Introduction
Cardiac fibroblasts play a pivotal role in the
development of cardiac fibrosis, which is closely associated with
numerous cardiovascular diseases, including hypertension,
myocardial infarction and cardiomyopathy (1,2).
Angiotensin II (Ang II) is considered to be a major factor in the
pathogenesis of cardiac fibrosis (3,4). Ang
II has been shown to induce cardiac fibrosis by stimulating the
proliferation of cardiac fibroblasts and promoting the aberrant
deposition of extracellular matrix in the myocardial interstitium
(5–7). It is well known that the majority of
the actions of Ang II are mainly mediated via angiotensin II
receptor type 1 (AGTR1) (8).
Currently, AGTR1 blockers such as Losartan are widely used in the
clinical setting for various cardiovascular diseases (8). However, the molecular mechanism
underlying the effects of Ang II on cardiac fibroblasts requires
elucidation by further studies.
MicroRNAs (miRNAs) are an endogenous conserved class
of small non-coding RNAs of 18–25 nucleotides, which are generally
considered to downregulate the expression of target genes at the
post-transcriptional level (9,10).
Several miRNAs have been reported to be involved in cardiac
fibrosis (11–17). Thum et al(11) reported that miRNA-21 (miR-21)
contributed to cardiac fibrosis by targeting transcript Sprouty1
which inhibits mitogen-activated protein kinase signaling in
cardiac fibroblasts. Roy et al(12) reported that miR-21 promoted cardiac
fibrosis by targeting the phosphatase and tensin homologue which
regulates the expression of matrix metalloproteinase 2 (MMP2).
However, Patrick et al(18)
observed that the genetic deletion of miR-21 did not affect the
response of the heart to pressure overload or other type of stress
and concluded that miR-21 was not essential for cardiac fibrosis.
Liang et al(19) recently
reported that the overexpression of miR-21 in cardiac fibroblasts
reduced transforming growth factor-β (TGFβ) RIII expression and
increased collagen content. Therefore, further studies are required
to elucidate the role of miR-21 in cardiac fibroblasts.
Our previous array study demonstrated that 33 miRNAs
were differentially expressed in cardiac fibroblasts in response to
treatment with Ang II (100 nM) for 24 h, including miRNA-224
(miR-224) (20). The increase of
miR-132, miR-125b-3p and miR-146b and the decrease of miR-300-5p,
miR-204* and miR-181b expression has been confirmed by quantitative
polymerase chain reaction (qPCR) (20). However, the level of miR-224 has not
been determined in cardiac fibroblasts. In order to elucidate the
effect of Ang II on the expression of miR-21 and miR-224, we
measured the levels of the two miRNAs with semi-quantitative
reverse transcription-polymerase chain reaction (RT-PCR) and qPCR
in adult rat cardiac fibroblasts. Our results demonstrated that
miR-21 was not induced by Ang II, whereas the expression of miR-224
was upregulated in Ang II-treated adult rat cardiac fibroblasts.
Furthermore, bioinformatic analysis revealed that the potential
target genes of miR-224 included SMAD4, SMAD5, cyclin-dependent
kinase 9 (CDK9) and early growth response 1/2 (EGR1/2), which
indicated the potential role of miR-224 in cardiac fibroblasts.
Materials and methods
Materials and animals
Collagenase, trypsin and Ang II were obtained from
Sigma (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium
(DMEM) and TRIzol reagent were obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA). The First Strand cDNA Synthesis
kit was purchased from Fermentas (Burlington, ON, Canada). SYBR
Premix Ex Taq™ II was purchased from Takara Bio, Inc. (Shiga,
Japan). Sprague-Dawley (SD) rats were supplied from the
Experimental Animal Center of Xi’an Jiaotong University. The animal
experiments were approved by the University Committee of Laboratory
Animal Care and Use and were performed according to the guidelines
of the National Animal Research Center.
Isolation and culture of cardiac
fibroblasts
Cardiac ventricular fibroblasts were obtained from
the hearts of adult male SD rats weighing 250–300 g, as previously
described (20). In brief,
following rapid excision of the hearts, the fibroblasts were
prepared by enzymatic digestion with a collagenase/trypsin
solution. After a 2-h period of attachment to uncoated culture
plates, the cells that were weakly attached or unattached were
rinsed free and discarded and the attached cells (mainly
fibroblasts) were washed and grown in DMEM supplemented with 10%
fetal bovine serum. The cardiac fibroblasts (passages 3–5) were
grown to 80–90% confluence and serum-starved for 24 h prior to
treatment.
Preparation of RNA
Following a 24-h serum starvation, adult rat cardiac
fibroblasts were treated with Ang II (100 nM) for 24 h. The cells
were then harvested for RNA extraction using TRIzol reagent as
previously described and RNAs were dissolved in RNase-free water
(20). The RNA quantity was
spectrophotometrically determined as A260 and A260/A280 ratio using
the NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific,
Wilmington, DE, USA) and RNA quality was assessed by
electrophoresis on a 1.2% agarose/formaldehyde gel. Isolated RNA
was stored at −70°C for subsequent quantitative analysis.
Semi-quantitative RT-PCR analysis of
miR-21
To measure the level of miR-21 in adult rat cardiac
fibroblasts and investigate the effect of Ang II on the expression
of miR-21, semi-quantitative RT-PCR was first used to measure the
level of miR-21. Briefly, total RNAs from six pairs of control and
Ang II-treated cardiac fibroblasts were extracted using TRIzol
reagent. Complementary DNAs (cDNAs) were synthesized from total
RNAs using the First Strand cDNA Synthesis kit with miRNA-specific
primers (Table I). The 20-μl
reactions were incubated for 60 min at 42°C and for 5 min at 70°C
and then stored at −20°C. Conventional PCR reactions were performed
at 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and at
60°C for 30 sec. The PCR products were submitted to electrophoresis
using a 2% agarose gel. U6 RNA was used as internal control to
normalize the level of miR-21 by densitometry using the ImageJ 1.37
freeware for Windows. The optical densitometry results were
expressed as means ± standard error of mean (SEM).
 | Table ImiRNA primer sequences. |
Table I
miRNA primer sequences.
| miRNA | Sequences |
|---|
| miR-21 | RT primer:
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA-3′ |
| PCR primer F:
5′-CGGCTAGCTTATCAGACTGA-3′
R: 5′-GTGCAGGGTCCGAGGT-3′ |
| miR-224 | RT primer:
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAACGG-3′ |
| PCR primer F:
5′-CGGCCAAGTCACTAGTGGTT-3′
R: 5′-GTGCAGGGTCCGA GGT-3′ |
| U6 | PCR primer F:
5′-CTCGCTTCGGCAGCACA-3′
R: 5′-AACGCTTCACGAATTTGCGT-3′ |
qPCR analysis of miR-21 and miR-224
To validate the level of miR-21 and confirm the
level of miR-224 in adult rat cardiac fibroblasts, we performed
stem-loop qPCR to quantify the levels of the two miRNAs. Briefly,
total RNAs from six pairs of control and Ang II-treated cardiac
fibroblasts were extracted using TRIzol reagent. cDNAs were
synthesized from total RNAs using the First-Strand cDNA Synthesis
kit with miRNA-specific primers, as mentioned above (Table I). qPCR was performed using SYBR
Premix Ex Taq II in an iQ5 real-time PCR detection system (Bio-Rad,
Hercules, CA, USA). The PCR reactions were performed at 95°C for 10
min, followed by 40 cycles at 95°C for 15 sec and at 60°C for 30
sec. The specificity of the PCR products was assessed by the
melting curve analysis. U6 was used as an internal control to
normalize miRNA. Relative quantitation of miR-21 and miR-224
expression was evaluated by the 2−ΔΔCt method.
Bioinformatic analysis and target
prediction
Four online software programs were used for
bioinformatic analysis and target prediction of miRNAs: TargetScan
(http://www.targetscan.org), microRNA.org
(http://www.microrna.org/microrna/home.do), miRDB
(http://mirdb.org/miRDB) and MicroCosm Targets
(http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/).
Statistical analysis
Data are presented as means ± SEM. The Student’s
t-test was used to compare data between the two groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
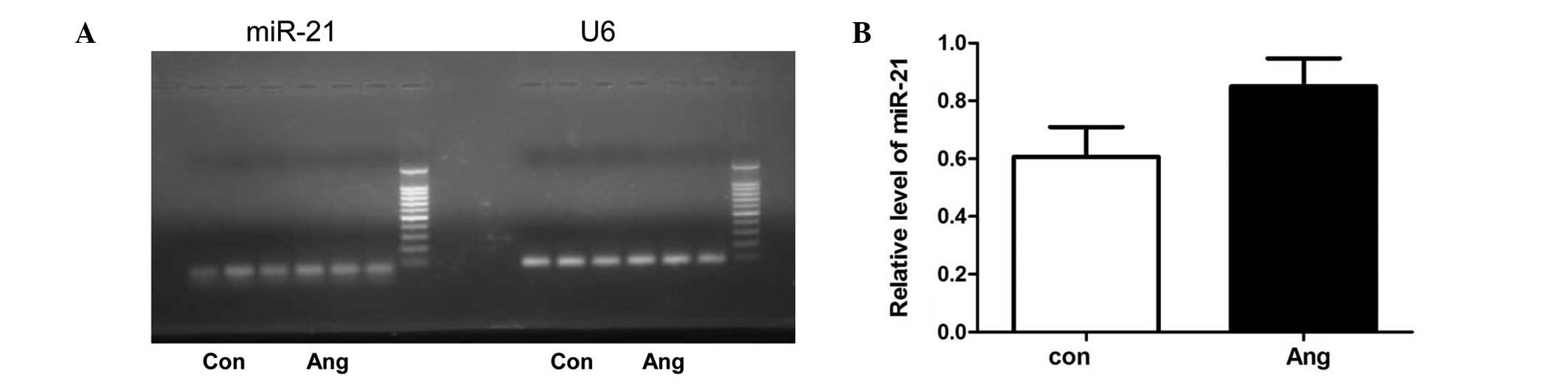
Effect of Ang II on miR-21 level in adult
rat cardiac fibroblasts
To investigate the effect of Ang II on the
expression of miR-21 in adult rat cardiac fibroblasts,
semi-quantitative RT-PCR was first used to measure the level of
miR-21. Although the level of miR-21 was not very low compared to
U6, stimulation with Ang II did not induce a significant increase
of miR-21 in cardiac fibroblasts (Fig.
1). Moreover, our previous miRNA array analysis also
demonstrated that Ang II increased the expression of miR-21 only by
1.056-fold in adult rat cardiac fibroblasts (20). Furthermore, qPCR demonstrated that
Ang II did not affect the level of miR-21 in adult rat cardiac
fibroblasts (Fig. 2).
Effect of Ang II on miR-224 level in
adult rat cardiac fibroblasts
Our previous miRNA array study (20) demonstrated that Ang II increased the
expression of miR-21 by 2.09-fold in adult rat cardiac fibroblasts.
To confirm the change of miR-224, qPCR was used to reassess the
level of miR-224 in adult rat cardiac fibroblast treated by Ang II
(100 nM) for 24 h. The qPCR analysis revealed that Ang II
significantly induced miR-224 expression in adult rat cardiac
fibroblasts (Fig. 2). The
upregulation of miR-224 induced by Ang II was verified by miRNA
arrays and qPCR.
Bioinformatic analysis and target
prediction of miR-224
After verifying that Ang II was able to induce a
significant increase of miR-224 expression in adult rat cardiac
fibroblasts, the potential target genes of miR-224 were analyzed
using the online software programs TargetScan, microRNA.org, miRDB
and MicroCosm Targets. The TargetScan analysis revealed that the
potential target genes of miR-224 include SMAD4, H3 histone, family
3B (H3F3B), EGR2, SMAD5 and CDK9. The microRNA.org revealed that
the potential target genes of miR-224 include EGR1, EGR2, H3F3B,
SMAD4, SMAD5, CDK9, a disintegrin and metallopeptidase domain 17
(ADAM17), angiotensin II receptor type 2 (AGTR2) and angiotensin II
receptor type 1a (AGTR1a). The miRDB analysis revealed that
apoptosis inhibitor 5 (API5), phosphoinositide-3-kinase, class 2, α
polypeptide (PIK3C2A), H3F3B, ADAM17 and EGR2 are the potential
target genes of miR-224. MicroCosm Targets analysis also indicated
that CDK9, MAPK8 and ADAM33 are the potential target genes of
miR-224 (Table II).
 | Table IIPotential target genes of miR-224. |
Table II
Potential target genes of miR-224.
| miR-224 | Potential
targets |
|---|
| TargetScan | SMAD4, H3F3B, EGR2,
SMAD5, CDK9 |
| microRNA.org | EGR1, EGR2, H3F3B,
SMAD4, SMAD5, CDK9, ADAM17, AGTR2, AGTR1a |
| miRDB | H3F3B, ADAM17, EGR2,
API5, PIK3C2A |
| MicroCosm
Targets | CDK9, MAPK8,
ADAM33 |
Discussion
Several studies demonstrated that miR-21 is involved
in the process of cardiac fibrosis by regulating the expression of
Sprouty1, MMP2 and TGFβ RIII (11,12,19).
However, evidence presented by other studies does not support the
role of miR-21 in cardiac fibrosis. Patrick et al(18) reported that genetic deletion of
miR-21 did not alter the response of the heart to pressure overload
or other type of stress and concluded that miR-21 was not essential
for cardiac fibrosis. Our semi-quantitative RT-PCR, miRNA arrays
and qPCR studies demonstrated that treatment with Ang II (100 nM)
for 24 h did not induce the increase of miR-21 expression in
cardiac fibroblasts, although the level of miR-21 in cardiac
fibroblasts was not considered to be low. Therefore, further
investigations are required to elucidate the role of miR-21 in
cardiac fibroblasts.
Our previous miRNA array studies and the qPCR
analysis demonstrated that Ang II significantly induced the
increase of miR-224 expression in adult rat cardiac fibroblasts
(Fig. 2). Furthermore,
bioinformatic analysis revealed that the potential target genes of
miR-224 included SMAD4, SMAD5, CDK9, API5, EGR1/2, H3F3B, AGTR2 and
AGTR1a, which indicated the potential role of miR-224 in cardiac
fibroblasts. It is well known that SMAD molecules are distinctly
associated with the TGFβ pathway and fibrosis, whereas CDK9, API5
and EGR1/2 are associated with cell proliferation. If AGTR2 and
AGTR1a are indeed target genes of miR-224, miR-224 may inhibit the
action of Ang II. However, this hypothesis requires confirmation by
further studies.
Several studies have confirmed potential target
genes, thus predicting the function of miR-224 (Table III): Wang et al(21) reported that miR-224 was
significantly upregulated in hepatocellular carcinoma and affected
cell apoptosis and proliferation through targeting API5; Wang et
al(22) reported that a high
miR-224 expression in hepatocellular carcinoma was regulated by
histone deacetylase 1, histone deacetylase 3 and E1A binding
protein p300; Yao et al(23)
reported that miR-224 promoted TGFβ1-induced granulosa cell
proliferation by targeting SMAD4; Liang et al(24) demonstrated that p53/p65 was able to
inhibit miR-224 expression in granulosa cells; Li et
al(25) observed that miR-224
was upregulated in HepG2 cells and was involved in cell
proliferation, migration and invasion; Huang et al(26) reported that miR-224 was
significantly upregulated in breast cancer cell lines and regulated
cell invasion and tumor metastasis by targeting Raf kinase
inhibitory protein; Zhang et al(27) reported that miR-224 in
hepatocellular carcinoma affected cell proliferation, migration and
exerted an anti-apoptotic effect; and Olaru et al(28) reported that miR-224 was highly
expressed in cancers associated with inflammatory bowel disease by
targeting p21.
 | Table IIIReported function of miR-224. |
Table III
Reported function of miR-224.
| miR-224 | Cell | Target genes | Function | Refs. |
|---|
| Up | Hepatocellular
carcinoma | API5 | Cell apoptosis and
proliferation | 21,22 |
| Up | Granulosa cell | SMAD4 | Cell
proliferation | 23,24 |
| Up | HepG2 cells | PAK4, MMP9 | Cell proliferation,
migration and invasion | 25 |
| Up | Human breast cancer
cells | RKIP | Cell invasion and
metastasis | 26 |
| Up | Hepatocellular
carcinoma | CDC42, CDH1, PAK2,
BCL2, MAPK1 | Cell proliferation,
migration, invasion 27 and anti-apoptosis | |
| Up | Cancer associated
with IBD | p21 | G1-S checkpoint | 28 |
The literature mentioned above demonstrated that
miR-224 regulates cell proliferation in tumor cells. In our study,
Ang II significantly upregulated the expression of miR-224 in adult
rat cardiac fibroblasts. Ang II has been shown to stimulate the
proliferation of cardiac fibroblasts, which is an important factor
in cardiac fibrosis (5–7). Therefore, we hypothesized that miR-224
may also be involved in the process of cardiac fibroblast
proliferation and cardiac fibrosis. The present study may provide a
starting point to investigate the target genes of miR-224 and
elucidate its role in cardiac fibrosis.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities (no. 08143023) and grants from
the National Science Foundation of China (no. 31100834).
References
|
1
|
Takeda N, Manabe I, Uchino Y, Eguchi K,
Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P,
Conway SJ and Nagai R: Cardiac fibroblasts are essential for the
adaptive response of the murine heart to pressure overload. J Clin
Invest. 120:254–265. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Porter KE and Turner NA: Cardiac
fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ren J, Yang M, Qi G, Zheng J, Jia L, Cheng
J, Tian C, Li H, Lin X and Du J: Proinflammatory protein CARD9 is
essential for infiltration of monocytic fibroblast precursors and
cardiac fibrosis caused by Angiotensin II infusion. Am J Hypertens.
24:701–707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang XR, Chung AC, Yang F, Yue W, Deng C,
Lau CP, Tse HF and Lan HY: Smad3 mediates cardiac inflammation and
fibrosis in angiotensin II-induced hypertensive cardiac remodeling.
Hypertension. 55:1165–1171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olson ER, Shamhart PE, Naugle JE and
Meszaros JG: Angiotensin II-induced extracellular signal-regulated
kinase 1/2 activation is mediated by protein kinase Cdelta and
intracellular calcium in adult rat cardiac fibroblasts.
Hypertension. 51:704–711. 2008. View Article : Google Scholar
|
|
6
|
Schellings MW, Vanhoutte D, van Almen GC,
Swinnen M, Leenders JJ, Kubben N, van Leeuwen RE, Hofstra L,
Heymans S and Pinto YM: Syndecan-1 amplifies angiotensin II-induced
cardiac fibrosis. Hypertension. 55:249–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lijnen PJ, Van Pelt JF and Fagard RH:
Stimulation of reactive oxygen species and collagen synthesis by
angiotensin II in cardiac fibroblasts. Cardiovasc Ther. 30:e1–e8.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang P, Su J, King ME, Maldonado AE, Park
C and Mende U: Regulator of G protein signaling 2 is a functionally
important negative regulator of angiotensin II-induced cardiac
fibroblast responses. Am J Physiol Heart Circ Physiol.
301:H147–H156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang X, Tsitsiou E, Herrick SE and
Lindsay MA: MicroRNAs and the regulation of fibrosis. FEBS J.
277:2015–2021. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diez J: Do microRNAs regulate myocardial
fibrosis? Nat Clin Pract Cardiovasc Med. 6:88–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thum T, Gross C, Fiedler J, Fischer T,
Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et
al: MicroRNA-21 contributes to myocardial disease by stimulating
MAP kinase signalling in fibroblasts. Nature. 456:980–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roy S, Khanna S, Hussain SR, Biswas S,
Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ and Sen CK: MicroRNA
expression in response to murine myocardial infarction: miR-21
regulates fibroblast metalloprotease-2 via phosphatase and tensin
homologue. Cardiovasc Res. 82:21–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Rooij E, Sutherland LB, Thatcher JE,
DiMaio JM, Naseem RH, Marshall WS, Hill JA and Olson EN:
Dysregulation of microRNAs after myocardial infarction reveals a
role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA.
105:13027–13032. 2008.PubMed/NCBI
|
|
14
|
Duisters RF, Tijsen AJ, Schroen B, et al:
miR-133 and miR-30 regulate connective tissue growth factor:
implications for a role of microRNAs in myocardial matrix
remodeling. Circ Res. 104:170–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai
B, Wang N, Li X, Feng T, Hong Y and Yang B: Downregulation of
miR-133 and miR-590 contributes to nicotine-induced atrial
remodelling in canines. Cardiovasc Res. 83:465–472. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Limana F, Esposito G, D’Arcangelo D, Di
Carlo A, Romani S, Melillo G, Mangoni A, Bertolami C, Pompilio G,
Germani A and Capogrossi MC: HMGB1 attenuates cardiac remodelling
in the failing heart via enhanced cardiac regeneration and
miR-206-mediated inhibition of TIMP-3. PLoS One. 6:e198452011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Huang W, Xu R, Nie Y, Cao X, Meng
J, Xu X, Hu S and Zheng Z: MicroRNA-24 regulates cardiac fibrosis
after myocardial infarction. J Cell Mol Med. 16:2150–2160. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patrick DM, Montgomery RL, Qi X, Obad S,
Kauppinen S, Hill JA, et al: Stress-dependent cardiac remodeling
occurs in the absence of microRNA-21 in mice. J Clin Invest.
120:3912–3916. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang H, Zhang C, Ban T, Liu Y, Mei L,
Piao X, Zhao D, Lu Y, Chu W and Yang B: A novel reciprocal loop
between microRNA-21 and TGFβRIII is involved in cardiac fibrosis.
Int J Biochem Cell Biol. 44:2152–2160. 2012.PubMed/NCBI
|
|
20
|
Jiang X, Ning Q and Wang J: Angiotensin II
induced differentially expressed microRNAs in adult rat cardiac
fibroblasts. J Physiol Sci. 63:31–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Lee AT, Ma JZ, Wang J, Ren J, Yang
Y, Tantoso E, Li KB, Ooi LL, Tan P and Lee CG: Profiling microRNA
expression in hepatocellular carcinoma reveals microRNA-224
up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific
target. J Biol Chem. 283:13205–13215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Toh HC, Chow P, Chung AY, Meyers
DJ, Cole PA, Ooi LL and Lee CG: MicroRNA-224 is up-regulated in
hepatocellular carcinoma through epigenetic mechanisms. FASEB J.
26:3032–3041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao G, Yin M, Lian J, Tian H, Liu L, Li X
and Sun F: MicroRNA-224 is involved in transforming growth
factor-β-mediated mouse granulosa cell proliferation and granulosa
cell function by targeting Smad4. Mol Endocrinol. 24:540–551.
2010.
|
|
24
|
Liang M, Yao G, Yin M, Lu M, Tian H, Liu
L, Lian J, Huang X and Sun F: Transcriptional cooperation between
p53 and NF-κB p65 regulates microRNA-224 transcription in mouse
ovarian granulosa cells. Mol Cell Endocrinol. 370:119–129.
2013.
|
|
25
|
Li Q, Wang G, Shan JL, Yang ZX, Wang HZ,
Feng J, Zhen JJ, Chen C, Zhang ZM, Xu W, Luo XZ and Wang D:
MicroRNA-224 is upregulated in HepG2 cells and involved in cellular
migration and invasion. J Gastroenterol Hepatol. 25:164–171. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang L, Dai T, Lin X, Zhao X, Chen X,
Wang C, Li X, Shen H and Wang X: MicroRNA-224 targets RKIP to
control cell invasion and expression of metastasis genes in human
breast cancer cells. Biochem Biophys Res Commun. 425:127–133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Takahashi S, Tasaka A, Yoshima T,
Ochi H and Chayama K: Involvement of microRNA-224 in cell
proliferation, migration, invasion, and anti-apoptosis in
hepatocellular carcinoma. J Gastroenterol Hepatol. 28:565–575.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olaru AV, Yamanaka S, Vazquez C, Mori Y,
Cheng Y, Abraham JM, Bayless TM, Harpaz N, Selaru FM and Meltzer
SJ: MicroRNA-224 negatively regulates p21 expression during late
neoplastic progression in inflammatory bowel disease. Inflamm Bowel
Dis. 19:471–480. 2013. View Article : Google Scholar : PubMed/NCBI
|