Introduction
Apoptosis is a physiological process that regulates
normal homeostasis and alterations of the apoptosis-related genes
are likely to contribute to the pathogenesis of malignant tumors
(1–3) and autoimmune diseases (4). FAS is a type of cell surface apoptotic
signal transmission receptor. When combined with its natural ligand
CD95L to initiate the death signal cascade, the complex leads to
apoptosis (5,6). The human FAS gene is one of members of
the tumor necrosis factor receptor superfamily (7) and is located in chromosome 10q24.1,
involving 9 exons and 8 introns. Previous studies (8–10)
reported that the downregulation of FAS may result in resistance to
death signals in several types of cancer. Nunobiki et
al(11) reported that the
transcriptional expression of the FAS gene was regulated by a
number of genetic elements located in the 5′ upstream promoter
region of the gene. In the promoter region, Huang et
al(12) reported that the
polymorphism involved an A-to-G substitution at the -670 nucleotide
position in the enhancer region (FAS-670 A>G, rs1800682) and the
heterozygous A/G alleles were observed in 52% of the normal
population, with a frequency of the G and A alleles of 0.49 and
0.51, respectively.
The FAS-670 polymorphism consists of the variant
genotypes FAS-670 G/G and FAS-670 A/G and the wild-type A/A. The
frequency range of FAS-670 A/A among healthy controls was reported
to be 25.5–43.6% and the frequency of the homozygous G/G variant
~12%, whereas the frequency range of the heterozygous A/G was
reported to be 44.2–60.5% (13,14).
Cervical cancer is the second most common cancer
among women worldwide (11,15), with a high incidence (>80%) in
developing compared to developed countries (15,16).
Cervical cancer is on the increase in Asia (17) and exhibits relatively higher
incidence and mortality rates in Hungary compared to those in other
European Union countries (18).
Moreover, cervical cancer was reported to constitute 23.3% of all
cancers among African women (19).
Human papillomavirus (HPV) is widely considered as
the key etiological agent in cervical carcinogenesis. A
meta-analysis of cross-sectional high-risk HPV type distribution in
115,789 HPV-positive women was performed, with HPV16 positivity in
particular increasing steeply from normal/atypical squamous cells
of undetermined significance/low-grade squamous intraepithelial
lesion (LSIL)/cervical intraepithelial neoplasia (CIN)1 (20–28%),
through CIN2/high-grade squamous intraepithelial lesion (HSIL)
(40/47%) to CIN3/invasive cervical cancer (58/63%) in different
regions (20). Furthermore,
previous epidemiological studies investigated the etiology of
cervical cancer in order to recommend preventive measures to reduce
the incidence of cervical carcinogenesis and identified certain
important environmental factors. Cervical cancer is considered to
be a multifactorial disease, with smoking and age being important
etiological factors contributing to increased risk (21,22).
Therefore, genetic as well as environmental factors may contribute
to cervical carcinogenesis.
Previous studies, including the 10 studies that we
included in the present meta-analysis, were conducted to estimate
the incidence of cervical carcinogenesis in association with the
FAS-670 polymorphism; however, a consensus was not reached
(13,23–31).
Zhang et al(32) conducted a
meta-analysis on FAS promoter polymorphisms and cancer risk, but
failed to demonstrate a significant association with FAS-670
polymorphism. Since then, no confirmed outcomes based on small
sample sizes or potential publication bias from the previous
studies was obtained. Therefore, an updating meta-analysis was
performed, using the accumulated data, to re-examine the
association between the risk of cervical carcinogenesis and FAS-670
polymorphism.
Materials and methods
Search strategy
A search for eligible studies was conducted in
PubMed, Embase and HuGNet electronic databases, using the following
key words and word combinations: ‘uterine cervical neoplasm’,
‘cervical cancer’, ‘cervical’, ‘cervix’, ‘FAS’ and ‘FAS-670’. The
last update of retrieval was March 25, 2012. The search was limited
to English language papers. Additional studies were identified
through the reference lists of the original studies. We selected
the articles with more information regarding the origin of cases
and controls and the ones with the largest number of subjects among
the overlapping reports.
Selection and exclusion criteria
The detailed selection criteria were as follows: i)
case-control studies evaluating the association between FAS-670
polymorphism and the risk of cervical carcinogenesis; ii) case
population including patients with precancerous lesions and
cervical cancer patients; iii) control population comprising
healthy individuals and not malignant tumor patients. The exclusion
criteria were as follows: i) if similar studies included
overlapping populations, only the most recent articles were
included and the remaining were excluded; ii) insufficient data;
iii) Hardy-Weinberg equilibrium (HWE) did not reach statistical
significance (P<0.05).
Data extraction
The information was extracted from the eligible
studies, including first author, year of publication, ethnicity,
area, sample size of cases and controls, source of cases and
controls, mean age of cases and controls and genotype frequency in
cases and controls.
Statistical analysis
The ORs with their corresponding 95% CIs were used
as the metric of choice. Based on the individual ORs, the pooled OR
was estimated. First, we investigated the distribution of genotypes
in the control groups under HWE to obtain evidence of population
stratification (HWE; P>0.05) (33). We also estimated the association
with cervical carcinogenesis risk with a complete overdominant
genotypic model (G/G + A/A vs. A/G). Second, to assess the
Pheterogeneity among different studies, a statistical
test for heterogeneity was conducted using the I2
statistic, with values between 0 and 100%, with higher values
leading to greater heterogeneity (no heterogeneity, I2:
0–25%; moderate heterogeneity, I2: 25–50%; significant
heterogeneity, I2: 50–75%; and extreme heterogeneity,
I2: 75–100%) (34). If
the effect sizes were homogeneous among the studies, the fixed
effects model was used to estimate the overall effect size.
Otherwise, a random effects model was used. Random effects may
incorporate an estimate of between-study variance to a great extent
and provide wider 95% CI.
To further investigate the source of heterogeneity,
we performed a subgroup analysis by grouping studies with similar
characteristics, such as ethnicity and sample size. The ethnic
subgroups were categorized into Caucasian and Asian. In addition, a
sensitivity analysis was employed. In the sensitivity analysis,
studies was excluded one at a time to determine the magnitude of
their effect on the overall summary estimate (35). Finally, publication bias was
assessed using funnel plots and Begg’s rank correlation test
(36). All the P-values were
two-sided. The statistical analysis was performed using Metagen and
Stata software, version 11.0 (Stata Corp, College Station, TX,
USA).
Results
Identification and characteristics
A total of 140 abstracts were retrieved through
searching PubMed, Embase and HuGNet databases. We identified 16
relevant studies that described the association between the FAS-670
polymorphism and cervical carcinogenesis. However, after reading
the full articles, one study was excluded as a letter (37), one as a review (11) and two due to the lack of raw data
(5,38). Two studies were overlapped (13,39)
and one was retained (13)
according to the criteria mentioned above. After calculating the
HWE for each of the remaining studies, one more was excluded
(14) and a total of 10 studies
were finally included in this meta-analysis.
All the articles were case-control
studies
Among the eligible studies, 6 were conducted on
Asian (13,24,25,27,28,30)
and 4 on Caucasian populations (23,26,29,31).
Two studies were classified as LSIL and HSIL (28,30)
and one study included HSIL and cervical cancer (27), whereas others exclusively included
cervical cancer patients (13,23–26,29,31).
Only one study reported the clinical stages (26). In all the studies, the majority of
the patients were recruited from hospitals by blood samples or
tissue specimens. Six studies mentioned the mean age of the
patients (13,23,24,27,29,30)
and the remaining 4 studies did not (25,26,28,31).
All the studies used polymerase chain reaction. Other detailed
information is presented in Table
I.
 | Table ISummary of the studies of cervical
cancer risk and FAS-670 polymorphism. |
Table I
Summary of the studies of cervical
cancer risk and FAS-670 polymorphism.
| Investigator
(year) |
Selection/characteristics of cases and
controls (mean age ± SD) | Race | Eligible
subjects | Source of
controls | Method | Refs. |
|---|
|
|
|---|
| Cases | Controls | Cases | Controls |
|---|
| Zucchi et
al(2009) | Histologically
confirmed diagnosis (52.5±11.9) | Randomly selected
healthy subjects (43.8±11.7) | Caucasian | 91 | 176 | Population | PCR-RFLP | (23) |
| Sun et
al(2005) | Histological and
gynecological diagnosis (43.5±9.8) | Randomly selected
healthy subjects (44.0±10.1) | Asian | 314 | 625 | Population | PCR-RFLP | (13) |
| Kang et
al(2008) | Histologically
confirmed diagnosis (47.8) | Age-matched healthy
subjects (48.2) | Asian | 155 | 160 | Hospital | PCR-RFLP | (24) |
| Ueda et
al(2006) | Histologically
confirmed diagnosis | Healthy
subjects | Asian | 259 | 95 | Population | PCR | (25) |
| Zoodma et
al(2005) | Histologically
confirmed diagnosis | Randomly selected
healthy subjects | Caucasian | 985 | 607 | Population | PCR | (26) |
| Lai et
al(2005) | Histological and
cytological diagnosis (45.7±12.9) (HSIL 45.5±13.0) (CC
54.2±12.9) | Age-matched to
patients | Asian | 318 | 318 | Hospital | PCR | (27) |
| Ueda et
al(2005) | Histologically
confirmed diagnosis | Normal patients
from hospital | Asian | 216 | 63 | Hospital | PCR-RFLP | (28) |
| Dybikowska et
al(2004) | Histologically
confirmed diagnosis (53.7) | Healthy women
(29.5) | Caucasian | 51 | 65 | Hospital | PCR-RFLP | (29) |
| Lai et
al(2003) | Histological and
cytological selected diagnosis (41.6±11.0) | One to one matched
to patients (within 3 years) | Asian | 411 | 411 | Hospital | PCR-RFLP | (30) |
| Chatterjee et
al(2009) | Histologically
confirmed diagnosis | Without cervical
cancer from hospitals and clinics | Caucasian | 447 | 424 | Hospital | Total nucleic acid
extraction technologies | (31) |
Main results and subgroup analysis
In total, the eligible studies included 3,247 cases
and 2,944 controls and a total of 2,901 cases and 2,831 controls
were genotyped. The summary ORs and 95% CIs for the FAS-670
polymorphism and the subgroup analysis are presented in Table II. The results indicated that
FAS-670 was not associated with the risk of cervical
carcinogenesis. The summary OR was 1.13 (95% CI: 0.95–1.34), with
between-study heterogeneity (I2=52.7%,
Pheterogeneity=0.03). All the analyses were based on
pooling of data from different populations. Therefore, a subgroup
analysis according to different ethnicities was performed. The OR
for Asians was 1.25 (6 comparisons, 95% CI: 1.05–1.48,
I2=23.5%, Pheterogeneity=0.03), whereas for
Caucasians, no significant association was observed between FAS-670
polymorphism and the risk of cervical carcinogenesis (4
comparisons, OR=0.96, 95% CI: 0.75–1.24, I2=45.9%,
Pheterogeneity=0.14) (Fig.
1).
 | Table IISummary ORs and 95% CIs for FAS-670
polymorphism and subgroup analysis. |
Table II
Summary ORs and 95% CIs for FAS-670
polymorphism and subgroup analysis.
| Subgroups and
FAS-670 polymorphism | Comparisons
(no.) | Genotype cases
(no.) | Genotype controls
(no.) | Random effects OR
(95% CI) |
Pheterogeneity | I2
(%) |
|---|
| Population |
| Asians | 6 | 1,496 | 1,662 | 1.25 (1.05,
1.48) | 0.03 | 23.5 |
| Caucasians | 4 | 1,405 | 1,169 | 0.96 (0.75,
1.24) | 0.14 | 45.9 |
| Sample |
| >200 | 6 | 2,522 | 2,335 | 1.13 (0.91,
1.41) | 0.01 | 69 |
| <200 | 4 | 290 | 496 | 1.13 (0.86,
1.48) | 0.42 | 0.0 |
| Overall | 10 | 2,901 | 2,831 | 1.13 (0.95,
1.34) | 0.03 | 52.7 |
In the sensitivity analysis, we applied the random
effects model (Fig. 2) to estimate
the risk of cervical cancer (OR=1.13, 95% CI: 0.95–1.34,
I2=52.7%, Pheterogeneity=0.03). However, in
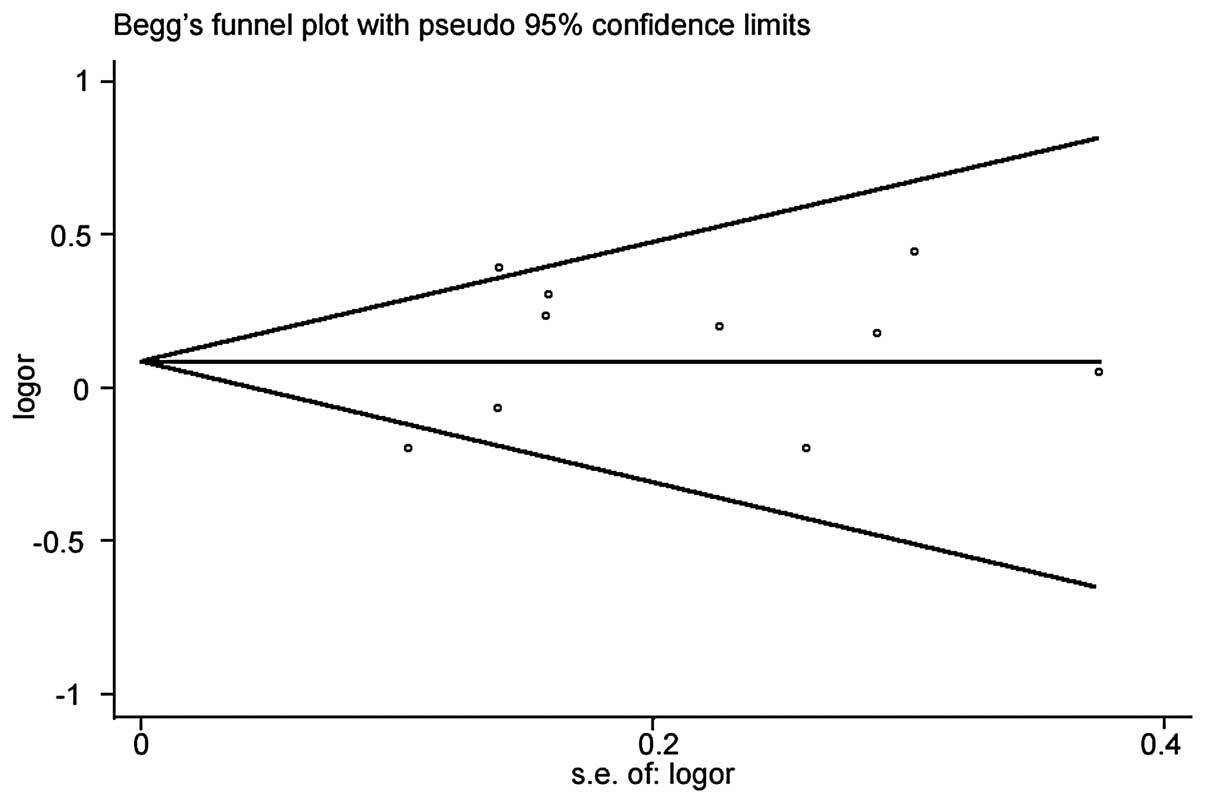
the funnel plot analysis of publication bias, the funnel plot
appeared to be symmetrical and the Egger’s test (P=0.343) revealed
no evidence of publication bias (Fig.
3).
Discussion
This meta-analysis, involving the comparison of a
total of 3,247 cases and 2,944 controls, investigated 10
case-control studies on FAS-670 and assessed the association of
FAS-670 polymorphism with the risk of cervical carcinogenesis.
There was no significant evidence supporting an association between
FAS-670 polymorphism and cervical cancer risk. In the
meta-analysis, heterogeneity was always estimated in a statistical
analysis. However, the tests appeared to be of low statistical
power. Thus, a subgroup meta-analysis was conducted based on
ethnicity and sample size. In the ethnicity subgroups, a positive
association between FAS-670 polymorphism and cervical cancer was
observed in Asian, but not in Caucasian populations. However, the
negative result in Caucasian must be assessed with caution, as the
relatively high between-study heterogeneity may due to a mixture of
populations of different races and from different geographical
regions. Moreover, regarding sample size subgroups, no association
between FAS-670 polymorphism and cervical cancer was observed in
the smaller or in the larger size subgroups.
Regarding our results, several limitations must be
mentioned. First, of all the eligible studies, there was inherent
bias in the study design. Selection bias is a possible major source
of heterogeneity in the acquisition of cancer samples and hospital
controls. Moreover, only two studies (27,30)
matched the number of subjects between the case and the control
groups and this lack of symmetry in the included subjects may lead
to deviations. All these factors may result in bias.
Second, the pathological classification and clinical
stages were not consistent. For example, some of the studies only
included samples of cervical cancer, whereas others included
cervical cancer and LSIL or HSIL. The potential deviation may
produce different outcomes.
Finally, the combined analysis of different ages and
races may lead to deviations. The mean age range of the eligible
subjects was 29–55 years in the case and control groups. However,
one study reported that the risk of cervical cancer increases with
advancing age (23). Thus, age may
be the cause of heterogeneity. Moreover, the incidence of cervical
cancer differs among different ethnicities (14,17,18);
therefore, a subgroup analysis according to race is required.
However, our meta-analysis was only focused on Asians and
Caucasians, which may have affected the outcome of the ethnicity
subgroup analysis.
References
|
1
|
Zörnig M, Hueber A, Baum W and Evan G:
Apoptosis regulators and their role in tumorigenesis. Biochim
Biophys Acta. 1551:F1–F37. 2001.
|
|
2
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lorenz HM, Herrmann M, Winkler T, Gaipl U
and Kalden JR: Role of apoptosis in autoimmunity. Apoptosis.
5:443–449. 2000. View Article : Google Scholar
|
|
5
|
Itoh N, Yonehara S, Ishii A, et al: The
polypeptide encoded by the cDNA for human cell surface antigen Fas
can mediate apoptosis. Cell. 66:233–243. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oehm A, Behrmann I, Falk W, et al:
Purification and molecular cloning of the APO-1 cell surface
antigen, a member of the tumor necrosis factor/nerve growth factor
receptor superfamily. Sequence identity with the Fas antigen. J
Biol Chem. 267:10709–10715. 1992.
|
|
7
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Butler LM, Hewett PJ, Butler WJ and Cowled
PA: Down-regulation of Fas gene expression in colon cancer is not a
result of allelic loss or gene rearrangement. Br J Cancer.
77:1454–1459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SH, Shin MS, Park WS, et al:
Alterations of Fas (APO-1/CD95) gene in transitional cell
carcinomas of urinary bladder. Cancer Res. 59:3068–3072.
1999.PubMed/NCBI
|
|
10
|
Shimonishi T, Isse K, Shibata F, et al:
Up-regulation of Fas ligand at early stages and down-regulation of
Fas at progressed stages of intrahepatic cholangiocarcinoma reflect
evasion from immune surveillance. Hepatology. 32:761–769. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nunobiki O, Ueda M, Toji E, et al: Genetic
polymorphism of cancer susceptibility genes and HPV infection in
cervical carcinogenesis. Patholog Res Int.
2011:3640692011.PubMed/NCBI
|
|
12
|
Huang QR, Morris D and Manolios N:
Identification and characterization of polymorphisms in the
promoter region of the human Apo-1/Fas (CD95) gene. Mol Immunol.
34:577–582. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun T, Zhou Y, Li H, et al: FASL -844C
polymorphism is associated with increased activation-induced T cell
death and risk of cervical cancer. J Exp Med. 202:967–974. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kordi Tamandani DM, Sobti RC and Shekari
M: Association of Fas-670 gene polymorphism with risk of cervical
cancer in North Indian population. Clin Exp Obstet Gynecol.
35:183–186. 2008.
|
|
15
|
Scarinci IC, Garcia FA, Kobetz E, et al:
Cervical cancer prevention: new tools and old barriers. Cancer.
116:2531–2542. 2010.PubMed/NCBI
|
|
16
|
Anorlu RI: Cervical cancer: the
sub-Saharan African perspective. Reprod Health Matters. 16:41–49.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moore MA, Manan AA, Chow KY, et al: Cancer
epidemiology and control in peninsular and island South-East Asia -
past, present and future. Asian Pac J Cancer Prev. 2:81–98.
2010.PubMed/NCBI
|
|
18
|
Langmár Z, Németh M and Kornya L: Cervical
cancer screening in Hungary - epidemiologic, historical and
methodologic aspects. Orv Hetil. 152:2063–2066. 2010.(In
Hungarian).
|
|
19
|
Parkin DM, Sitas F, Chirenje M, Stein L,
Abratt R and Wabinga H: Part I: Cancer in indigenous Africans -
burden, distribution, and trends. Lancet Oncol. 9:683–692. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guan P, Howell-Jones R, Li N, Bruni L, de
Sanjosé S, Franceschi S and Clifford GM: Human papillomavirus types
in 115,789 HPV-positive women: a meta-analysis from cervical
infection to cancer. Int J Cancer. 131:2349–2359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pate Capps N, Stewart A and Burns C: The
interplay between secondhand cigarette smoke, genetics, and
cervical cancer: a review of the literature. Biol Res Nurs.
10:392–399. 2009.PubMed/NCBI
|
|
22
|
Dikshit R, Gupta PC, Ramasundarahettige C,
et al: Cancer mortality in India: a nationally representative
survey. Lancet. 12:1807–1816. 2012. View Article : Google Scholar
|
|
23
|
Zucchi F, da Silva ID, Ribalta JC, et al:
Fas/CD95 promoter polymorphism gene and its relationship with
cervical carcinoma. Eur J Gynaecol Oncol. 30:142–144.
2009.PubMed/NCBI
|
|
24
|
Kang S, Dong SM, Seo SS, Kim JW and Park
SY: FAS -1377 G/A polymorphism and the risk of lymph node
metastasis in cervical cancer. Cancer Genet Cytogenet J. 180:1–5.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueda M, Terai Y, Kanda K, et al: Fas gene
promoter -670 polymorphism in gynecological cancer. Int J Gynecol
Cancer. 16(Suppl 1): 179–182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zoodsma M, Nolte IM, Schipper M, et al:
Interleukin-10 and Fas polymorphisms and susceptibility for
(pre)neoplastic cervical disease. Int J Gynecol Cancer. 3:282–290.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai HC, Lin WY, Lin YW, Chang CC, Yu MH,
Chen CC and Chu TY: Genetic polymorphisms of FAS and FASL
(CD95/CD95L) genes in cervical carcinogenesis: an analysis of
haplotype and gene-gene interaction. Gynecol Oncol. 99:113–118.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueda M, Hung YC, Terai Y, et al: Fas gene
promoter -670 polymorphism (A/G) is associated with cervical
carcinogenesis. Gynecol Oncol. 98:129–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dybikowska A, Sliwinski W, Emerich J and
Podhajska AJ: Evaluation of Fas gene promoter polymorphism in
cervical cancer patients. Int J Mol Med. 14:475–478.
2004.PubMed/NCBI
|
|
30
|
Lai HC, Sytwu HK, Sun CA, et al: Single
nucleotide polymorphism at Fas promoter is associated with cervical
carcinogenesis. Int J Cancer. 103:221–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chatterjee K, Engelmark M, Gyllensten U,
et al: Fas and FasL gene polymorphisms are not associated with
cervical cancer but differ among black and mixed-ancestry South
Africans. BMC Res Notes. 26:2382009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Xue H, Gong W, Wang M, Yuan L,
Han S and Zhang Z: FAS promoter polymorphisms and cancer risk: a
meta-analysis based on 34 case-control studies. Carcinogenesis.
30:487–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weir BS: Genetic data analysis II: Methods
for discrete population genetic data. Sunderland Mass: Sinauer
Associates; 1996
|
|
34
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zintzaras E, Chatzoulis DZ, Karabatsas CH
and Stefanidis I: The relationship between C677T
methylenetetrahydrofolate reductase gene polymorphism and
retinopathy in type 2 diabetes: a meta-analysis. J Hum Genet.
50:267–275. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Engelmark MT, Renkema KY and Gyllensten
UB: No evidence of the involvement of the Fas -670 promoter
polymorphism in cervical cancer in situ. Int J Cancer.
112:1084–1085. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lerma E, Romero M, Gallardo A, et al:
Prognostic significance of the Fas-receptor/Fas-ligand system in
cervical squamous cell carcinoma. Virchows Arch. 452:65–74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li H, Guo HY, Sun T, Zhou YF, Lin DX,
Zhang WH and Qiao J: Association between Fas/Fas L genes promoter
polymorphisms and pathogenic risk of cervical cancer. Chin J Oncol.
31:38–41. 2009.(In Chinese).
|