Introduction
Cardiovascular complications are likely to occur at
an early stage in uremic patients undergoing hemodialysis (1,2).
Common risk factors, such as hypertension, rarely account for this
phenomenon (3,4).
Activation of inflammatory response centered on the
excretion of cytokines is closely associated with cardiovascular
complications in uremic cases (5,6),
particularly by T cells (7).
IL-10-producing CD4+CD25+Foxp3+
regulatory T cells (Tregs) contribute to anti-inflammatory response
(8), while the IL-17-induced
T-helper cells (Th17s) exhibit a pro-inflammatory role (9). IL-10 and IL-6 play a pivotal role in
altering the Treg/Th17 balance. The aim of this study was to
examine the association between Treg/Th17 balance and
cardiovascular comorbidity in maintenance hemodialysis (MHD).
Subjects and methods
Patients
This study conformed to the approved institutional
guidelines of the Ethics Committee of our Hospital. Informed
consent in accordance with the Declaration of Helsinki protocol was
obtained from each participant. Of the 96 patients diagnosed with
uremia from July, 2010 to January, 2011, 30 cases (18 men and 12
women, mean age 50.33±16.12 years) presented with no history of
cardiovascular complications (WHD) nor underwent MHD, while the
remaining 66 cases (40 men and 26 women, mean age 53.02±12.73
years) had undergone MHD for at least 3 months, with a mean
duration of dialysis of 30.28±21.24 months.
The exclusion criteria for this study were: i) the
presence of preceding infection, connective tissue diseases,
malignant tumors, progressive hepatopathy, hepatitis B, hepatitis
C, AIDS, valvular heart disease, as well as bleeding and
coagulation dysfunction; ii) installation of heart pacemakers; iii)
the administration of steroids or non-steroidal anti-inflammatory
drugs and; iv) the serum level of parathyroid hormone (PTH) ≥400
pg/ml. The underlying diseases of the WHD group included chronic
glomerulonephritis (n=12), hypertensive nephrosclerosis (n=7),
diabetic nephropathy (n=4), polycystic kidney (n=2), chronic
interstitial nephritis (n=3) and 2 cases with unknown origin, while
those of the MHD group included 24 cases with chronic
glomerulonephritis, 16 with hypertensive nephrosclerosis, 10 with
diabetic nephropathy, 6 with chronic interstitial nephritis, 4 with
polycystic kidney, 4 with cerebrovascular disease and 2 with
unknown origin. A total of 66 cases of MHD were further divided
depending on presence of cardiovascular diseases. Consequently, 36
cases (MHD1) were identified with unstable angina (n=6), left
ventricular hypertrophy (n=10), severe arrhythmia (n=14),
congestive heart failure (n=6), while no cardiovascular diseases
were identified in the remaining 30 cases (MHD2). Moreover, 20
healthy individuals (10 men and 10 women, mean age 43.89±8.72
years) comprised the control group (CON). Patient data were
collected for statistical analysis including age, gender, duration
of dialysis, smoking, history of diabetes mellitus,
hyperlipidaemia, blood pressure, body mass index (BMI), laboratory
and echocardiographical parameters, as well as medications. BMI was
calculated as height/weight2.
Hemodialysis strategy
Hemodialysis patients in the MHD group were treated
using polysulfone membranes, with heparin serving as an
anticoagulant. The hemodialysis blood flow rate was set at 200–250
ml/min, while the dialysate flow rate was 500 ml/min. Hemodialysis
was conducted at a regular frequency of 2–3 times per week, lasting
4–5 h each time.
Peripheral mononuclear cell (PMNC)
preparation
Fresh serum samples were obtained from the ulnar
vein on an empty stomach from all the patients and healthy
volunteers. Samples were collected in tubes containing heparin. The
levels of haemoglobin, albumin, serum creatinine, blood urea
nitrogen (BUN), lipid, glucose, PTH and CK-MB in the blood were
measured. PMNCs were purified by Ficoll-Hypaque (Sigma Chemical,
St. Louis, MO, USA). Cells were separated using density-gradient
centrifugation and cultured in RPMI-1640 medium (Gibco-BRL Life
Technologies, Merelbeke, Belgium) supplemented with 10% FCS
(Hyclone, Logan, UT, USA), 2 mM L-glutamine, 100 U/ml penicillin,
and 100 μg/ml streptomycin, in a humidified incubator with
5% CO2 at 37°C (Shellab 2306, USA).
Flow cytometry
Flow cytometry and cell sorting were performed on
the BD FACSAria Cell-Sorting System (BD Biosciences, San Jose, CA,
USA). Separated cells were washed with FACS buffer
(phosphate-buffered saline supplemented with 0.1% sodium azide and
2% fetal bovine serum), fixed with 4% paraformaldehyde for 10 min,
and permeabilized with 0.1% Triton X-100. The cells were incubated
with anti-CD4, anti-CD80 or anti-CD86 conjugated with PE, as well
as anti-CD25 and Anti-Foxp3 labeled with FITC (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) according to the
manufacturer’s instructions. The cells were then washed with the
FACS buffer and underwent flow cytometric analysis with CellQuest
Pro (BD Biosciences). The results were presented as the geometric
mean fluorescence intensity.
Magnetic bead cell sorting
Using CD4 and CD25 microbead selection (Miltenyi
Biotec, Auburn, CA, USA), Treg cells were obtained as
CD4+CD25+ and Th17 cells as
CD4+CD25−, as previously described (10,11).
Similarly, peripheral monocytes, isolated from PMNC using
CD14+ microbead selection (Miltenyi Biotec), were
respectively seeded into an anti-CD3-coated round-bottomed 96-well
plate at 1×105/well in 200 μl and subdivided into
four subgroups: i) exclusive culture of CD14+ cells; ii)
coculture with CD4+CD25+ T cells at a ratio
of 1:1; iii) coculture with CD4+CD25− T cells
at a ratio of 1:1 in the absence of anti-IL-17Ab and; iv) coculture
with CD4+CD25− T cells at a ratio of 1:1 in
the presence of anti-IL-17Ab. Cells were cultured for 3 days at
37°C in RPMI-1640 medium. Experiments were repeated three
times.
Cytokine secretion
The supernatants of cultured systems were collected
and stored at −80°C for subsequent experiments. Prior to use,
samples were treated for 30 min at 37°C with 25 U/ml of
hyaluronidase (Sigma-Aldrich, Zwijndrecht, The Netherlands).
Soluble IL-6 and IL-10 levels were measured by enzyme-linked
immunosorbent assay (ELISA) using the commercially available kits
(R&D Systems, Minneapolis, MN, USA). Optical densities were
measured at 450 nm (Spectra II; SLT, Vienna, Austria) and cytokine
concentrations were determined from the optical density value
according to standard curves.
Statistical analysis
Data were assessed using the SPSS software 16.0
(Statistical Package for the Social Sciences, version 13.0; SPSS
Inc., Chicago, IL, USA). The two-tailed unpaired Student’s t-test
was used for comparison between groups and subgroups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Subjects
No significant differences were found among the
MHD1, MHD2 and WHD groups with regards to characteristics such as
age, gender, risk factors, therapeutic regimens, ejection factor,
left ventricular end-diastolic diameter and left ventricular
end-systolic diameter (LVEDD and LVESD) (P>0.05). The levels of
BUN and serum creatinine were significantly higher in the WHD group
when compared with the MHD1 and MHD2 groups undergoing hemodialysis
(P<0.05). In the MHD1 group, the left ventricular mass index of
189.00±35.38 was the highest, followed by 120.05±2.59 in the WHD
and 102.34±11.30 in the MHD2 groups (P<0.05). In the MHD2 group,
the fractional shortening ratio of 33.79±2.08 was the highest,
compared to 31.72±3.39 in the WHD and 26.86±1.82 in the MHD1 groups
(P<0.05). No significant differences were found in the
traditional risk factors of atherosclerotic cardiovascular disease,
such as smoking, hypertension, diabetes mellitus, hyperlipidaemia,
anemia, left ventricular hypertrophy, and increased age, as well as
other baseline characteristics among in any of the uremic subjects
(Table I).
 | Table IBaseline characteristics of the study
population. |
Table I
Baseline characteristics of the study
population.
| Characteristics | CON (n=20) | WHD (n=30) | MHD1 (n=36) | MHD2 (n=30) |
|---|
| Age (years) | 43.89±8.72 | 50.33±16.12 | 55.70±12.64 | 46.85±11.02 |
| Gender
(male:female) | 10:10 | 18:12 | 24:12 | 16:14 |
| Months since
initiating dialysis | - | - | 30.28±23.04 | 30.30±17.20 |
| Risk factors | | | | |
| Smoking, n (%) | 4 (20) | 10 (33) | 11 (31) | 8 (27) |
| Diabetes mellitus,
n (%) | 0 (0) | 4 (13) | 6 (17) | 4 (13) |
| Hyperlipidaemia, n
(%) | 2 (10) | 11 (37) | 12 (33) | 8 (27) |
| Systolic blood
pressure (mmHg) | 120.44±8.88 | 147.14±16.15 | 149.10±15.60 | 141.15±16.88 |
| Diastolic blood
pressure (mmHg) | 72.94±6.48 | 88.52±15.55 | 83.70±9.83 | 86.31±9.22 |
| Body mass
index | 20.99±1.81 | 22.54±2.43 | 21.75±3.39 | 22.09±1.71 |
| Haemoglobin
(g/l) | 130.56±11.08 | 93.40±21.90 | 93.63±22.81 | 105.62±14.14 |
| Albumin (g/l) | 37.47±5.56 | 32.57±5.06 | 35.47±4.61 | 37.69±6.64 |
| Other laboratory
parameters | | | | |
| Blood urea nitrogen
(mmol/l) | 5.86±0.90 | 31.16±10.59 | 22.68±8.94a | 20.26±8.91a |
| Serum creatinine
(μmol/l) | 89.5±15.21 | 997.10±325.25 | 728.54±288.06a | 710.88±249.38a |
| Echocardiography | | | | |
| Left ventricular
systolic lumen (mm) | 31.33±2.99 | 34.00±4.68 | 34.97±2.58 | 31.77±2.28 |
| Left ventricular
diastolic lumen (mm) | 43.39±3.70 | 43.64±5.06 | 49.70±3.39 | 42.91±4.56 |
| Ejection fraction
(%) | 58.82±2.72 | 57.73±4.69 | 57.77±7.68 | 58.95±3.21 |
| Fractional
shortening (%) | 37.27±4.73 | 31.72±3.39 | 26.86±1.82a | 33.79±2.08b |
| Left ventricular
mass index (g/m2) | 84.71±6.94 | 120.05±2.59b | 189.00±35.38a |
102.34±11.30a,b |
| Medications | | | | |
| rHu-EPO, n (%) | 0 (0) | 18 (60) | 22 (61) | 20 (67) |
| β-blockers, n
(%) | 0 (0) | 9 (30) | 10 (28) | 10 (33) |
| Statins, n (%) | 2 (10) | 9 (30) | 12 (33) | 8 (27) |
Enhanced Th17 proliferation and reversal
of the Treg/Th17 ratio in uremic patients, particularly for the WHD
and MHD1 groups
As shown in Table II
and Fig. 1, the frequencies of Treg
and Th17 cells were significantly higher in the uremic groups when
compared with the CON. By contrast, the ratio of Treg/Th17 was
lower in the uremic groups compared with the CON (P<0.05). No
significant differences of the Treg frequencies were identified
among the WHD, MHD1 and MHD2 subgroups (P>0.05). The WHD
subgroup had a markedly higher frequency of Th17 cells and a
notably lower Treg/Th17 ratio compared with the MHD2 subgroup
(P<0.05). Between the MHD subgroups, the Th17 frequencies of the
MHD1 subgroup were notably higher, while the Treg/Th17 ratio was
notably lower compared with the MHD2 group (P<0.05).
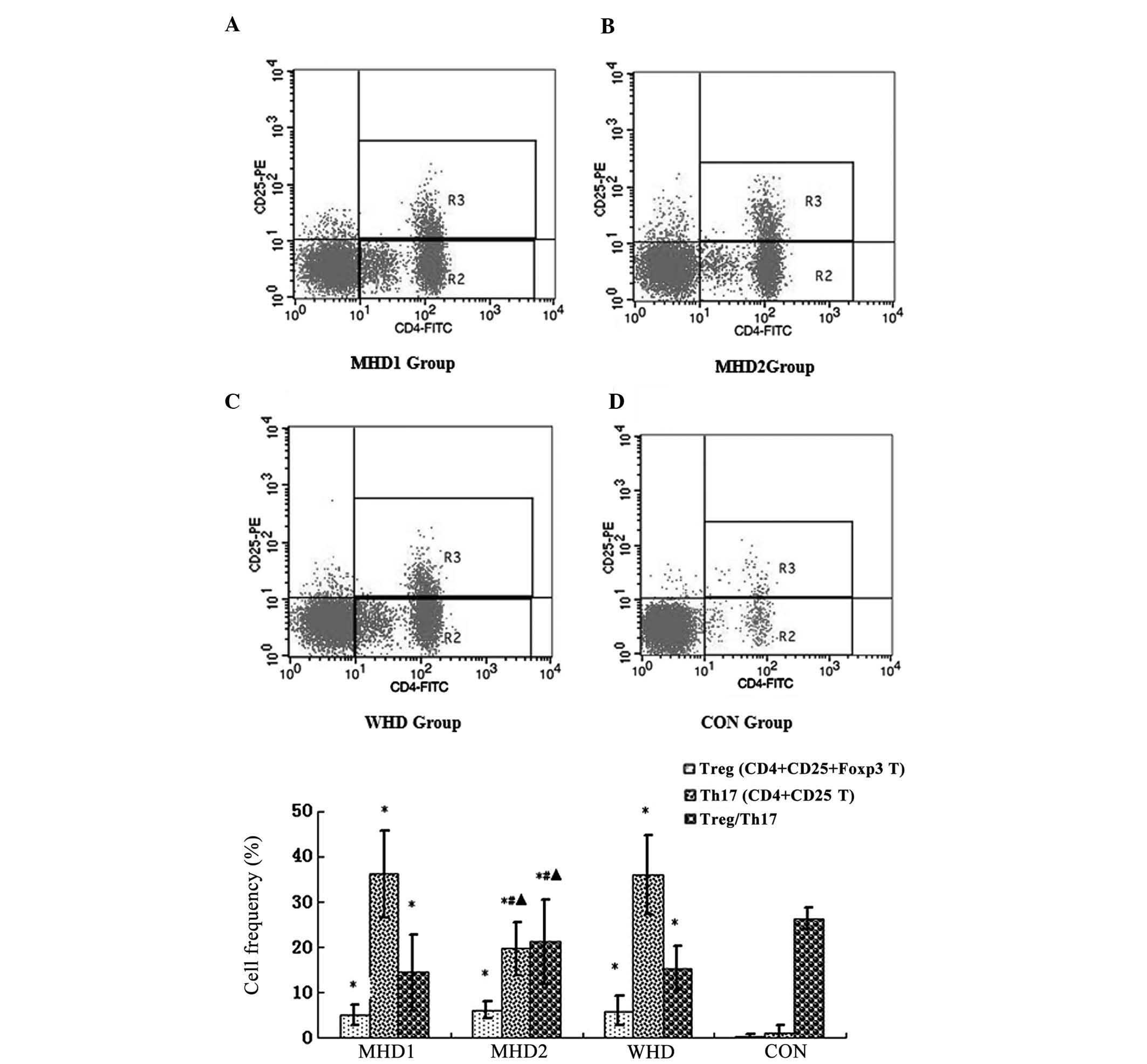 | Figure 1As detected by flow cytometry,
CD4+CD25+ populations were counted as Treg
cells, while CD4+CD25− populations were
counted as Th17 cells. (A) Frequencies of Treg and Th17 cells in
the MHD1, (B) MHD2, (C) WHD and CON groups. As shown in (E),
compared to the control group, the frequencies of Treg and Th17
cells were significantly higher and the ratio of Treg/Th17 was
lower in the uremic groups (P<0.05), compared to the MHD2 group.
The WHD and MHD1 subgroups had markedly higher frequencies of Th17
cells and a notably lower Treg/Th17 ratios (P<0.05).
*P<0.05, compared with the CON group;
#P<0.05, comparison between the WHD group and the MHD
group. ▴P<0.05, comparison between the MHD1 group and
the MHD2 group. MHD, maintenance hemodialysis; MHD1, group
presenting with cardiovascular complications during MHD; MHD2,
group lacking cardiovascular complications during MHD; WHD, group
with neither cardiovascular complications nor maintenance
hemodialysis, CON, healthy control group; Treg, regulatory T cell;
Th17; IL-17-induced T-helper cell |
 | Table IIComparison of Treg, Th17 frequencies
and Treg/Th17 ratios among groups (mean ± standard deviation). |
Table II
Comparison of Treg, Th17 frequencies
and Treg/Th17 ratios among groups (mean ± standard deviation).
| MHD1 | MHD2 | WHD | CON |
|---|
| Treg (%) | 5.05±2.38a | 6.06±1.79a | 5.92±3.29a | 0.32±0.44 |
| Th17 (%) | 36.27±9.62a |
19.64±5.97a,b,c | 35.98±8.85a | 1.12±1.52 |
| Treg/Th17 | 14.43±8.28a |
21.21±10.28a,b,c | 15.35±4.86a | 26.31±2.26 |
Restoration of Th17-induced IL-6
overproduction by anti-IL-17 in cocultured supernatants of uremic
cohort
Expression of IL-6 and IL-10 was detected in the
cocultured supernatants. Compared with the healthy controls, IL-6
concentration levels of the subgroups of each uremic group were
significantly higher, respectively (P<0.05) (Table III). By contrast, compared with the
MHD2 group, IL-6 concentration levels of the MHD1 and WHD subgroups
were significantly higher (P<0.05). However, no significant
differences were observed when comparing the MHD1 group with the
WHD group (P>0.05). Treg cells inhibited, while Th17 cells
increased IL-6 expression in the WHD, MHD1 and MHD2 subgroups,
although no statistically significant differences were detected
(P>0.05). Additionally, in the MHD1 and WHD groups, the addition
of anti-IL-17 was found to suppress the upregulation of IL-6
induced by Th17 cells (P<0.05). By contrast, compared with the
healthy or MHD2 groups, the IL-10 concentrations of the cocultured
subgroups were significantly decreased in the MHD1 and WHD groups,
respectively (P<0.05) (Table
IV).
 | Table IIIIL-6 concentration in cocultured
supernatants by ELISA. |
Table III
IL-6 concentration in cocultured
supernatants by ELISA.
| IL-6 (pg/ml) | CD14+
monocytes | Monocyte-Treg
cocultures | Monocyte-Th17
cocultures | Monocyte-Th17
cocultures in the presence of anti-IL-17 |
|---|
| MHD1 (n=36) | 75.04±3.25a | 56.12±1.49a |
82.74±3.28a,d |
57.71±2.87a,b |
| MHD2 (n=30) |
44.17±4.75a,c |
35.15±0.72a,c |
49.58±4.26a,c |
37.02±1.22a,c |
| WHD (n=30) | 78.94±5.44a | 61.18±1.36a |
88.94±1.83a,d |
64.47±1.98a,e |
| CON (n=20) | 15.56±1.58 | 14.68±1.66 | 16.98±1.25 | 16.04±0.83 |
 | Table IVIL-10 concentration in cocultured
supernatants by ELISA. |
Table IV
IL-10 concentration in cocultured
supernatants by ELISA.
| IL-10 (pg/ml) | CD14+
monocytes | Monocyte-Treg
cocultures | Monocyte-Th17
cocultures | Monocyte-Th17
cocultures in the presence of anti-IL-17 |
|---|
| MHD1 (n=36) |
12.62±1.90a,b |
17.47±1.62a,b |
19.92±2.53a,b |
17.52±2.71a,b |
| MHD2 (n=30) | 21.75±1.17 | 23.29±2.36 | 23.48±1.35 | 23.22±2.11 |
| WHD (n=30) |
11.27±2.22a,b |
13.63±2.45a,b |
18.58±1.91a,b |
15.36±1.18a,b |
| CON (n=20) | 26.94±0.54 | 24.15±1.50 | 27.62±0.68 | 23.77±2.31 |
Mild alteration of costimulatory
molecules CD80 and CD86 on monocytes when cocultured with Treg or
Th17 cells in the presence or absence of anti-IL-17
To mimic signaling via the B7 family of
costimulatory molecules, the monocyte surface expression of the
costimulatory molecules CD80 and CD86 was detected by flow
cytometry (Table V). When compared
with the healthy group, a significantly elevated percentage of
monocytes expressing either CD80 or CD86 was detected in the
pathological cohort between the same paired subgroups (P<0.05).
Although the tendency of CD80 and CD86 to induce Treg
downregulation and Th17 upregulation was identified, no significant
differences were observed (P>0.05). The addition of anti-IL-17
mAb to the monocyte-Th17 cocultures partly resulted in the
suppression of Th17-induced CD80 and CD86 activation in all the
studied subsets, although no statistically significant differences
were noted (P>0.05).
 | Table VExpression of costimulatory molecules
CD80 and CD86 on monocytes when cocultured with Treg or Th17 cells
in the absense or presense of anti-IL-17. |
Table V
Expression of costimulatory molecules
CD80 and CD86 on monocytes when cocultured with Treg or Th17 cells
in the absense or presense of anti-IL-17.
| CD14+
monocytes | Monocyte-Treg
cocultures | Monocyte-Th17
cocultures | Monocyte-Th17
cocultures in the presence of anti-IL-17 |
|---|
| CD80 | | | | |
| MHD1 | 53.32±0.75a | 51.29±1.28a | 57.14±1.07a | 54.00±0.79a |
| MHD2 | 49.55.±0.72a | 47.85±0.92a | 52.86.±0.18a | 50.22±1.10a |
| WHD | 59.50±0.62a | 56.76±1.65a | 66.70±1.54a | 61.29±1.59a |
| CON | 23.53±2.52 | 23.50±1.45 | 27.97±0.27 | 25.58±0.45 |
| CD86 | | | | |
| MHD1 | 42.98±0.31a |
40.82±1.51as | 47.21±0.56a | 43.73±0.37a |
| MHD2 | 40.58±0.54a | 39.42±0.36a | 43.56.±0.97a | 42.11±0.04a |
| WHD | 47.93±0.33a | 44.88±1.18a | 50.44±1.63a | 46.83±1.02a |
| CON | 23.06±1.40 | 23.02±1.48 | 28.06±0.50 | 25.56±1.34 |
Discussion
Cardiovascular disease, an inflammatory disorder
regulated by T lymphocytes, is the leading cause of mortality in
chronic kidney diseases and the uremic state. One of the risk
factors for potential cardiovascular complications in uremic adults
is the autoimmune component, which has been characterized by the
excretion of inflammatory cytokines (IL-1, IL-6, IL-10 and TNF-α),
particularly by T cells at an early stage. Homeostasis of uremic
patients is disturbed by a complex network of immune T-cell
subpopulations. Recently, Treg and Th17 cells, two distinct subsets
from Th1 and Th2 cells, have been described to have the opposite
effects on autoimmunity (12).
Treg/Th17 balance controls inflammation and may therefore be
crucial in the pathogenesis of plaque destabilization as well as
the onset of acute coronary syndrome, which includes unstable
angina and acute myocardial infarction (?). To demonstrate whether
Treg/Th17 is associated with cardiovascular disease in uremic
patients during MHD, we detected Treg/Th17 functions on different
levels including cell frequencies, related cytokine secretion and
key costimulatory molecules.
It is noteworthy that the Treg/Th17 cell function
disequilibrium might act synergistically with calcification in the
high incidence of cardiovascular disease subsequent to
hemodialysis, which has been indirectly demonstrated in a previous
study with the aid of ex vivo intervention of recombinant
human bone morphogenetic protein-2 (13). However, the previous study was
completely based on hemodialysis patients, who did not have uremia
or were not assessed for baseline cardiovascular disease.
Consequently, findings of that study render it difficult to
distinguish whether the Treg/Th17 imbalance was mainly affected by
uremia-related inflammation, cardiovascular disease-related
conditions during hemodialysis or other pathological alterations,
such as calcification. Therefore, the present study selected 30
uremic cases with no cardiovascular complications or MHD as the WHD
baseline control.
The results of this study indicate that uremic
patients, particularly those in the MHD1 and WHD cohort, exhibited
significant increases of peripheral Th17 frequencies and
Th17-related cytokines (IL-6) levels. Additionally, a mild
alteration of Treg frequencies and Treg-related cytokine (IL-10)
levels was observed when compared with the healthy cohort. These
findings suggest that the disturbed Treg/Th17 numerical and
functional balance has a potential role in the uremic cases and in
the development of adverse cardiovascular events for this cohort,
to some extent, mainly via IL-6-mediated Th17 differentiation
rather than Treg. This observation is concordance with those of
recent studies which described that the function fo Treg was
defective in certain human autoimmune diseases, including multiple
sclerosis (14), type 1 diabetes
mellitus, and rheumatoid arthritis (15). Consistent with the previous
conclusion that Treg cells were able to induce
CD4+CD25− naive T cells or Treg cells
themselves to differentiate into Th17 in the presence of IL-6 alone
without exogenous TGF-β (16), we
observed that IL-6 rather than IL-10 might be the key switch
immunoregulatory factor contributing to the shift of the Th17/Treg
ratio in the uremic cases and in the development of adverse
cardiovascular complications for this cohort. Moreover, IL-6 is a
pleiotropic cytokine involved in determining the relative balance
of inflammation-promoting Th17 vs. anti-inflammatory Treg cells by
inducing the development of Th17 cells from naive T cells. Our
results have demonstrated that MHD effectively improved the
pathological Treg/Th17 imbalance and IL-6 overproduction for the
uremic cohort. These findings suggest that blocking IL-6-induced
Th17 differentiation is a promising therapeutic target that is
likely to mitigate myocardial inflammation and reduce the risk of
adverse cardiovascular events. By contrast, anti-IL-17
significantly restored the Th17-induced IL-6 secretion in uremic
cases, particularly for the MHD1 and WHD cohorts, without altering
the costimulatory molecules CD80 and CD86 expression on the surface
of monocytes. Thus, peripheral blood Th17 likely plays a crucial
role in the pathogenesis of cardiovascular complications for uremic
patients in a costimulatory molecule independent pattern, although
the concrete mechanisms remain unclear. In this study, blocking
IL-6 seems to be a more optimal target compared with either CD80 or
CD86 in preventing adverse cardiovascular events for uremic
patients during MHD.
A larger patient sample and more isolated Treg cells
for functional studies should be considered for future studies.
Moreover, a workup of Treg/Th17 cytokines, including TGF-β and more
costimulatory molecules, which were unavailable in our study, may
provide a more precise tool for determining the correlation between
inflammation status and various cardiovascular complications during
hemodialysis.
In summary, our findings provided evidence that
Treg/Th17 imbalance plays a potential role in the pathogenesis of
cardiovascular complications for uremic patients undergoing
hemodialysis. Additionally, blockage of Th17 differentiation
activated by IL-17-induced IL-6 overproduction may be an attractive
therapeutic goal. However, this remains to be proven in a larger
scale patient cohort. The precise mechanism of Treg/Th17 imbalance
in the pathogenesis of cardiovascular complications for uremic
patients undergoing hemodialysis should also be investigated.
References
|
1
|
Carrero JJ, de Jager DJ, Verduijn M, et
al: Cardiovascular and noncardiovascular mortality among men and
women starting dialysis. Clin J Am Soc Nephrol. 6:1722–1730. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
House AA and Ronco C: The burden of
cardiovascular risk in chronic kidney disease and dialysis patients
(cardiorenal syndrome type 4). Contrib Nephrol. 171:50–56. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liebman SE, Lamontagne SP, Huang LS, et
al: Smoking in dialysis patients: a systematic review and
meta-analysis of mortality and cardiovascular morbidity. Am J
Kidney Dis. 58:257–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dheir H, Ozkahya M, Kircelli F, et al:
Glycosylated hemoglobin levels are associated with cardiovascular
events in nondiabetic peritoneal dialysis patients. J Nephrol.
25:107–112. 2012. View Article : Google Scholar
|
|
5
|
Singh AK, Singh V, Pal Singh M, et al:
Effect of immunosenescence on the induction of cardiovascular
disease pathogenesis: role of peripheral blood mononuclear cells.
Immunopharmacol Immunotoxicol. 30:411–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bacharaki D, Thodis E, Passadakis P, et
al: Comparative in vitro study of different peritoneal dialysis
solutions on cytokine production by peripheral blood mononuclear
cells. Nephron Clin Pract. 113:c321–c329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Hua G, Zhang X, et al: Regulatory
T cells/T-helper cell 17 functional imbalance in uraemic patients
on maintenance haemodialysis: a pivotal link between
microinflammation and adverse cardiovascular events. Nephrology.
15:33–41. 2010. View Article : Google Scholar
|
|
8
|
Ohkusu-Tsukada K, Toda M, Udono H, et al:
Targeted inhibition of IL-10-secreting CD25− Treg via
p38 MAPK suppression in cancer immunotherapy. Eur J Immunol.
40:1011–1021. 2010.PubMed/NCBI
|
|
9
|
Korn T, Mitsdoerffer M, Croxford AL, et
al: IL-6 controls Th17 immunity in vivo by inhibiting the
conversion of conventional T cells into Foxp3+
regulatory T cells. Proc Natl Acad Sci USA. 105:18460–18465. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu L, Kitani A, Fuss I and Strober W:
Cutting edge: regulatory T cells induce
CD4+CD25−Foxp3− T cells or are
self-induced to become Th17 cells in the absence of exogenous
TGF-beta. J Immunol. 178:6725–6729. 2007.PubMed/NCBI
|
|
11
|
Walker LS: CD4+
CD25+ Treg: divide and rule? Immunology. 111:129–137.
2004.
|
|
12
|
Weaver CT and Hatton RD: Interplay between
the TH17 and TReg cell lineages: a (co-)evolutionary perspective.
Nat Rev Immunol. 9:883–889. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen D, Huang X, Yang M, et al: Treg/Th17
functional disequilibrium in Chinese uremia on hemodialysis: a link
between calcification and cardiovascular disease. Ren Fail.
34:697–702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haas J, Fritzsching B, Trübswetter P, et
al: Prevalence of newly generated naive regulatory T cells (Treg)
is critical for Treg suppressive function and determines Treg
dysfunction in multiple sclerosis. J Immunol. 79:1322–1330. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamagiwa T, Fukunishi S, Tachibana T, et
al: Abrogation of Treg function deteriorates rheumatoid arthritis.
Mod Rheumatol. 22:80–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura A and Kishimoto T: IL-6: regulator
of Treg/Th17 balance. Eur J Immunol. 40:1830–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|















