Introduction
Breast cancer is a malignancy that affects women
worldwide. Despite developments in surgery and chemotherapeutics,
early diagnosis and prognosis of breast cancer remain poor.
Previous studies have investigated Muc-1 gene-related protein
biomarkers CA15.3 and CA27.29 (1,2),
Her-2/neu (3) and CEA (4), however, each of these potential
biomarkers has limitations with regard to sensitivity, specificity
or the scale of applicability. At present, no single protein serves
as a biomarker for the screening of breast cancer. The combined
application of biomarkers in biochemical detection, such as
CEA-TPA-CA15.3 (5), ameliorates the
weakness of each biomarker. However, the cost and complicated
evaluation process involved in the detection of this type of cancer
suggests a combined application is not feasible. Therefore, it is
crucial to identify biomarkers with high diagnostic value.
Neutrophil gelatinase-associated lipocalin (NGAL)
also known as lipocalin 2 is a 25-kDa glycoprotein, which was
originally identified as a covalent complex with matrix
metalloproteinase-9 (MMP-9) in human neutrophil (6,7). NGAL
has recently been investigated in a variety of physiological and
pathological conditions. As a biomarker, the diagnostic value of
NGAL has been identified in acute kidney injury (8). Additionally, NGAL is involved in
various types of human cancer. NGAL expression is upregulated in
the majority of human cancers, including colorectal neoplasia
(9–11), gastric cancer (12), oesophageal squamous cell carcinoma
(13), lung adenocarcinoma
(14), primary liver carcinoma
(15) and thyroid neoplasia
(16). However, this expression is
downregulated in cancers such as pancreatic (17–19)
and prostate (20), as well as
chronic myeloid leukemia (21,22).
The NGAL gene in human is highly expressed in
luminal epithelial cells compared to myoepithelial cells (23). Subsequently, the majority of breast
carcinomas were thought to develop from luminal epithelial cells.
Thus, NGAL may actively participate in breast cancer progression
(24). NGAL levels were also
strongly correlated with poor histological grading, lymph node
metastasis, high carcinoma proliferation ability and weak prognosis
of breast cancer patients (25).
However, no specific study has sufficiently
evaluated the correlation between NGAL expression and the risk of
breast cancer. Therefore, in the present study, we aimed to
estimate the possibility of NGAL as a biomarker in the early
diagnosis of breast cancer via a meta-analysis of published
literatures.
Materials and methods
Data sources and search strategy
The meta-analysis of Observational Studies in
Epidemiology (MOOSE) guidelines for the conduct of meta-analyses of
observational cohort studies were followed. Two invesgators (Y.W.
and T.T.Z.) conducted a literature search using PubMed, OVID,
ScienceDirect and the China National Knowledge Infrastructure
(CNKI) databases, including all published papers until November
2012 using a combination of the following terms: neutrophil
gelatinase-associated lipocalin, NGAL, Lipocalin 2 and breast
cancer. There were no language restrictions.
Publication selection
The investigators Y.W. and T.T.Z. independently
reviewed potentially associated publications by checking their
titles and abstracts and then procured the most relevant papers for
further examination. Moreover, the reference lists of the selected
studies were also screened for any potential information. The
criteria used to select studies for the meta-analysis were: i)
studies focusing on the association of NGAL with breast cancer; ii)
observational studies; iii) studies that reported breast cancer
pathological diagnoses and sources of cases and controls; iv) test
methods [immunohistochemistry (IHC) or ELISA] and v) completeness
of data, or availability of information that proved useful in
deriving results. Exclusion criteria included: i) different design
and definition of experiments; ii) source of cases and controls and
other important information could not be obtained; iii) animals or
in vitro experiments; iv) reviews and repeated
literature.
Data extraction and study quality
assessment
Data including author, publication year, region,
study population and the measurement method of NGAL were extracted
by two independent reviewers (Y.W. and T.T.Z.) and entered into a
database. For conflicting evaluations, agreement was achieved
following a discussion. The quality of each included study was
assessed using the diagnostic accuracy tool, quality assessment for
studies of diagnostic accuracy (QUADAS; maximum score, 14).
Statistical analysis
The studies were analysed using a Chi-square-based Q
statistic test to assess heterogeneity and I2 to
estimate the degree of heterogeneity. Statistically significant
heterogeneity was considered when P<0.05 and the I2
value was >50%. If there was significant heterogeneity, the
random-effect model (DerSimonian and Laird) was used. Otherwise,
the fixed-effect model (Mantel-Haenszel) was employed.
The bivariate model was applied for the diagnostic
meta-analysis in order to perform the pooled sensitivity,
specificity, as well as the positive likelihood (PLR), negative
likelihood (NLR) and diagnostic odds (DOR) ratios. Pooled estimates
with the corresponding 95% CI were initially calculated using the
appropriate statistical analysis model. In addition, summary
receiver operator characteristic (sROC) curves were constructed.
The area under the curve (AUC) value with the Q-value was also
calculated to present an overall summary of test performance in
order to differentiate between a diseased and non-diseased
participant. Spearman’s correlation coefficient of sensitivity and
1-specificity was calculated to estimate the threshold effect. The
publication bias of included studies was assessed using the
effective sample-size funnel plot and Egger’s test.
Statistical analysis was implemented by MetaDisc 1.4
and Stata 11.0 software.
Results
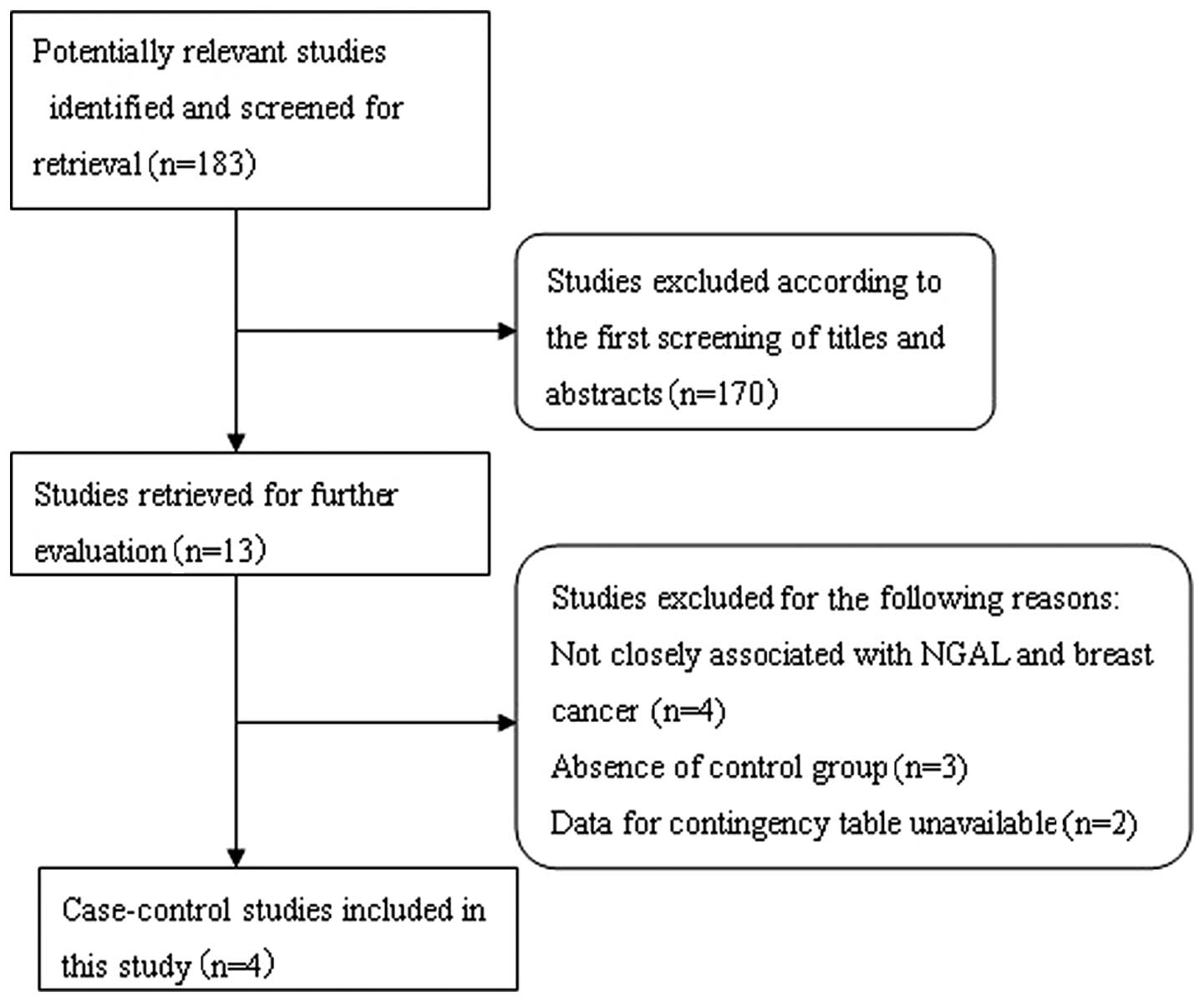
Search results and study
characteristics
The systematic literature search generated a total
of 183 references based on the search strategy. We excluded 170
studies after screening the titles and abstracts, as the majority
of these studies did not fulfill the criteria for our
meta-analysis, while others were excluded due to duplication or
review articles. A careful review of the remaining 13 studies
revealed that 4 studies did not focus on the association between
NGAL and breast cancer, and were excluded. Subsequently, a further
5 studies were excluded; 3 studies were excluded as they were
performed on tissue microarrays and lacked control groups (25–27)
and 2 studies were excluded as they did not interpret the IHC
results with regard to negative/positive and had insufficient data
for constructing the 2×2 contingency tables (24,28).
Following exclusion of the abovementioned studies, 4 studies were
included in this meta-analysis (29–32). A
flow chart showing the study selection procedure is given in
Fig. 1.
A database was established based on the extracted
information from these 4 studies (Table
I). These 4 studies were single-center trials conducted in
China and included 332 breast cancer patients. NGAL expression was
analyzed in paraffin sections by IHC. The quality of each study was
appraised according to QUADAS. The results are shown in Table I.
 | Table ICharacteristics of the studies
included in the meta-analysis. |
Table I
Characteristics of the studies
included in the meta-analysis.
| | | | | | | Age range (mean,
years)
| | | |
|---|
| Authors | Year | Method | No. of patients | Histological type of
control | No. of control | Type of control | Patients | Control | Country | QUADAS | Ref. |
|---|
| Zhang et
al | 2008 | IHC | 127 | 102 IDC, 17 ILC, 8
other | 20 | Breast
fibroadenoma | NA | NA | China | 9 | (29) |
| Lv and Qi | 2011 | IHC | 85 | IDC | 60 | 30 mammary benign
hyperplasia, 30 adjacent normal tissue | 23 72 (47) | NA | China | 11 | (30) |
| Wang et
al | 2011 | IHC | 60 | 28 DC, 21 LC, 11
other | 10 | Normal mammary
gland | 36 69 (55) | 31 58 (43) | China | 10 | (31) |
| Qiu | 2012 | IHC | 60 | IBC | 52 | Mammary benign
hyperplasia | 20 78 (48) | 17 75 (40) | China | 13 | (32) |
Diagnostic accuracy analyses
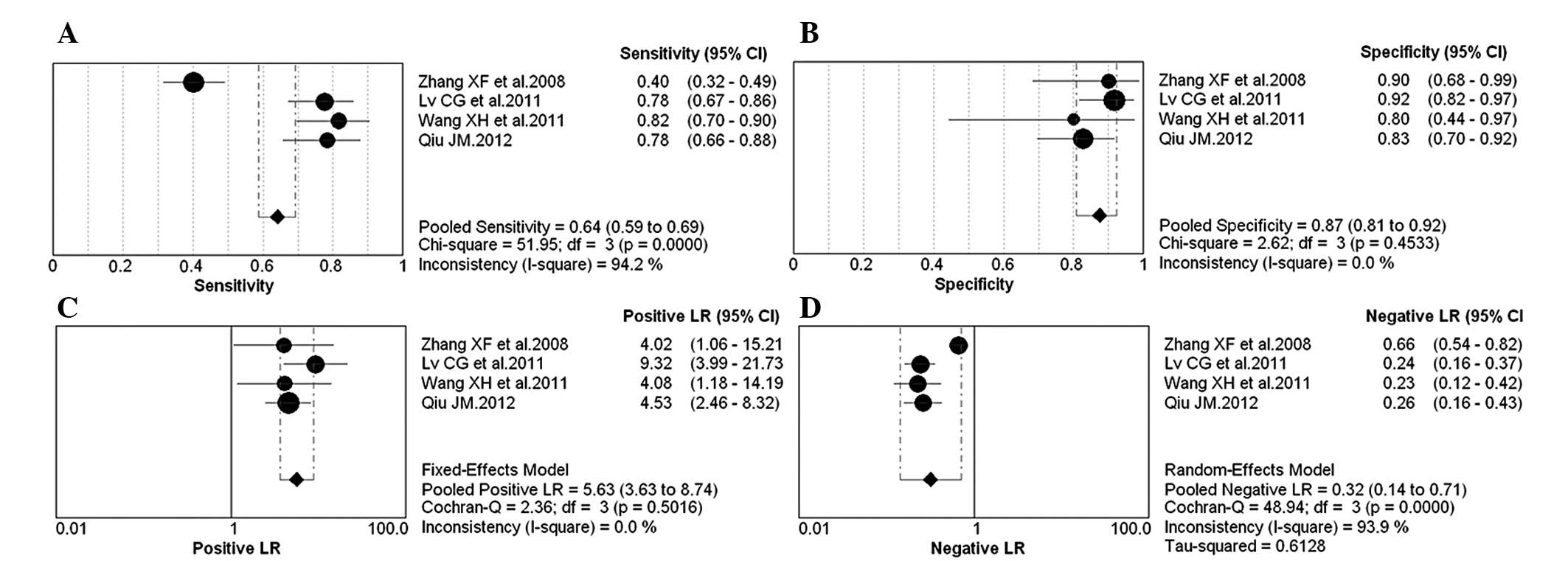
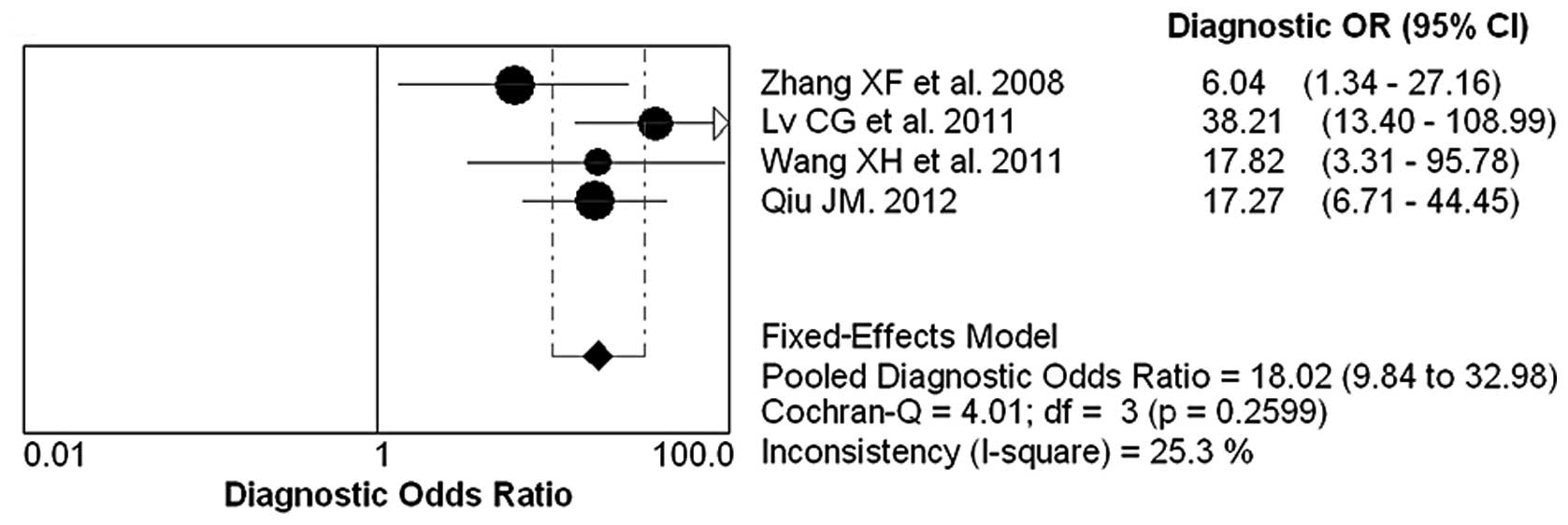
The forest plot of sensitivity, specificity, PLR,
NLR and DOR for NGAL test in breast cancer diagnosing is shown in
Figs. 2 and 3. The overall pooled sensitivity and
specificity of all studies were 64% (95% CI, 0.59–0.69) and 87%
(95% CI, 0.81–0.92), respectively. The overall pooled PLR and NLR
were 5.63 (95% CI, 3.63–8.74) and 0.32 (95% CI, 0.14–0.71). The
pooled DOR was 18.02 (95% CI, 9.84–32.98).
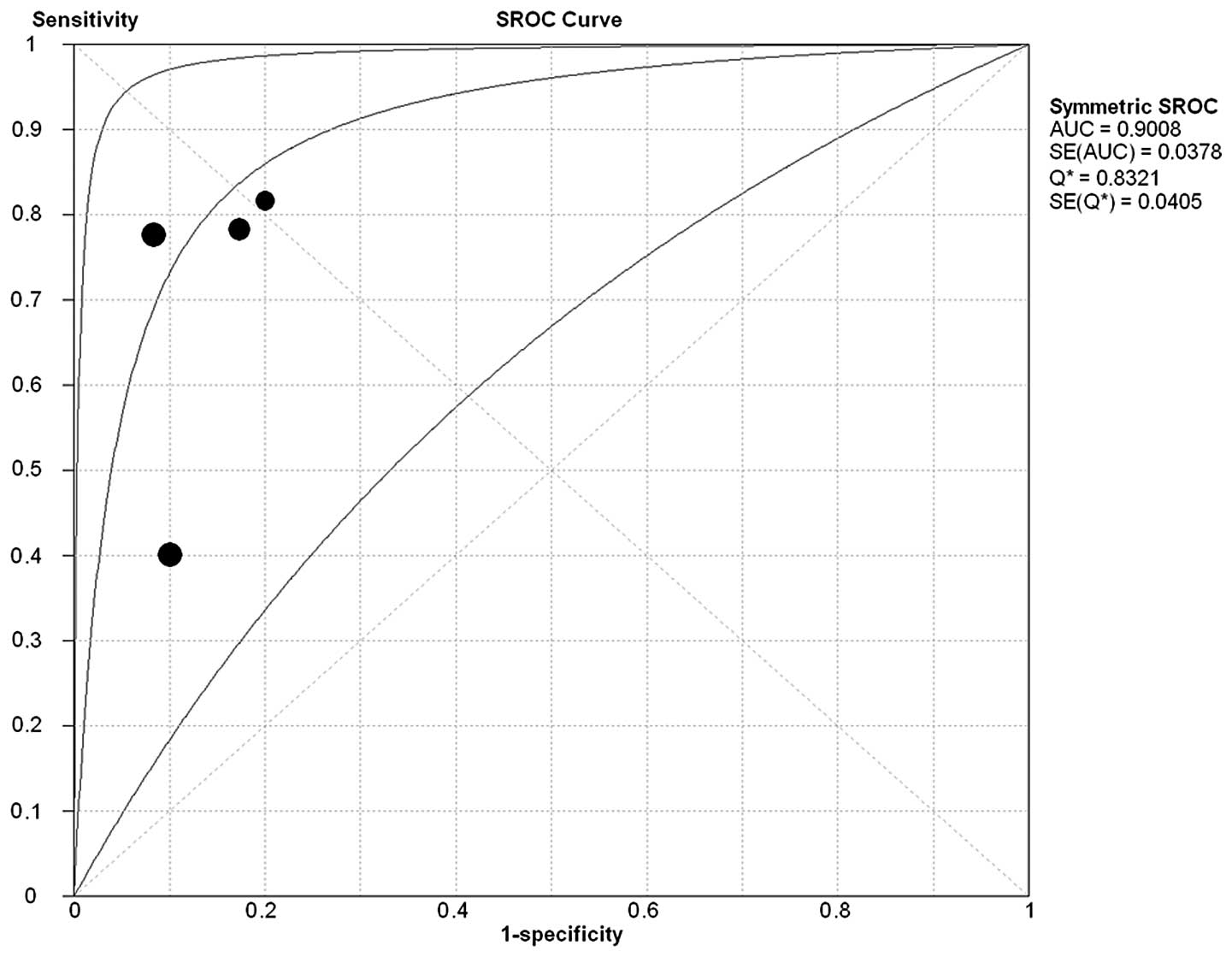
Summary receiver operating
characteristics
The sROC curve for NGAL expression showing
true-positive rates against false-positive rates from each study
demonstrates the trade-off between sensitivity and specificity. The
studies were included to construct the sROC curve (Fig. 4). The AUC for the diagnosis of
breast cancer was 0.9008 and the Q*-value was
0.8321.
Test of heterogeneity
A threshold analysis was performed to explore the
threshold effect, which was evaluated with the Spearman’s
correlation coefficient, using the Moses model weighted by inverse
variance. Not statistically significant difference was observed
(Spearman’s correlation coefficient, 0.8, P=0.2).
Cochran’s Q test and the I2 statistic
were used to evaluate the presence of statistical heterogeneity in
the 4 studies examined (Figs. 2 and
3), and the following results were
identified: pooled sensitivty (Chi-square=51.95,
I2=94.2%, P<0.001), specificity (Chi-square=2.62,
I2=0, P>0.05), PLR (Chi-square=2.36, I2=0,
P>0.05), NLR (Chi-square=48.94, I2=93.9%, P=0.001)
and DOR (Chi-square=4.01, I2=25.3, P>0.05). By
meta-regression analysis, the number of cases in control group or
QUADAS score was not the heterogeneity source.
Publication bias
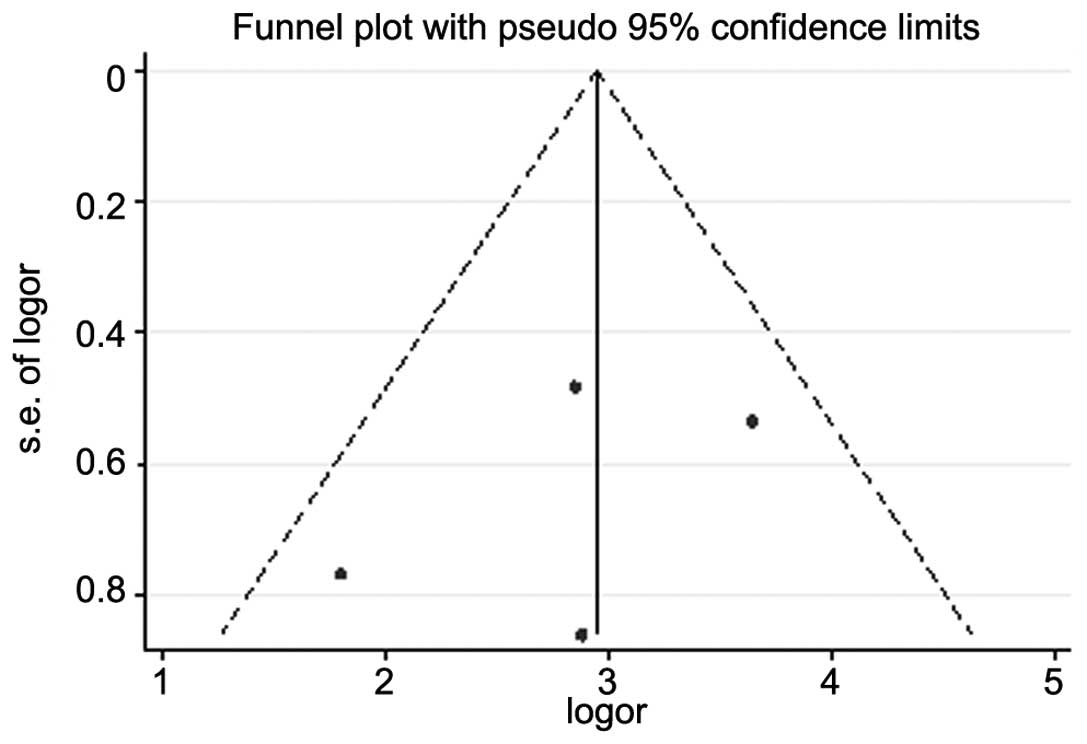
Funnel plot and Egger’s test were performed to
access the publication bias of these studies. The shape of funnel
plots showed symmetry (Fig. 5). The
P-value of Egger’s test was 0.5. The result did not suggest any
evidence of publication bias.
Discussion
NGAL is a small, secreted glycoprotein with proposed
functions in cell proliferation, survival and morphogenesis. NGAL
is expressed in a variety of tumor types including breast cancer
(25). In normal human mammary
epithelial cells, NGAL expression is under estrogen control
(33), while in malignant human
mammary epithelial cells, NGAL appears to escape from hormonal
regulation, since this protein is most abundant in ER-negative
breast cancer cell lines and primary tumor samples (25).
In this study, we clarified the diagnostic accuracy
of NGAL for breast cancer by meta-analysis of 4 studies and the
results suggested a relationship between NGAL and breast cancer.
Using the bivariate model for diagnostic meta-analysis, we found a
summary AUC of 0.9008. The DOR is a single indicator used to
evaluate the diagnostic value of proposed tests. The pooled DOR of
NGAL was 18.02, representative of the odds ratio between breast
cancer patients and controls. The pooled sensitivity and
specificity were 64 and 87%, respectively, indicating that the
assay may result in 36% false-negative and 13% false-positive test
results. The overall results indicated that NGAL test may be useful
in the diagnosis of breast cancer. However, the low sensitivity but
high specificity suggested that a patient with a positive result
needed to undergo further laboratory evaluation and imaging.
When PLR>10 or NLR<0.1, the possibility of
approving or negating a diagnosis of a disease is significantly
increased. In our study, the pooled PLR was 5.63, indicating that
the NGAL test was 5.63 times more likely to achieve a correct
NGAL-positive test result in the breast cancer group compared with
the controls. The pooled NLR was 0.32, indicating that the
possibility of the NGAL test achieving an incorrect NGAL-negative
test result in the breast cancer group was 32% compared with the
controls. The overall results indicated that the NGAL protein test
had a certain value in the diagnosis of breast cancer.
The heterogeneity of the 4 studies was analyzed.
Results of the Spearman’s correlation coefficient indicated that
heterogeneity was not correlated with the threshold effect. The
factors, number of controls and QUADAS score were added to the
meta-regression, but these did not explain the heterogeneity. We
hypothesized that the heterogeneity was due to the limited sample
size of the 4 selected studies in this meta-analysis.
Limitations of the present meta-analysis should also
be considered. First, the number of studies meeting the inclusion
criteria was limited as the focus was on China alone. Studies from
other countries were excluded due to the absence of control groups
(25–27) or a lack of particular data. Second,
IHC was utilized for the detection of NGAL in the 4 included
primary studies. One study pertaining to NGAL quantified by ELISA
assay of blood sample was not included as the information was not
interpreted using odds ratios (28). Third, certain studies used a limited
sample size. Thus, due to the limitations of this present
meta-analysis, more worldwide studies are required to confirm the
value of the NGAL test for breast cancer diagnosis in the
future.
In summary, the association of NGAL and breast
cancer was assessed by pooling the included data via a systematic
meta-analysis. The results of the present study demonstrated that
NGAL is a potential biomarker for the diagnosis of breast
cancer.
References
|
1
|
Gion M, Mione R, Leon AE and Dittadi R:
Comparison of the diagnostic accuracy of CA27.29 and CA15.3 in
primary breast cancer. Clin Chem. 45:630–637. 1999.
|
|
2
|
Gion M, Mione R, Leon AE, Luftner D,
Molina R, Possinger K and Robertson JF: CA27.29: a valuable marker
for breast cancer management. A confirmatory multicentric study on
603 cases. Eur J Cancer. 37:355–363. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luftner D, Luke C and Possinger K: Serum
HER-2/neu in the management of breast cancer patients. Clin
Biochem. 36:233–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Fang F, Shi C, et al: Evaluation
of a method for the simultaneous detection of multiple tumor
markers using a multiplex suspension bead array. Clin Biochem.
45:1394–1398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nicolini A, Carpi A, Ferrari P and Rossi
G: Immunotherapy prolongs the serum CEA-TPA-CA15.3 lead time at the
metastatic progression in endocrine-dependent breast cancer
patients: a retrospective longitudinal study. Cancer Lett.
263:122–129. 2008. View Article : Google Scholar
|
|
6
|
Flower DR: The lipocalin protein family:
structure and function. Biochem J. 318(Pt 1): 1–14. 1996.
|
|
7
|
Triebel S, Blaser J, Reinke H and
Tschesche H: A 25 kDa alpha 2-microglobulin-related protein is a
component of the 125 kDa form of human gelatinase. FEBS Lett.
314:386–388. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haase M, Bellomo R, Devarajan P,
Schlattmann P and Haase-Fielitz A: Accuracy of neutrophil
gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis
in acute kidney injury: a systematic review and meta-analysis. Am J
Kidney Dis. 54:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kjeldsen L, Johnsen AH, Sengelov H and
Borregaard N: Isolation and primary structure of NGAL, a novel
protein associated with human neutrophil gelatinase. J Biol Chem.
268:10425–10432. 1993.PubMed/NCBI
|
|
10
|
Hu L, Hittelman W, Lu T, et al: NGAL
decreases E-cadherin- mediated cell-cell adhesion and increases
cell motility and invasion through Rac1 in colon carcinoma cells.
Lab Invest. 89:531–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang XF, Zhang Y, Zhang XH, et al:
Clinical significance of Neutrophil gelatinase-associated lipocalin
(NGAL) expression in primary rectal cancer. BMC Cancer. 9:1342009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kubben FJ, Sier CF, Hawinkels LJ, et al:
Clinical evidence for a protective role of lipocalin-2 against
MMP-9 autodegradation and the impact for gastric cancer. Eur J
Cancer. 43:1869–1876. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Xu L, Xiao D, et al: Upregulation
of neutrophil gelatinase-associated lipocalin in oesophageal
squamous cell carcinoma: significant correlation with cell
differentiation and tumour invasion. J Clin Pathol. 60:555–561.
2007. View Article : Google Scholar
|
|
14
|
Friedl A, Stoesz SP, Buckley P and Gould
MN: Neutrophil gelatinase-associated lipocalin in normal and
neoplastic human tissues. Cell type-specific pattern of expression.
Histochem J. 31:433–441. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li LG, Zhou T and Hou YM: Expression and
functional analysis of NGAL gene in human hepatocellular carcinoma.
J Fudan Univ (Nat Sci). 51:91–98. 2012.
|
|
16
|
Iannetti A, Pacifico F, Acquaviva R, et
al: The neutrophil gelatinase-associated lipocalin (NGAL), a
NF-kappaB-regulated gene, is a survival factor for thyroid
neoplastic cells. Proc Natl Acad Sci USA. 105:14058–14063. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furutani M, Arii S, Mizumoto M, Kato M and
Imamura M: Identification of a neutrophil gelatinase-associated
lipocalin mRNA in human pancreatic cancers using a modified signal
sequence trap method. Cancer Lett. 122:209–214. 1998. View Article : Google Scholar
|
|
18
|
Laurell H, Bouisson M, Berthelemy P, et
al: Identification of biomarkers of human pancreatic
adenocarcinomas by expression profiling and validation with gene
expression analysis in endoscopic ultrasound-guided fine needle
aspiration samples. World J Gastroenterol. 12:3344–3351. 2006.
|
|
19
|
Tong Z, Kunnumakkara AB, Wang H, et al:
Neutrophil gelatinase-associated lipocalin: a novel suppressor of
invasion and angiogenesis in pancreatic cancer. Cancer Res.
68:6100–6108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahadevan NR, Rodvold J, Almanza G, Perez
AF, Wheeler MC and Zanetti M: ER stress drives Lipocalin 2
upregulation in prostate cancer cells in an NF-κB-dependent manner.
BMC Cancer. 11:2292011.PubMed/NCBI
|
|
21
|
Villalva C, Sorel N, Bonnet ML, et al:
Neutrophil gelatinase-associated lipocalin expression in chronic
myeloid leukemia. Leuk Lymphoma. 49:984–988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leng X, Lin H, Ding T, et al: Lipocalin 2
is required for BCR-ABL-induced tumorigenesis. Oncogene.
27:6110–6119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jones C, Mackay A, Grigoriadis A, et al:
Expression profiling of purified normal human luminal and
myoepithelial breast cells: identification of novel prognostic
markers for breast cancer. Cancer Res. 64:3037–3045. 2004.
View Article : Google Scholar
|
|
24
|
Yang J, Bielenberg DR, Rodig SJ, et al:
Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci
USA. 106:3913–3918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bauer M, Eickhoff JC, Gould MN, Mundhenke
C, Maass N and Friedl A: Neutrophil gelatinase-associated lipocalin
(NGAL) is a predictor of poor prognosis in human primary breast
cancer. Breast Cancer Res Treat. 108:389–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wenners AS, Mehta K, Loibl S, et al:
Neutrophil gelatinase-associated lipocalin (NGAL) predicts response
to neoadjuvant chemotherapy and clinical outcome in primary human
breast cancer. PLoS One. 7:e458262012. View Article : Google Scholar
|
|
27
|
Stoesz SP, Friedl A, Haag JD, Lindstrom
MJ, Clark GM and Gould MN: Heterogeneous expression of the
lipocalin NGAL in primary breast cancers. Int J Cancer. 79:565–572.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Provatopoulou X, Gounaris A, Kalogera E,
et al: Circulating levels of matrix metalloproteinase-9 (MMP-9),
neutrophil gelatinase-associated lipocalin (NGAL) and their complex
MMP-9/NGAL in breast cancer disease. BMC Cancer. 9:3902009.
View Article : Google Scholar
|
|
29
|
Zhang XF, Zhou SM, Wan L, et al:
Relationship between NGAL expression and progression, metastasis
and prognosis of breast cancer. J Pract Oncol. 23:206–210.
2008.
|
|
30
|
Lv CG and Qi FJ: Expression of NGAL and
MMP-9 protein in invasive breast cancer and its clinical
application. Guangdong Med J. 32:1287–1289. 2011.
|
|
31
|
Wang XH, Guo YY and Gou XM: Expression of
NGAL in breast cancer and its clinical application. China Pract
Med. 6:43–45. 2011.
|
|
32
|
Qiu JM: Clinical significance of
determination in tumor cells NGAL levels of breast cancer patients
with immuno-histochemistry. J Radioimmunol. 25:546–548. 2012.
|
|
33
|
Stuckey R, Aldridge T, Lim FL, et al:
Induction of iron homeostasis genes during estrogen-induced uterine
growth and differentiation. Mol Cell Endocrinol. 253:22–29. 2006.
View Article : Google Scholar
|