinal options for the prevention and treatment of influenza A
virus infection are accompanied by relatively severe side effects
and development of drug resistance with their wide range of
applications. Furthermore, the vaccines against the new influenza A
virus are ineffective. Therefore, effective anti-influenza drugs
used in Chinese medicine should be considered. Chinese medicine
enhances the immunity against influenza virus infection, mainly by
regulating the immune system (3).
Previous studies demonstrated that quercetin
possesses significant antitumor, anti-inflammatory, antioxidant and
other biological properties (4,5).
Several previous studies demonstrated that quercetin directly
interacts with different types of viruses, reducing their infective
potential (6,7). It was previously reported that
quercetin interfered with the gene signals that enable hepatitis C
virus production, indicating that quercetin may allow for
dissection of the viral life cycle and has potential therapeutic
use by reducing virus production with low-associated toxicity
(8). Another study demonstrated
that quercetin may reduce influenza A viral replication by directly
interacting with viral particles (9). It was also previously reported that
quercetin significantly boosted the respiratory antioxidant defense
system in mice exposed to influenza A, providing significant
protection for the lungs under high-stress conditions (10).
A previous study by Zhang et al(11) reported that overexpression of
cyclin-dependent kinase (CDK4) was statistically significant in
Epstein-Barr virus-associated nasopharyngeal carcinoma tissues
compared to non-cancerous nasopharyngeal epithelial tissues
(P<0.05) by quantitative reverse-transcription polymerase chain
reaction (RT-PCR) and tissue microarray.
At present, there are no studies available on the
antagonizing action of quercetin towards the CDK4 induction caused
by H1N1 influenza virus. The aim of this study was to investigate
the effect of quercetin on the expression of CDK4 mRNA and protein
in H1N1-infected A549 cells, elucidate the mechanism underlying the
anti-influenza action of quercetin and provide experimental
evidence that may lead to the development of novel anti-influenza
drugs.
Materials and methods
Virus and cells
Influenza A H1N1 (A1/Qian fang/166/85), was provided
by the Chinese Academy of Traditional Chinese Medicine and stored
at −75°C in the laboratory with the hemagglutination titer virus
stock solution of 2−7. The A549 human lung epithelial
cell line was purchased from the Cell Culture Center of Peking
Union Medical College and cultured prior to use.
Drugs
Quercetin (lot no. 100081-200907, purchased from The
Control of Pharmaceutical and Biological Products, Beijing; HPLC
purity 96.5%), was stored in incomplete DMEM containing 1/1000
dimethyl sulfoxide (DMSO) to create a 2-g/l stock. Ribavirin
particles (lot no. 100725, Sichuan Baili Pharmaceutical Co., Ltd.,
Sichuan, China), were stored in incomplete DMEM to create a 10-g/l
stock. The drugs mentioned above were filtered through a 0.22-μm
Millipore filter (Millipore, Billerica, MA, USA), packaged and
stored at 4°C.
Reagents
The reagents used are listed below: fetal calf serum
(Hyclone, Logan, UT, USA), DMEM and 0.25% trypsin digestion
solution (Neuron Biotech Co., Ltd), Thiazolyl Blue Tetrazolium
Bromide (MTT) and DMSO (Sigma, St. Louis, MO, USA), TRIzol kit
(Invitrogen, Carlsbad, CA, USA), M-MLV reverse transcription kit
(Takara Bio, Inc., Shiga, Japan), qPCR amplification kit (Beijing
Zeping Bioscience & Technologies Co., Ltd., Beijing, China),
SYBR-Green PCR mix (Bio-Rad, Hercules, CA, USA; cat no.: 170-8880,
lot no.: 20220B), agarose (Promega, Madison, WI, USA), diethyl
pyrocarbonate (DEPC, Sigma), cell lysis buffer (Beyotime, Nanjing,
China), CDK4 primary antibody (Millipore; cat no.: MAB8879, lot
no.: NG1753071), mouse anti-human β-actin (Boshide Bioengineering
Co., Ltd., Wuhan, China; cat no.: BM0627) and goat anti-mouse IgG
secondary antibody (Beijing Zhongshan Golden Bridge BioTecnology
Co., Ltd., Beijing, China; cat no.: ZB-2305 lot no.: 92580). The
western blot analysis reagents were prepared in the laboratory. The
PCR primer sequences were synthesized by Shanghai Sangon Biological
Engineering Technology and Service Co., Ltd., Shanghai, China, and
were as follows: CDK4 (154 bp): F: 5′-CACCCGTGGTTGTTACACTC-3′ and
R: 5′-AACTGGTCGGCTTCAGAGTT-3′; GAPDH (143 bp): F:
5′-GGGTGTGAACCATGAGAAGT-3′ and R: 5′-GGCATGGACTGTGGTCATGA-3′.
Main equipment
The equipment used is listed below: ABI 7500
Real-Time PCR instrument (Applied Biosystems, Foster City, CA,
USA), Steady Current and Voltage Electrophoresis system (Bio-Rad),
Gel Imaging Analysis System BINTA 2020D (Beijing BINTA Instrument
Technology Co., Ltd., Beijing, China), Graphic Image Analyzer
(Image-Pro Plus Analysis Software 6.0), Low-Temperature High-Speed
Centrifuge Series (Thermo Fisher Scientific, West Palm Beach, FL,
USA), Sartorius BS224S electronic scale (Sartorius, Goettingen,
Germany), BioPhotometer nucleic acid UV spectrophotometer
(Eppendorf, Hamburg, Germany), Vertical Electrophoresis slot
(Bio-Rad) and Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell
(Bio-Rad).
Determination of H1N1 virulence
A549 cells were digested with 0.25% trypsin
solution, adjusted to 3×105 cells/ml, transferred into a
96-well cell culture plate (100 μl per well), incubated at 37°C in
a 5% CO2 incubator until cells grew into a monolayer and
the liquid medium was discarded. H1N1 was diluted to 10
concentrations by a 10X gradient with a maintenance medium, using 4
wells per concentration and 100 μl per well. An additional 4 wells
were used as the normal control group (without addition of virus)
and the cells were cultured for 3 days at 37°C in a 5%
CO2 incubator. The cultures were observed daily, cell
lesions were determined by MTT and the TCID50 was
calculated according to the Reed-Muench formula (12).
Determination of quercetin and ribavirin
cytotoxicity
A total of 3×105 cells/ml were placed in
96-well plates, discarding the liquid medium in the well when the
cells grew into a monolayer. The drugs were diluted to 10
concentrations by a 2X gradient with a complete DMEM medium, using
4 wells per concentration and 100 μl per well. An additional 4
wells were used as the normal control group, cultured in a 37°C, 5%
CO2 incubator for 2 days. Drug cytotoxicity was
determined by MTT. The drug concentration in which the cell
survival rate was >50% was defined as the maximum non-toxic
concentration (TC0).
Inhibitory action of quercetin on A549
lesions induced by H1N1
A total of 3×105 cells/ml were placed in
96-well plates and the liquid medium in the well was discarded when
the cells grew into a monolayer. After exposing the cells to 100
TCID50 for 2 h, the virus solution was removed and the
cells were washed twice with PBS. The drugs were diluted from 160
mg/l to 10 concentrations by a 2X gradient with a cell maintenance
medium, using 4 wells per concentration and 100 μl per well, with
established normal control and H1N1 groups (4 wells per group),
cultured in a 37°C, 5% CO2 incubator for 2 days. The
inhibitory action of quercetin on the A549 lesions induced by H1N1
was determined by MTT.
Grouping and treatment of A549 cells
A549 cells at a concentration of 2×105/ml
were placed in 96 Petri dishes (diameter 60-mm), including the
normal control, H1N1, ribavirin-positive control and
quercetin-treated groups, using 6 dishes per group (3 dishes for
RNA extraction and the remaining 3 for protein analysis), setting 4
time points (4, 12, 24 and 48 h).
The excess fluid in the wells was discarded when the
cells grew into a monolayer and the cells were washed twice with
PBS. After exposing the cells to 100 TCID50 for 2 h
(except for the normal group), the virus solution was discarded.
Maintenance medium (4 ml) was added to the normal control and H1N1
groups; maintenance medium (4 ml) containing 20 mg/l ribavirin was
added to the ribavirin group; maintenance medium (4 ml) containing
10 mg/l quercetin was added to the quercetin group.
mRNA assay of CDK4 by fluorescent
qPCR
The Petri dishes were removed at each time point and
the cells were washed twice with PBS, followed by the addition of 1
ml TRIzol to every dish and mixing the cell mixture by pipetting up
and down several times. The cells were incubated for 10 min at room
temperature and the total RNA was extracted according to the method
described by Deng et al(13).
The reverse transcription system (25 μl) was as
follows: 3 μl RNA, 1 μl oligo(dT), 5 μl 5X buffer, 5 μl dNTP (10
mM), 0.5 μl RNAse inhibitor, 1 μl M-MLV and 9.5 μl
ddH2O. The mixture was incubated at 42°C for 60 min and
then at 70°C for 10 min. The fluorescent qPCR system (20 μl) was as
follows: 1.5 μl cDNA, 0.5 μl primers F (10 pmol/μl), 0.5 μl primers
R (10 pmol/μl), 10 μl SYBR-Green mix and 7.5 μl ddH2O.
The qPCR conditions were as follows: pre-denaturation at 94°C for
15 min, denaturation at 94°C for 15 sec, annealing at 60°C for 34
sec, extension at 72°C for 15 sec and, after a total of 40 cycles,
extension at 72°C for 10 min.
Protein assay of CDK4 by western blot
analysis
The Petri dishes were removed at each time point,
the cells were washed twice with PBS and 600 μl of cell lysate
solution was added to each dish, followed by continued lysis on ice
for 15 min. The cell lysate was collected in EP tubes (1.5 ml) and
centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was
then transferred to a new EP tube. Western blot analysis was
performed following determination of the protein concentration by
the Bradford method.
Statistical analysis
The CDK4 and β-actin gray values were measured using
Image-Pro Plus 6.0 software and the ratio of gray value was
relative to the protein expression level. Experimental data were
statistically analyzed by SPSS software, version 13.0. The two
groups were compared by t-test and data are presented as mean ±
standard error. P≤0.05 was considered to indicate a statistically
significant difference.
Results
H1N1 virulence
Following infection of A549 cells with H1N1 for 48
h, the cells of the normal control group appeared to be in good
condition when observed under the inverted microscope. Compared to
the normal control group, the cells of the virus group appeared to
be rounder, smaller and detached from the sidewalls; the
intercellular connections were broken or had disappeared,
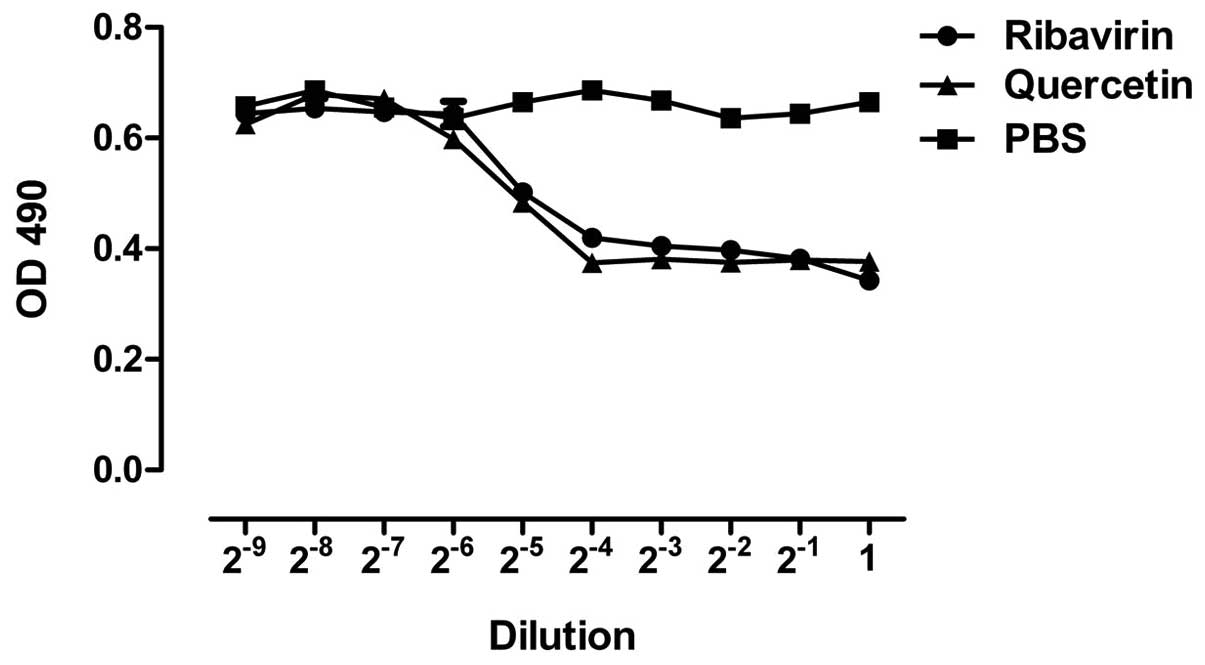
demonstrating the cytopathic effect (Fig. 1A). According to MTT and the
Reed-Muench formula, the TCID50 of H1N1
(A1/Qianfang/166/85) on A549 cells was 10−4.75 (Fig. 1B).
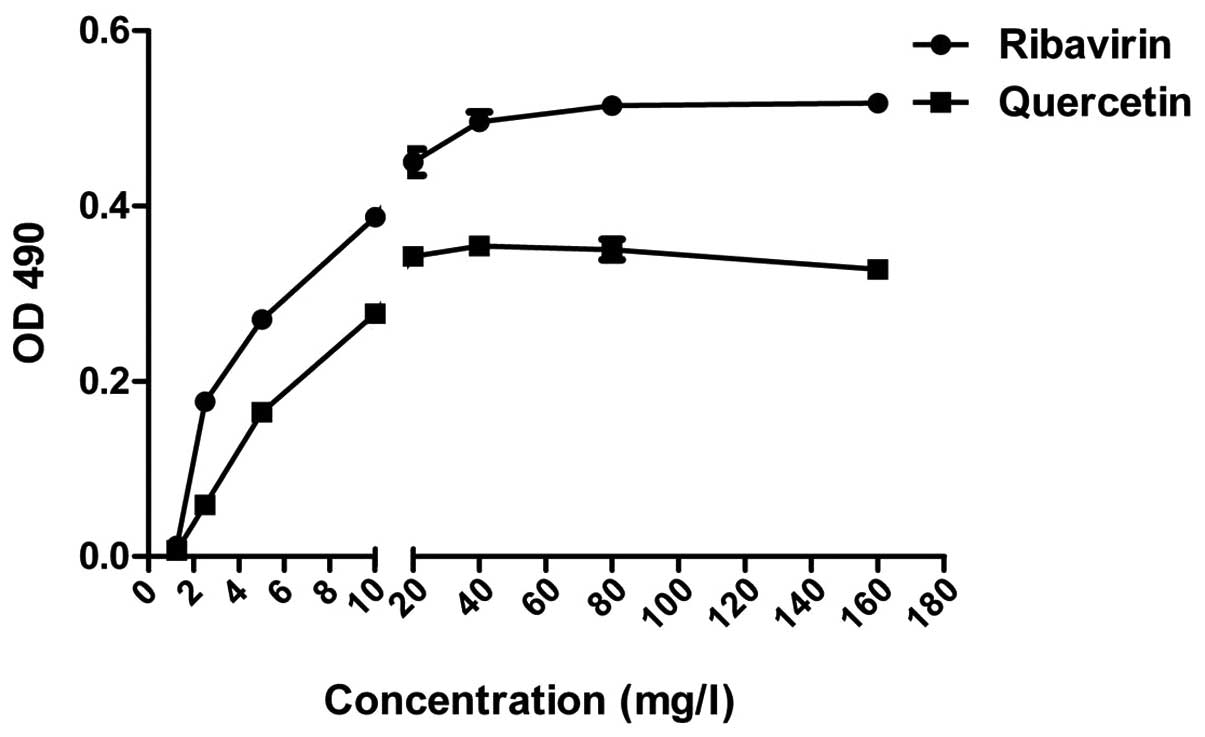
Cytotoxicity of quercetin and
ribavirin
Following culture for 48 h with 3 repetitions,
according to MTT, the TC0 of quercetin and ribavirin on
A549 cells was calculated to be 30–60 and 150–300 mg/l,
respectively (Fig. 2).
Inhibitory effects of quercetin on A549
cell lesions induced by H1N1
The cell shape and size and the number of cells
adherent to the sidewalls in the quercetin groups (80, 40, 20 and
10 mg/l) were better compared to those in the H1N1 and the
ribavirin groups (160, 80, 40 and 20 mg/l) and were similar to
those of the normal group (Fig. 3).
The minimum effective concentration of quercetin and ribavirin on
A549 cell lesions induced by H1N1 were 10 and 20 mg/l,
respectively.
Effects of quercetin on CDK4 mRNA
expression
The method described by Livak and Schmittgen
(14) was used to assess the
effects of quercetin on CDK4 mRNA expression and the results are
presented in Table I. Compared to
the normal control group, mRNA expression in the H1N1 groups was
significantly higher (P<0.01) at all 4 time points (4, 12, 24
and 48 h), which indicated that H1N1 infection may induce the
expression of CDK4 mRNA in A549 cells. Compared to the H1N1 groups,
CDK4 mRNA expression in the ribavirin and the quercetin groups was
significantly lower (P<0.01) at 4 treatment time points (2, 10,
22 and 46 h), indicating that CDK4 mRNA expression in A549 cells
infected with H1N1 within 48 h may be inhibited by quercetin (10
mg/l).
 | Table IRelative expression of CDK4 mRNA in
each group. |
Table I
Relative expression of CDK4 mRNA in
each group.
| Groups | 4 h,
2−ΔΔCT | 12 h,
2−ΔΔCT | 24 h,
2−ΔΔCT | 48 h,
2−ΔΔCT |
|---|
| Normal | 1.000±0.112 | 1.001±0.118 | 0.937±0.106 | 1.082±0.121 |
| H1N1 | 2.343±0.235a | 2.510±0.225a | 3.947±0.442a | 3.647±0.333a |
| Ribavirin | 1.354±0.142b | 1.572±0.131b | 2.455±0.262b | 1.851±0.138b |
| Quercetin | 1.614±0.124b | 1.761±0.136b | 2.588±0.232b | 2.341±0.227b |
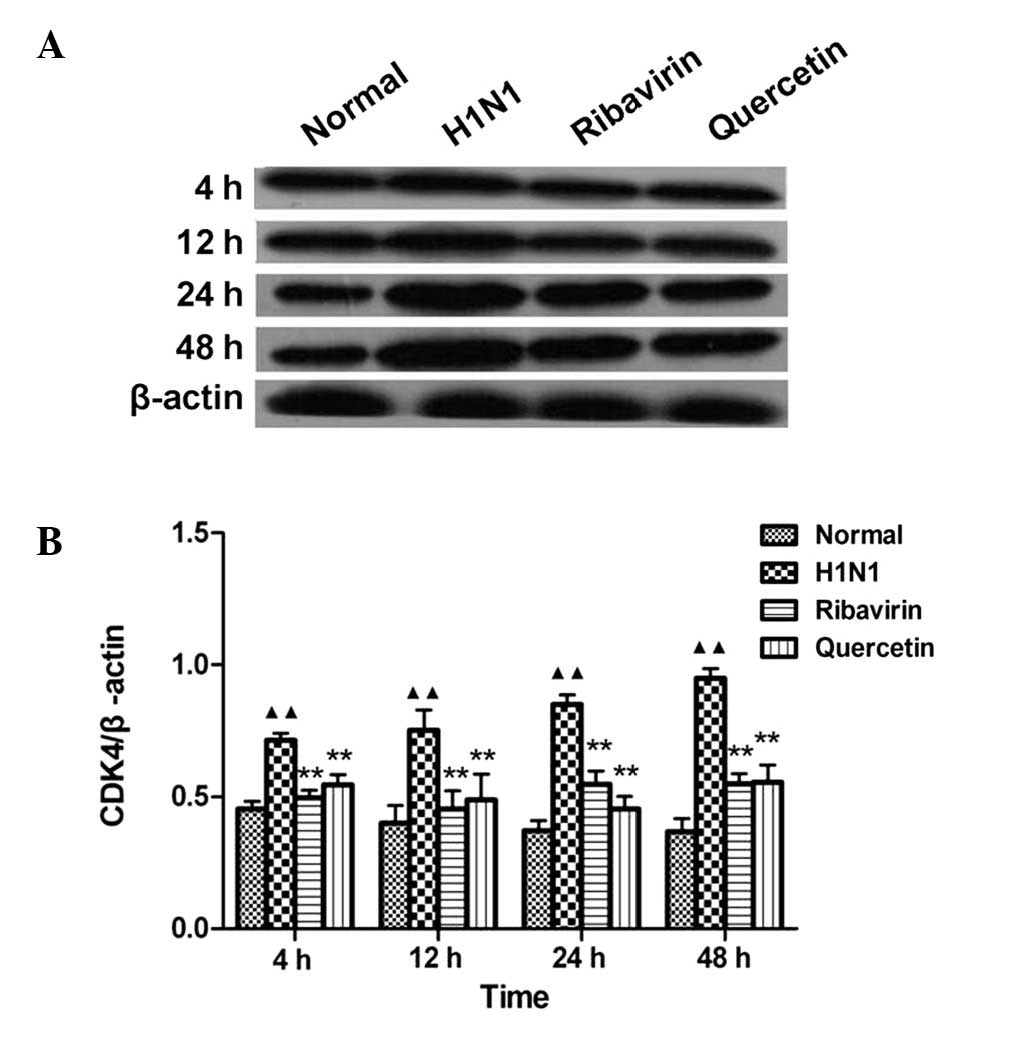
Effects of quercetin on CDK4 protein
expression
Compared to the normal group, the CDK4 protein
expression in the H1N1-infected group was significantly increased
(P<0.01) at 4 time points (4, 12, 24 and 48 h), which indicates
that H1N1 may induce the expression of CDK4 in A549 cells. Compared
to the H1N1 groups, CDK4 protein expression was significantly
decreased (P<0.01) at four treatment time points (2, 10, 22 and
46 h) (Fig. 4B). This result
indicates that CDK4 expression in A549 cells infected by H1N1
within 48 h may be inhibited by quercetin (10 mg/l).
Discussion
Influenza is an acute respiratory infectious
disease. The epithelial cells of the respiratory tract are a
vulnerable target for H1N1. Based on the different characteristics
of H1N1 proliferation in different cell lines in vitro, the
A549 human lung cancer epithelial cell line was selected to conduct
this experiment.
The eukaryotic cell cycle is under the control of
precise and complex protective regulatory mechanisms and consists
of four distinct phases: G1, S (synthesis), G2 and M (mitosis).
Several factors, such as the cyclin/cyclin-dependent kinase
(cyclin/CDK) complex, closely regulate all the processes of each
phase (15). If cell proliferation
is disturbed by infection with the influenza A virus, the host
cells are arrested in the G0/G1 phase (16) and if the DNA damage is not repaired,
the cells enter the apoptotic process (17).
The results of this study have demonstrated that the
CDK4 mRNA and protein expression was significantly increased in the
H1N1 group (P<0.01) at four time points (4, 12, 24 and 48 h).
However, CDK4 expression was significantly decreased in the
quercetin-treated group (P<0.01) at four treatment time points
(2, 10, 22 and 46 h). It is possible that quercetin in A549 cells
infected with H1N1 within 48 h is able to induce the DNA repair
mechanism, thus contributing to the host cell proliferation.
However, additional studies are required to support this
hypothesis. This study has demonstrated that, although the direct
antiviral effect of quercetin on H1N1 was not as potent as that of
ribavirin, it was less toxic and acted by inhibiting the mRNA and
protein expression of CDK4 induced by H1N1 infection. Therefore, it
is concluded that quercetin possesses a potential antiviral
clinical application value, although further investigations are
required to elucidate the mechanism of action and time limit of
quercetin on H1N1-infected cells.
Acknowledgements
This study was funded by the Special Talents Project
of Ningxia Medical University (XT 2012004) and the Provincial
Project (NGY 2012067).
References
|
1
|
Fraser C, Donnelly CA, Cauchemez S and
Hanage WP: Pandemic potential of a strain of influenza A (H1N1):
early findings. Science. 324:1557–1561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Romanowska M, Stefanska I, Donevski S and
Brydak LB: Infections with A(H1N1)2009 influenza virus in Poland
during the last pandemic: experience of the National Influenza
Center. Adv Exp Med Biol. 756:271–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma U, Bala M, Saini R, et al:
Polysaccharide enriched immunomodulatory fractions from
Tinospora cordifolia(Willd) miers ax hook. f. & Thoms.
Indian J Exp Biol. 50:612–617. 2012.PubMed/NCBI
|
|
4
|
Gacche RN, Shegokar HD, Gond DS, Yang Z
and Jadhav AD: Evaluation of selected flavonoids as antiangiogenic,
anticancer, and radical scavenging agents: an experimental and in
silico analysis. Cell Biochem Biophys. 61:651–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang W, Ahmed M, Tasawwar B, et al:
Combination radiofrequency (RF) ablation and IV liposomal heat
shock protein suppression: reduced tumor growth and increased
animal endpoint survival in a small animal tumor model. J Control
Release. 160:239–244. 2012. View Article : Google Scholar
|
|
6
|
Karl TN, Middleton E Jr and Ogra PL:
Antiviral effect of flavonoids on human viruses. J Med Virol.
15:71–79. 1985. View Article : Google Scholar
|
|
7
|
Ganesan S, Faris AN, Comstock AT, et al:
Quercetin inhibits rhinovirus replication in vitro and in vivo.
Antiviral Res. 94:258–271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gonzalez O, Fontanes V, Raychaudhuri S, et
al: The heat shock protein inhibitor Quercetin attenuates hepatitis
C virus production. Hepatology. 50:1756–1764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi HJ, Song JH and Kwon DH:
Quercetin3-rhamnoside exerts antiinfluenza A virus activity in
mice. Phytother Res. 26:462–464. 2012.PubMed/NCBI
|
|
10
|
Uchide N and Toyoda H: Antioxidant therapy
as a potential approach to severe influenza-associated
complications. Molecules. 16:2032–2052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Zeng Z, Zhou Y, et al:
Identification of aberrant cell cycle regulation in Epstein-Barr
virus-associated nasopharyngeal carcinoma by cDNA microarray and
gene set enrichment analysis. Acta Biochim Biophys Sin (Shanghai).
41:414–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo YJ and Chen XW: Influenza virus and
its experimental techniques. China Three Gorges Press; Beijing:
1997, pp. 94–96
|
|
13
|
Deng LH, Luo MW, Zhang CF and Zeng HC:
Extraction of high-quality RNA from rubber tree leaves. Biosci
Biotechnol Biochem. 76:1394–1396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
15
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He Y, Xu K, Keiner B, et al: Influenza a
virus replication induces cell cycle arrest in G0/G1 phase. J
Virol. 84:12832–12840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Odell ID, Wallace SS and Pederson DS:
Rules of engagement for base excision repair in chromatin. J Cell
Physiol. 228:258–266. 2013. View Article : Google Scholar : PubMed/NCBI
|