Introduction
It has been demonstrated that glucose variability,
independent of the glycosylated hemoglobin (HbA1c) levels, may
significantly affect the risk for chronic diabetic complications
(1,2). Therefore, it is recommended that
glucose variability is considered along with HbA1c levels in the
management of diabetes (3,4).
It was hypothesized that the postprandial plasma
glucose variability is associated with the hypoglycemic drug
mechanisms and the types of meals, particularly regarding the
proportion of carbohydrate. Therefore, two different anti-diabetic
drugs with different mechanisms of action in different types of
meal tests were designed. Nateglinide and acarbose specifically
target postprandial plasma glucose with different hypoglycemic
mechanisms. To the best of our knowledge, there has been no
head-to-head trial comparing the effects of these two agents on
glycemic excursions in different types of meal tests. Therefore,
the different effects of the two drugs on metabolic control and
glucose variability were assessed in drug-naïve type 2 diabetic
patients, with the aim to determine a treatment strategy that may
be more effective in Chinese type 2 diabetic patients.
Materials and methods
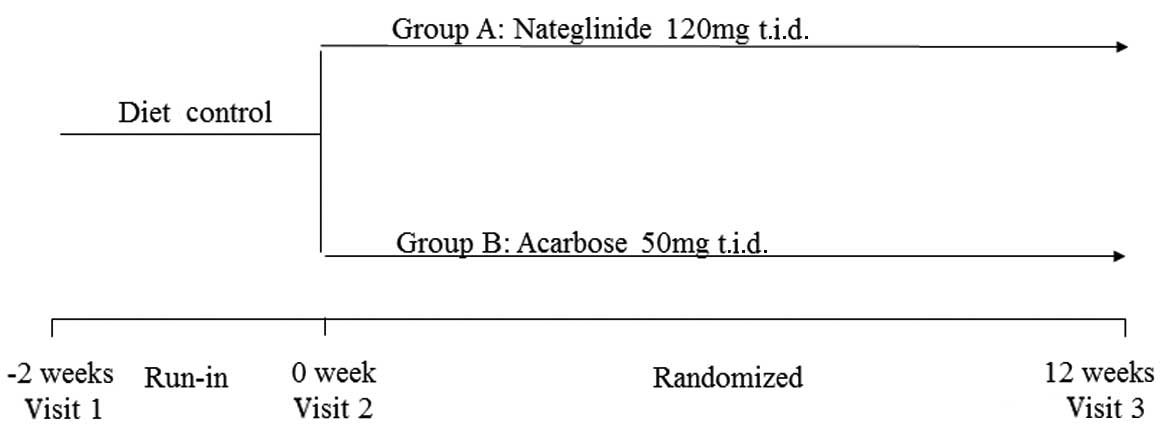
Study design
A total of 39 drug-naïve type 2 diabetic patients
(16 males and 23 females, aged 56.7±10.2 years) were screened and
included in this study. The main inclusion criteria were as
follows: fasting plasma glucose ≤9 mmol/l, HbA1c 6.5–9.0% and
treatment with diet alone for a minimum of 2 weeks without any
concurrent medication prior to enrolment. After 2 weeks of
treatment with diet alone, the subjects were randomized into groups
A and B. The patients in group A were administered nateglinide 120
mg, three times daily (t.i.d.); the patients in group B were
administered acarbose 50 mg, t.i.d (Fig. 1). A 70-g carbohydrate standardized
meal and a consecutive three-day mixed-meal (30 kcal/kg ideal
weight/day, based on dietitian recommendations, including 55% of
calories from carbohydrates, 25% from fat and 20% from proteins,
with a meal distribution of 1/5, 2/5 and 2/5) tests were performed
at baseline and at the end of study. In the carbohydrate
standardized meal test, blood samples were collected at fasting,
15, 30, 60, 90 and 120 min to calculate the postprandial glucose
excursion (PPGE), which was defined as the mean difference between
preprandial and postprandial glucose values within 2 h after the
standardized meal. In the consecutive three-day mixed-meal test,
the patients underwent continuous glucose monitoring system (CGMS)
measurements. They were instructed to perform four glucose
calibration measurements and mark the time of each meal by pressing
the input button on the CGMS device.
The study protocol was approved by the Ethics
Committee of The First Affiliated Hospital of Sun Yat-Sen
University and all the subjects provided written informed
consent.
Parameters derived from CGMS
All the parameters of continuous glucose monitoring
were calculated from each CGMS output, which was extracted using
the CGMS 3.0 software package [Medtronic MiniMed, MMT-7310 version
3.0C (3.0.128)]. The mean glucose level was calculated as the mean
of all the consecutive sensor readings, from which the standardized
deviation (SD) was also calculated. The largest amplitude of
glycemic excursion (LAGE) was defined as the maximal sensor glucose
level minus the minimal sensor glucose level during each day. The
mean amplitude of glycemic excursion (MAGE) was used for assessing
the intraday glucose variability. MAGE was calculated by measuring
the arithmetic mean of the differences in consecutive peaks and
nadirs, which were taken into consideration only if they exceeded
one standard of deviation from the mean. The mean of the daily
differences (MODD), calculated as the average absolute difference
of paired sensor glucose values during two successive 24-h periods,
was used to assess the interday glucose variability.
Statistical analyses
Values are expressed as means ± standard deviation.
The statistical significance of variation between means was
assessed using the two-tailed paired Student’s t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patients
A total of 16 male and 23 female patients with newly
diagnosed type 2 diabetes [mean age 56.7 years (SD, 10.2), body
mass index 26.3 kg/m2 (SD, 2.8)] were randomly assigned
to the nateglinide (n=19) and the acarbose groups (n=20). The
clinical characteristics, glucose levels and lipid profiles at
baseline were similar between the two groups (Table I).
 | Table IPatient characteristics at
baseline. |
Table I
Patient characteristics at
baseline.
| Treatment group | Nateglinide
(n=19) | Acarbose (n=20) | P-value |
|---|
| Age (years) | 54.6 ±8.6 | 58.6±11.1 | 0.75 |
| Gender
(male/female) | 6/13 | 10/10 | 0.27 |
| BMI | 25.9±2.6 | 26.7±2.9 | 0.61 |
| SBP (mmHg) | 126.2±15.1 | 134.9±13.4 | 0.29 |
| DBP (mmHg) | 77.6±10.0 | 81.9±10.2 | 0.56 |
| HbA1c (%) | 7.3±0.6 | 7.4±0.6 | 0.49 |
| FPG (mmol/l) | 6.5±1.0 | 6.5±0.8 | 0.87 |
| 2-h PPG (mmol/l) | 12.8±2.3 | 12.4±1.9 | 0.22 |
| TC (mmol/l) | 5.91±1.40 | 5.55±0.76 | 0.53 |
| TG (mmol/l) | 1.79±1.44 | 1.51±0.99 | 0.97 |
| LDL-C (mmol/l) | 3.77±1.43 | 3.39±0.73 | 0.20 |
| HDL-C (mmol/l) | 1.20±0.17 | 1.22±0.21 | 0.84 |
Effects of nateglinide vs. acarbose on
postprandial glycemic excursion in the standardized carbohydrate
and mixed-meal tests
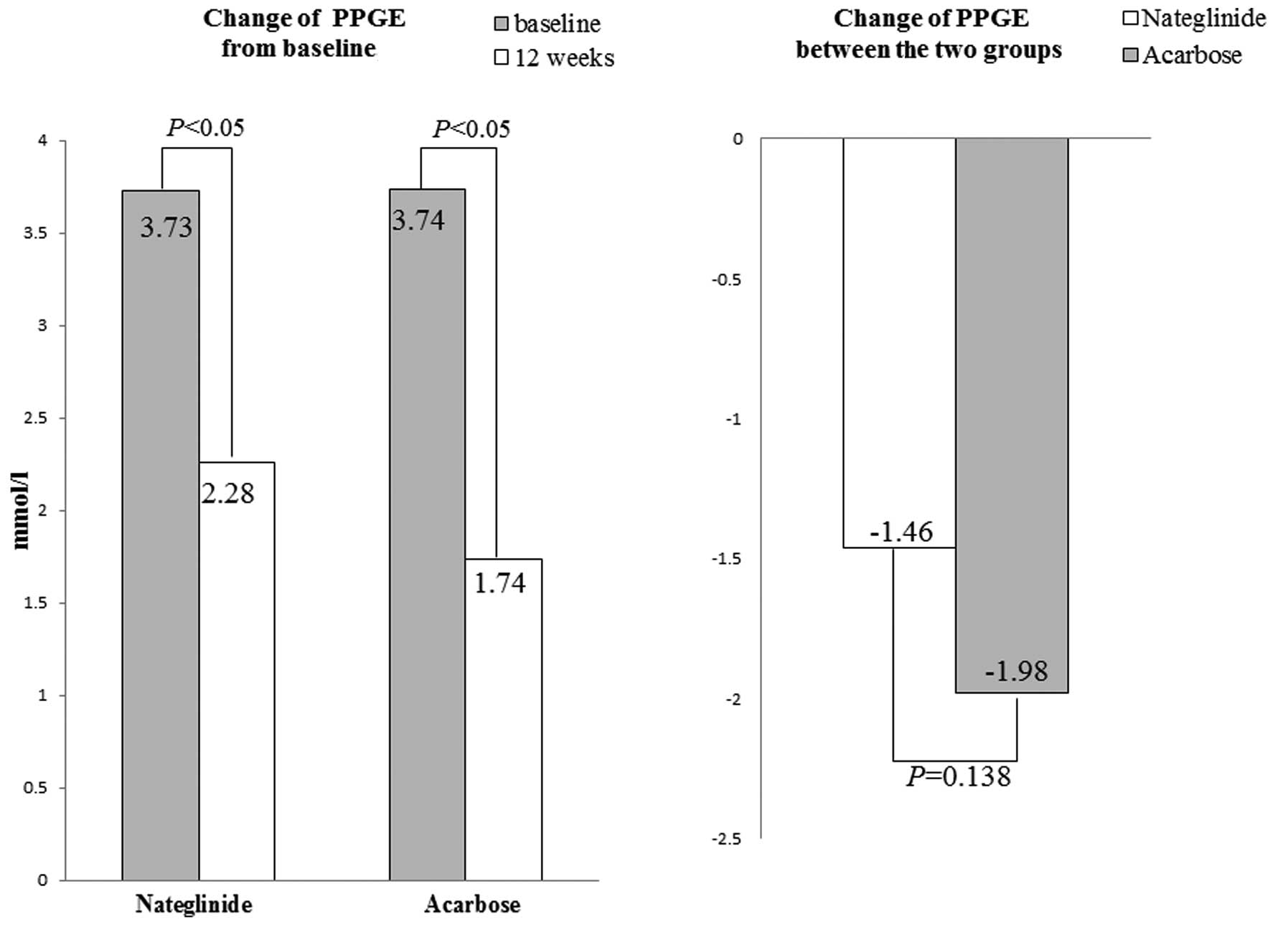
Standardized carbohydrate meal test: at baseline,
the PPGE levels in the nateglinide and acarbose groups were 3.73
and 3.74 mmol/l, respectively, without a statistically significant
difference (P=0.86). After 12 weeks of treatment, PPGE was
significantly reduced in the two groups (P<0.05), with acarbose
exerting a more potent effect compared to nateglinide (−1.98±1.18
versus −1.46±0.98 mmol/l, P=0.138) (Fig. 2).
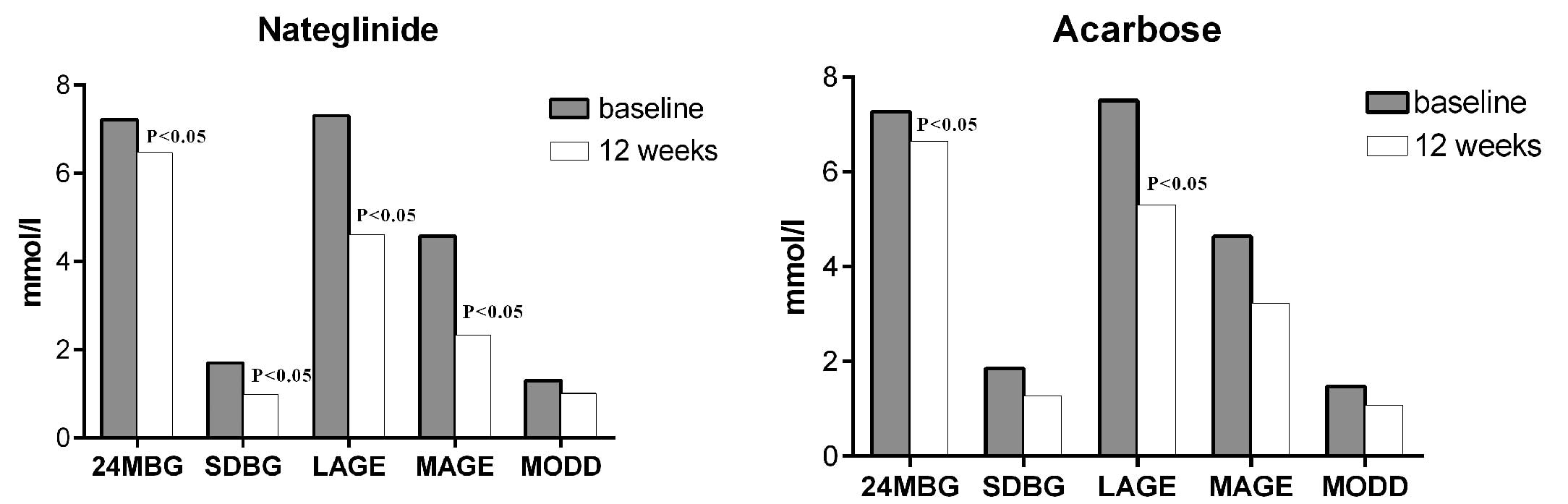
Mixed-meal test: the parameters of glucose
variability derived from the CGMS were well-matched between the two
treatment groups prior to randomization. Analysis of the data
calculated from the CGMS output demonstrated that the mean sensor
glucose values (24-h MBG) had decreased significantly in the two
groups after 12 weeks of treatment. The parameters of glucose
excursions, including SD, LAGE, MAGE and MODD, were reduced in the
two groups and the reductions in SD, LAGE and MAGE in the
nateglinide group were statistically significant. However, in the
acarbose group, only the decrease in LAGE was considered to be
statistically significant (Table
II and Fig. 3).
 | Table IIParameters of continuous glucose
monitoring system in both group at baseline and at 12 weeks. |
Table II
Parameters of continuous glucose
monitoring system in both group at baseline and at 12 weeks.
| Nateglinide
(n=19) | Acarbose (n=20) |
|---|
|
|
|
|---|
| Parameters | Baseline | 12 weeks | Baseline | 12 weeks |
|---|
| 24-h MBG
(mmol/l) | 7.22±1.14 | 6.47±1.17a | 7.27±1.41 | 6.65±1.19a |
| SDBG (mmol/l) | 1.70±0.82 | 0.98±0.48a | 1.85±0.78 | 1.28±0.22 |
| LAGE (mmol/l) | 7.30±2.70 | 4.62±2.42a | 7.51±3.5 | 5.34±1.8a |
| MAGE (mmol/l) | 4.58±2.09 | 2.34±1.67a | 4.64±1.59 | 3.22±0.79 |
| MODD (mmol/l) | 1.31±0.37 | 1.01±0.40 | 1.47±0.64 | 1.07±0.21 |
Effects of nateglinide versus acarbose on
postprandial glycemic profiles in the standardized carbohydrate
meal test
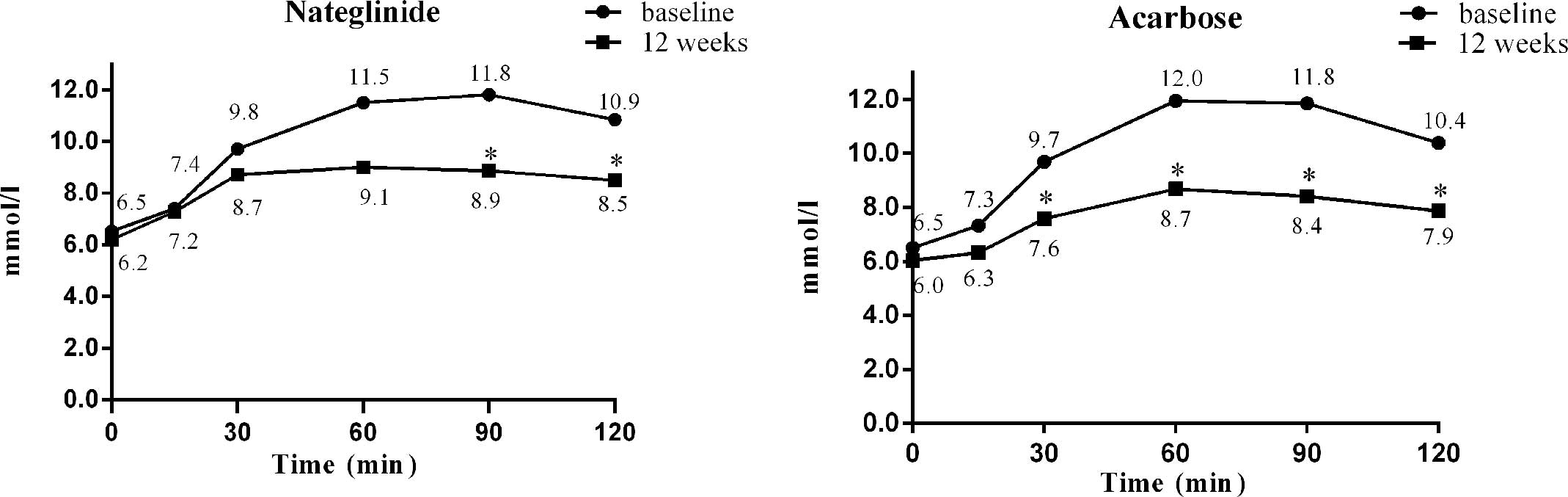
The efficiencies of nateglinide and acarbose in
lowering postprandial 120-min hyperglycemia were similar (−2.35 vs.
−2.52 mmol/l, P=0.82). The mean variations in glucose at fasting,
15, 30, 60, 90 and 120 min after a standardized meal from baseline
to week 12 in the nateglinide treatment group were −0.32, −0.18,
−1.02, −2.50, −2.94 and −2.35 mmol/l, respectively, with
significant differences at 90 and 120 min (P<0.05, Fig. 4). The corresponding changes in the
acarbose treatment group were −0.47, −1.01, −2.10, −3.2, −3.45 and
−2.52 mmol/l, respectively, with significant differences at 30, 60,
90 and 120 min after a standardized meal (P<0.001, Fig. 4). The intergroup differences in the
intragroup changes were significant at 15, 30 and 60 min after a
standardized meal, with acarbose treatment being superior to
nateglinide treatment at 15, 30 and 60 min after a standardized
meal (P<0.05).
Effects of nateglinide versus acarbose on
fasting and postprandial lipid profiles after 12 weeks of
treatment
Nateglinide exhibited a tendency to increase the
high-density lipoprotein cholesterol (HDL-C) levels and decrease
the total cholesterol (TC), low-density lipoprotein cholesterol
(LDL-C) and triglyceride (TG) levels after 12 weeks of treatment,
with a significant reduction (0.44 mmol/l) of fasting TC compared
to baseline (P<0.03). However, this tendency was not observed in
the acarbose group (Table
III).
 | Table IIIFasting and postprandial lipid
profiles at baseline and at 12 weeks. |
Table III
Fasting and postprandial lipid
profiles at baseline and at 12 weeks.
| Nateglinide | Acarbose |
|---|
|
|
|
|---|
| Lipid profiles | Baseline | 12 weeks | Baseline | 12 weeks |
|---|
| TC (mmol/l) |
| Fasting | 5.91±1.40 | 5.46±1.30a | 5.15±0.76 | 5.18±0.90 |
| Postprandial | 5.56±1.398 | 5.19±1.18 | 4.93±0.70 | 4.83±0.84 |
| TG (mmol/l) |
| Fasting | 1.79±1.44 | 1.37±0.63 | 1.51±0.99 | 1.57±0.83 |
| Postprandial | 2.13±1.47 | 1.61±0.71 | 1.75±1.21 | 1.79±1.37 |
| LDL-C (mmol/l) |
| Fasting | 3.77±1.43 | 3.73±1.14 | 3.39±0.73 | 3.37±0.88 |
| Postprandial | 3.58±1.32 | 3.13±1.11 | 3.08±0.70 | 3.15±0.73 |
| HDL-C (mmol/l) |
| Fasting | 1.20±0.17 | 1.25±0.20 | 1.12±0.21 | 1.11±0.20 |
| Postprandial | 1.13±0.16 | 1.25±0.28 | 1.03±0.19 | 1.01±0.16 |
Discussion
The present study has demonstrated that nateglinide
and acarbose exert similar hypoglycemic effects on postprandial 120
min glucose, which was consistent with the findings of previous
studies (5–9). We demonstrated that acarbose may be
more efficient in controlling early (30 and 60 min) postprandial
glucose and PPGE in the carbohydrate meal. However, as observed in
the mixed-meal test by CGMS, the efficiency of nateglinide in
reducing glycemic excursions in mixed meals was superior to that of
acarbose.
In type 2 diabetic patients, the early phase of
insulin secretion in response to a meal is delayed and blunted,
which is crucial in the regulation of postprandial blood glucose
levels, with this deficiency resulting in excessive meal-related
glucose excursions (10–13). Restoration of early insulin
secretion following a meal may exert a beneficial effect on
postprandial glucose control and glycemic excursions (14–16).
The results of the present study are consistent with those of
previous studies, which demonstrated that nateglinide effectively
decreases mealtime plasma glucose excursions (17–20).
Nateglinide reduces postprandial plasma glucose levels by promoting
early short-term insulin secretion, which may exert a beneficial
effect on controlling postprandial glucose and excursions.
In the mixed-meal tests evaluated by CGMS, the
efficiency of nateglinide in reducing glycemic excursions after a
mixed meal was superior to that of acarbose. However, in the
standardized carbohydrate meal test acarbose appeared to be
superior to nateglinide in controlling PPGE. This paradox may be
explained by the differences in the hypoglycemic mechanisms of the
two drugs. Acarbose inhibits the breakdown of carbohydrate by
binding competitively to α-glucosidase; therefore, since in the
standardized carbohydrate meal test the patients only ingested
carbohydrates, acarbose was more efficient in controlling early
(15, 30 and 60 min) postprandial glucose and PPGE.
The effects of antidiabetic drugs on blood lipid
levels were also investigated in this study. It was demonstrated
that nateglinide exhibited a tendency to increase HDL-C and
decrease LDL-C levels after 12 weeks of treatment. However,
acarbose did not affect the fasting or postprandial lipid profiles.
It is well known that blood lipid metabolism is affected by various
factors and primarily associated with insulin secretion dysfunction
in type 2 diabetes mellitus. The effect of nateglinide on lipid
profiles may be attributed to its mechanism of action,
significantly improving insulin secretion in the early phase.
However, acarbose does not exert any direct effect on insulin
secretion (21).
In conclusion, the results of our study demonstrated
that nateglinide and acarbose may effectively improve postprandial
glycemic control. Acarbose may be more effective in controlling
early (30 and 60 min) postprandial glucose and excursions in the
carbohydrate meal test, whereas in the mixed-meal test nateglinide
was superior to acarbose in controlling postprandial glucose
excursions. In addition, nateglinide exerted beneficial metabolic
effects by improving lipid profiles, which may be associated with
the restoration of early-phase insulin secretion.
References
|
1
|
Brownlee M and Hirsch IB: Glycemic
variability: a hemoglobin A1c-independent risk factor for diabetic
complications. JAMA. 295:1707–1708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch IB: Glycemic variability: it’s not
just about A1C anymore! Diabetes Technol Ther. 7:780–783. 2005.
|
|
3
|
Ceriello A and Ihnat MA: ‘Glycaemic
variability’: a new therapeutic challenge in diabetes and the
critical care setting. Diabet Med. 27:862–867. 2010.
|
|
4
|
Monnier L, Colette C and Owens DR:
Glycemic variability: the third component of the dysglycemia in
diabetes. Is it important? How to measure it? J Diabetes Sci
Technol. 2:1094–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gribble FM, Manley SE and Levy JC:
Randomized dose ranging study of the reduction of fasting and
postprandial glucose in type 2 diabetes by nateglinide (A-4166).
Diabetes Care. 24:1221–1225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim MK, Suk JH, Kwon MJ, et al:
Nateglinide and acarbose for postprandial glycemic control after
optimizing fasting glucose with insulin glargine in patients with
type 2 diabetes. Diabetes Res Clin Pract. 92:322–328. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hazama Y, Matsuhisa M, Ohtoshi K, et al:
Beneficial effects of nateglinide on insulin resistance in type 2
diabetes. Diabetes Res Clin Pract. 71:251–255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiasson JL, Josse RG, Hunt JA, et al: The
efficacy of acarbose in the treatment of patients with
non-insulin-dependent diabetes mellitus. A multicenter controlled
clinical trial. Ann Intern Med. 121:928–935. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feinbock C, Luger A, Klingler A, et al:
Prospective multicentre trial comparing the efficacy of, and
compliance with, glimepiride or acarbose treatment in patients with
type 2 diabetes not controlled with diet alone. Diabetes Nutr
Metab. 16:214–221. 2003.PubMed/NCBI
|
|
10
|
Calles-Escandon J and Robbins DC: Loss of
early phase of insulin release in humans impairs glucose tolerance
and blunts thermic effect of glucose. Diabetes. 36:1167–1172. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pratley RE and Weyer C: The role of
impaired early insulin secretion in the pathogenesis of type II
diabetes mellitus. Diabetologia. 44:929–945. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Del Prato S: Loss of early insulin
secretion leads to postprandial hyperglycaemia. Diabetologia.
46:M2–M8. 2003.PubMed/NCBI
|
|
13
|
Owens DR, Cozma LS and Luzio SD:
Early-phase prandial insulin secretion: its role in the
pathogenesis of type 2 diabetes mellitus and its modulation by
repaglinide. Diabet Nutr Met. 15(Suppl 6): 19–27. 2002.PubMed/NCBI
|
|
14
|
Bruttomesso D, Pianta A, Mari A, et al:
Restoration of early rise in plasma insulin levels improves the
glucose tolerance of type 2 diabetic patients. Diabetes. 48:99–105.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dimitriadis G, Boutati E, Lambadiari V, et
al: Restoration of early insulin secretion after a meal in type 2
diabetes: effects on lipid and glucose metabolism. Eur J Clin
Invest. 34:490–497. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pratley RE, Foley JE and Dunning BE: Rapid
acting insulinotropic agents: restoration of early insulin
secretion as a physiologic approach to improve glucose control.
Curr Pharm Des. 7:1375–1397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanefeld M, Bouter KP, Dickinson S and
Guitard C: Rapid and short-acting mealtime insulin secretion with
nateglinide controls both prandial and mean glycemia. Diabetes
Care. 23:202–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saloranta C, Guitard C, Pecher E, et al:
Nateglinide improves early insulin secretion and controls
postprandial glucose excursions in a prediabetic population.
Diabetes Care. 25:2141–2146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hollander PA, Schwartz SL, Gatlin MR, et
al: Importance of early insulin secretion comparison of nateglinide
and glyburide in previously diet-treated patients with type 2
diabetes. Diabetes Care. 24:983–988. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saloranta C, Hershon K, Ball M, et al:
Efficacy and safety of nateglinide in type 2 diabetic patients with
modest fasting hyperglycemia. J Clin Endocrinol Metab.
87:4171–4176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao HW, Xie C, Wang HN, et al: Beneficial
metabolic effects of nateglinide versus acarbose in patients with
newly-diagnosed type 2 diabetes. Acta Pharmacol Sin. 28:534–539.
2007. View Article : Google Scholar : PubMed/NCBI
|