Introduction
Soluble interleukin-2 receptor (sIL-2R) is a
glycoprotein which derived from α chain of interleukin 2 receptors
of the mononuclear cell membranes as well as the T-cell membranes
and the molecular weight is 45 kDa (?). sIL-2R is crucial in
lymphokines in the lymphocyte culture fluid and blood circulation,
and plays a significant role in regulating cell immune function.
The secretion of sIL-2R increased when lymphocytes stimulated by a
specific antigen or mitogen. Regulation of the expression of the
sIL-2R in IL/sIL-2R system is crucial and is generally considered
as another important indicator involved in the activation of the
immune system (1,2). Imbalance of the IL-2/sIL-2R system is
capable of inducing abnormalities of the immune function of tumor
cells in the body, resulting in the immune escape of tumors.
Previous studies showed that the expression of sIL-2R levels was
altered in the serum of malignant solid tumors, autoimmune diseases
and skin melanoma patients and was associated with the immune state
of the body (3–6). This study aimed to determine the sIL-2
serum level in 40 hamster cheek pouch carcinoma models and
investigated the effect of heavy-ion beam irradiated tumor-bearing
golden hamster immune status.
Materials and methods
Reagents
Reagents and equipment used in the present study
included: 7,12-dimethyl-1,2-benzanthracene (DMBA) 090 (Sigma, St.
Louis, MO, USA), acetone of analytical grade (Columbine Canton of
Bose National Instrument Co., Ltd., ?), ELISA kit (Wuhan Boster
Biological Technology Ltd., Wuhan, China), lotion self with 0.01 M
PBS (1,000ml H2O followed by Tris 1.2 g NaCl 8.5 g, with
the pH adjusted to 7.2–7.6 and concentrated hydrochloric acid, ~700
μl), EL×800 microplate reader (BioTek Instruments Inc., Winooski,
VT, USA), EL×50 microplate strip washer (?), G5-2A low-speed
centrifuge (Beijing Medical Centrifuge Factory), 200 μl pipettes
NPX-200 (Nichiryo Co., Ltd., Koshigaya, Japan), 75% ethanol
(Tianjin Hong Yan Chemical Reagent Factory, Dongli, China), 1 ml of
medical syringe (Changzhou Chunguang Medical Equipment Co., Ltd.,
Changzhou, China), as well as calipers, dividers and ruler (The
Beijing Jingxicheng Optical Instrument Co., Ltd., Beijing,
China).
Experimental animals
Selection and grouping of the
animals
The study included 48 (n=24 males and females,
respectively, per group) normal 6- to 8-week-old Syrian golden
hamsters (purchased from the Lanzhou Institute of Biological
Products, Lanzhou, Gansu, China), with a weight of 80–100 g. The
animals were raised at a room temperature of 24±2°C, under 12-h
light and dark cycle conditions. The hamsters were randomly divided
into five groups prior to irradiation, at the proposed irradiation
doses of 0, 4, 6, 8 and 12 Gy.
Establishment of the experimental
animal model
DMBA acetone (0.5%) was prepared according to a
previously published method (7).
The prepared DMBA acetone liquid was coated on the right side of
the golden hamster cheek pouch mucosa with a 4 Brush Pen, three
times a week and consecutively for 16 weeks in order to establish
the tumor model. Right cheek pouch tissue was removed prior to the
experiment and analyzed using hematoxylin and eosin staining as per
the histological grade determination criteria [according to the WHO
(1996) of oral precancerous lesions and the Banoczy method]
(8,9).
Heavy-ion irradiation
Heavy ion was irradiated on a biological irradiation
terminal device located at the heavy-ion irradiation facility
laboratory (HIRFL) of the Lanzhou Institute of Modern Physics
Laboratory, China. A 12C6+ ion beam was
produced and used for animal irradiation. The energy generated was
235 MeV, linear transfer energy (LET) was 155 keV/μm and the
absorbed dose rate was 3 Gy/min respectively. The dose was
monitored using an air ionization chamber.
Methods
Specimen collection
In the first four weeks following heavy-ion
irradiation all 48 animals were fasted. Venous blood (2 ml),
without anticoagulant, was drawn and incubated overnight at 4°C,
then centrifuged for 10 min at 1,000 rpm. Serum was then collected
and stored at −20°C.
Detection methods
Serum sIL-2R was measured by radioimmunoassay (RIA)
using a radioimmunoassay kit (Beijing Huaying Biotechnology
Research Institute, Peking, China); GC-911 gamma counter (HKUST
Innovation Co., Ltd., Zhongjia, China); and KDC-2044 low-speed
refrigerated centrifuge (HKUST Innovation Co., Ltd.). Measurements
were monitored by quality control.
Statistical analysis
SPSS 15.0 statistics was used for data analysis.
Data were expressed as mean ± standard deviation (SD). Statistical
analysis was performed using the Student’s t-test and variance
analysis. Spearman correlation analysis was used to determine the
correlation between the groups at a signficance level of
α=0.05.
Results
Expression levels of serum sIL-2R in the
tumor and control groups
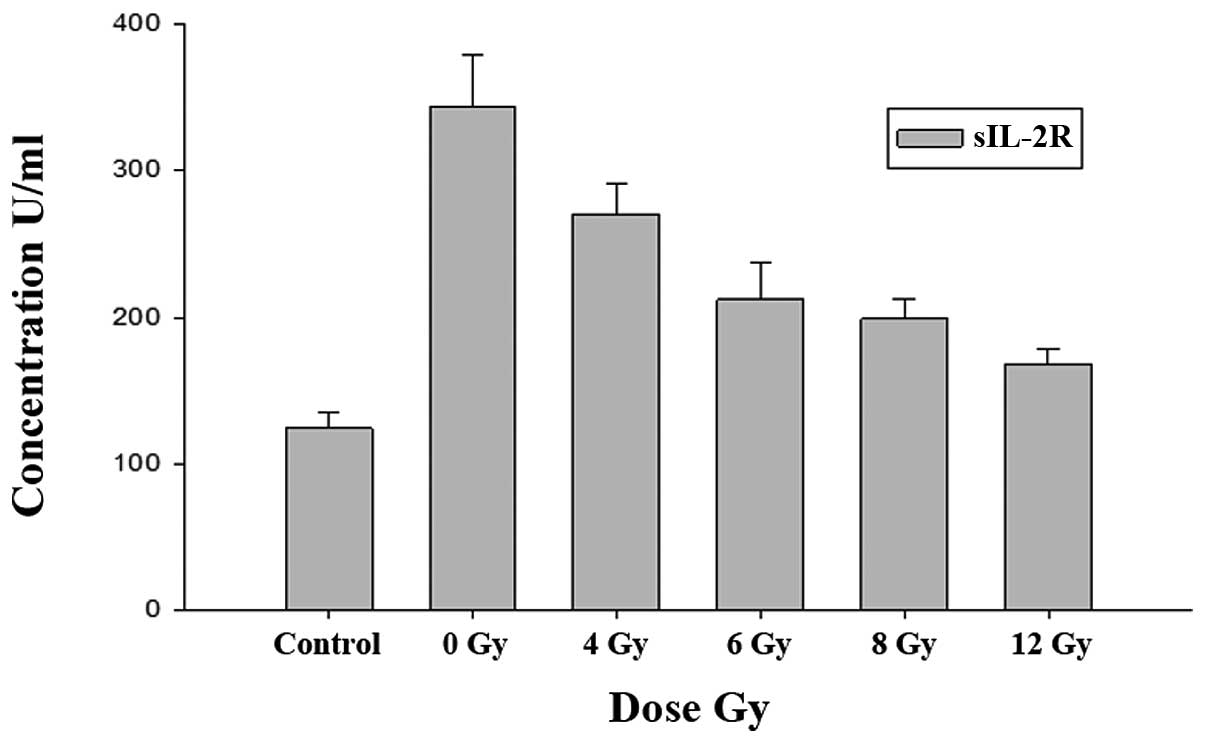
The results showed that sIL-2R serum levels of the
simple tumor-bearing and blank control groups were: (343.84±34.58)
and (124.26±10.41) μ/ml respectively. sIL-2R levels of the simple
tumor-bearing group were significantly higher than those of the
blank control group (P<0.01) (Table
I).
 | Table IComparison of serum soluble
interleukin-2 receptor (sIL-2R) levels in the three groups (mean ±
SD) (U/ml). |
Table I
Comparison of serum soluble
interleukin-2 receptor (sIL-2R) levels in the three groups (mean ±
SD) (U/ml).
| Group | No. | Dose (Gy) | sIL-2R |
|---|
| Blank control | 8 | - | 124.26±10.41 |
| Simple
tumor-bearing | 8 | 0 | 343.84±34.58a |
| 8 | 4 | 270.32±20.27 |
| Tumor-bearing and
irradiation | 8 | 6 | 212.10±25.68b |
| 8 | 8 | 198.83±13.64b |
| 8 | 12 | 167.43±11.50b |
Serum sIL-2R expression between the tumor
and irradiation groups
The sIL-2R serum levels of the simple tumor-bearing
group (0 Gy) and irradiation group (4, 6, 8 and 12 Gy) were:
(343.84±34.58), (270.32±20.27), (212.10±25.68), (198.83±13.64) and
(167.43±11.50) μ/ml respectively. The results show a decrease in
the sIL-2R serum level with the increase of the irradiation dose
between the two groups. Statistically significant differences were
observed when compared with the blank control group (P<0.05)
(Table I).
Association of serum sIL-2R level changes
in the different irradiation dose groups
An increase in the irradiation dose resulted in a
gradual decrease in sIL-2R serum levels in the irradiation groups.
Expression levels of sIL-2R and the irradiation dose were
positively correlated (rs=−0.949, P=0.001<0.01), as analyzed by
the Spearman correlation analysis. Statistically significant
differences were observed between the simple tumor-bearing and
blank control groups (Fig. 1).
Discussion
The occurrence and development of tumors are closely
associated with immune function and patients with malignant tumors
experience immune dysfunction. Tumor cells secrete various
immunosuppressive factors by themselves or are induced by these
factors, resulting in a decrease in the immunogenicity of the tumor
cells and leading to immune escape of the tumors. sIL-2R is a
glycoprotein shedding from membrane interleukin-2 receptor (mIL-2R)
which is on the the surface of cytomembrane together with the
enzyme activity. Only a small number of sIL-2R were shed from the
surface of activated normal lymphocytes, most of which were from
malignant tumor cells, and circulated in the blood. Therefore,
sIL-2R is regarded as an active biological response marker of the
host and malignant tumours and is one of the significant signals in
the activated immune system of the organism.
Recent studies (?) have shown that sIL-2R has a
bidirectional immunoregulatory action, and competes with IL-2R when
combined with IL-2, reducing the blood concentration of IL-2 and
lymphocyte activation, while releasing IL-2 when IL-2 is at a low
level, thereby achieving a self-protective function in order to
maintain the balance of the immune response. It is believed
(10,11) that sIL-2R competes with mIL-2R when
combined with IL-2, similar to blocking agents, thereby
neutralizing IL-2 activated T cells and regulating the immune
response of the IL-2 antagonist effect, and weakening the
organism’s autocrine effect. Furthermore, the production of sIL-2R
involves shedding of the membrane receptor mIL-2R p55 chain. An
increase of sIL-2R accelerates the expurgation of sIL-2 and then
restrains the activation and multiplication of T cells. sIL-2R
shedding from membranes resulted in activated lymphocytes
recovering to a static condition or lymphocyte exhaustion, thereby
reducing the immunocompetence of T cells and leading to abnormal
immune function.
The cell immune response has an inhibitory role in
malignant tumors. Immune cells lose the ability to regulate the
expression of IL-2R chain, resulting in abnormal immune cells being
highly expressed in IL-2R chain and released into the bloodstream,
resulting in high levels of serum sIL-2R. sIL-2R levels are usually
positively correlated with the progress of clinical stages and
prognosis, although the mechanism by which this occurs is not
clear. Tumors are known to activate the immune cells, such as T
cells, in vivo and release excessive amounts of sIL-2R,
resulting in a high expression of sIL-2R. sIL-2R levels decreased
when the tumors disappeared, although its content increased when
tumor relapse occurred. In their study, Li Ling et al
(13) demonstrated that high sIL-2R
serum levels were significantly decreased following removal of the
tumors. Tartour et al (14),
Murakami et al (15) and
Grotowski and Piechota (16)
reported that the sIL-2R serum levels of head and neck, gastric and
colorectal cancer patients were significantly higher compared to
the control groups and showed an increase with progression of
clinical stage. Lai et al (17) revealed that sIL-2R serum levels in
nasopharyngeal carcinoma patients were significantly higher
compared to the normal controls and was significantly increased
with progression of clinical stage, with stage III-IV>I-II
sIL-2R serum levels being lower subsequent to radiotherapy. In the
present study, results showed that an increase in the irradiation
dose following heavy-ion beam irradiation resulted in a decrease in
the sIL-2R serum levels of the tumor-bearing golden hamsters. Our
findings are in concordance with those of Xu Mei et al
(18) who reported changes in
sIL-2R serum levels in esophageal cancer patients prior to and
following radiotherapy.
We analyzed the related factors and possible reasons
for heavy-ion beam irradiation affecting changes in sIL-2R levels
(1). sIL-2R is involved in the
regulation of the body’s immune status as when it is significantly
expressed in tumors it is able to reverse the activated state of
immune cells to a dormant one. As heavy-ion beam irradiation
removed the tumor burden of tumor-bearing golden hamsters, the
secretion of sIL-2R was reduced by blocking tumors to the
activation of T lymphocytes; however, the content of IL-2
increased. Heavy-ion beam irradiation resulted in the tumor losing
its ability to remove immune suppression more than direct
immunosuppressive effects and was beneficial for immune regulation.
Heavy-ion radiation damaged the DNA of the target cells, leading to
cell mutation or death (19,20),
and triggered the body to make feedback regulation for this signal
and the result was activated, initiating the IL-R/IL-2R system,
make sIL-2R combine with IL-2 increased, to reduce the content of
serous sIL-2R and activation effect of T lymphocyte. Together with
the disappearance of the tumor burden, autocrine effects in the
body weakened, and the sIL-2R serum levels decreased. This
suggested that irradiation potentially activates and starts the
body stress repair system and enhances the immune effect.
sIL-2R is a crucial immunologically reactive
substance, which together with other factors including mutual
regulation and mutual restraint, forms a complex signaling pathway
network. Heavy-ion irradiation generated significant radiation
damage effects on tumors, alterated tumor microenvironment and
influenced metabolites, thereby agitating immune cells to the
expression of the IL-2R chain. The content of the expression
appeared abnormal, as well as the progress of the clinical stage,
degree of immune inhibition and the body’s own secretion amount of
IL-2; irradiation dose, sensitivity of the body to the irradiated
and irradiated site be related. sIL-2R is an indicator for the
assessment of immune status of the tumor body cells and that
real-time monitoring of sIL-2R levels may provide an understanding
of the development of tumors and the immune status of the body.
Thus, sIL-2R can also be used as an objective indicator to evaluate
the efficacy of heavy-ion beam as cancer treatment and its effects
on clinical prognosis.
References
|
1
|
Rubin LA, Galli F, Greene WC, et al: The
molecular basis for the generation of the human soluble interleukin
2 receptor. Cytokine. 2:330–336. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubin LA, Kurman CC, Fritz ME, et al:
Soluble interleukin 2 receptors are released from activated human
lymphoid cells in vitro. J Immunol. 135:3172–3177. 1985.PubMed/NCBI
|
|
3
|
Gansauge F, Steinbach G, Gansauge S, et
al: Prognostic significance of soluble interleukin-2 receptor-alpha
in adenocarcinoma of the pancreas. Cancer Lett. 134:193–199. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takasaki S, Hayashida K, Morita C, et al:
Elevated serum soluble CD8 level in autoimmune hepatitis and the
effect of corticosteroid therapy. Hepatol Res. 15:521999.
View Article : Google Scholar
|
|
5
|
Vuoristo MS, Laine S, Huhtala H, et al:
Serum adhesion molecules and interleukin-2 receptor as markers of
tumour load and prognosis in advanced cutaneous melanoma. Eur J
Cancer. 37:1629–1634. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma Z: Clinical significance of
measurements of changes of serum IL-2, SIL-2 R, TNF-α levels after
chemotherapy in patients with ovary cancer. Radioimmunology.
18:34–36. 2005.
|
|
7
|
Salley JJ: Experimental carcinogenesis in
the cheek pouch of the Syrian hamster. J Dent Res. 33:253–262.
1954. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banoczy J and Sugar L: Longitudinal
studies in oral leukoplakias. J Oral Pathol. 1:265–272. 1972.
View Article : Google Scholar
|
|
9
|
Zhou ZT, Zhang SL and Wang Y: The light
microscopic observing analysis of 12-item pathological features on
golden hamster cheek pouch dysplasia. Shanghai Kou Qiang Yi Xue.
6:32–35. 2005.(In Chinese).
|
|
10
|
Safwat A, Schmidt H, Bastholt L, et al: A
phase II trial of low-dose total body irradiation and subcutaneous
interleukin-2 in metastatic melanoma. Radiother Oncol. 77:143–147.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao Y, Liu Y, Zhang K, et al: The
examination and clinical significance of TNF-α, IL-6, IL-8 and
sIL-2R in serum in patients with primary hepatic cancer. Chin J Lab
Diagn. 12:101–104. 2008.(In Chinese).
|
|
12
|
Ottaiano A, Leonardi E, Simeone E, et al:
Soluble interleukin-2 receptor in stage I–III melanoma. Cytokine.
33:150–155. 2006.PubMed/NCBI
|
|
13
|
Marino P, Cugno M, Preatoni A, Cori P,
Rosti A, Frontini L and Cicardi M: Increased levels of soluble
interleukin-2 receptors in serum of patients with lung cancer. Br J
Cancer. 61:434–435. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tartour E, Mosseri V, Jouffroy T, et al:
Serum soluble interleukin-2 receptor concentrations as an
independent prognostic marker in head and neck cancer. Lancet.
357:1263–1264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murakami S, Sakata H, Tsuji Y, et al:
Serum soluble interleukin-2 receptor as a predictor of lymph node
metastasis in early gastric cancer. Dig Surg. 19:9–13. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grotowski M and Piechota M: Receptors of
selected cytokines and angiokine bFGF in patients with colorectal
cancer (a preliminary study). Pol Merkur Lekarski. 11:398–401.
2001.(In Polish).
|
|
17
|
Lai KN, Ho S, Leung JCK and Tsao SY:
Soluble interleukin-2 receptors in patients with nasopharyngeal
carcinoma. Cancer. 67:2180–2185. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang Y, Cheng Y, Liu Q, et al: The study
of sIL-2R in esophageal carcinoma patients before and after
radiotherapy. Cancer Res Prev Treat. 28:42–43. 2001.
|
|
19
|
Mcmillan TJ and Peacock JH: Molecular
determinants of radiosensitivity in mammalian cells. Int J Radiat
Biol. 65:49–55. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chetioui A, Despiney I, Guiraud L, et al:
Possible role of inner-shell ionization phenomena in cell
inactivation by heavy ions. Int J Radiat Biol. 65:511–522. 1994.
View Article : Google Scholar : PubMed/NCBI
|