1. Introduction
An estimated 90% of cancer deaths are the result of
metastasis. Therefore, elucidating the mechanisms involved in this
process is crucial. Metastasis is considered to begin with
epithelial-to-mesenchymal transition (EMT), a cascade of events
during which tumor cells lose their epithelial characteristics and
acquire mesenchymal cell characteristics (1). The change in the tumor cells is
accompanied by an increase in motility and matrix invasion. Once
the malignant cells become detached from the primary tumor site and
enter the bloodstream or lymphatic vessels, they become circulating
tumor cells (CTCs). Several patients with early-stage cancer have a
poor prognosis, since CTCs may reach a secondary organ prior to the
onset of clinical symptoms. To exploit the window of opportunity
for therapeutic intervention between initial dissemination and
eventual metastatic recurrence, a better understanding of the
biological behavior of CTCs is required.
2. CTCs and EMT
EMT, a transient and reversible process, is
considered to enhance the capacity of cancer cells to invade,
access the vasculature, metastasize and resist apoptosis (2). Primary tumors may recruit various
cells into their microenvironment and secrete transforming growth
factor-β (TGF-β), which is considered to be the most potent inducer
of EMT. EMT promotes a patchy asynchronous development that
involves relatively small numbers of primary cancer cells (3). These transitioning cancer cells then
acquire an invasive phenotype and translocate from the primary
tumor site to the vasculature (4).
However, the microenvironment of CTCs is clearly different from
their primary counterpart and there is currently some debate
regarding whether EMT is involved in the biological events of
CTCs.
Accumulating evidence indicates that CTCs share many
morphological and phenotypical traits with cells undergoing EMT
(5). The majority of CTCs obtained
from the peripheral blood of patients with breast or prostate
cancer co-express epithelial and mesenchymal markers, including
E-cadherin, cytokeratin (CK), vimentin and N-cadherin (6,7).
EMT-related antigens are also found in
CK−/CD45− cells, suggesting that these cells
may represent CTCs that have undergone complete EMT (8,9).
Inhibition of pivotal elements in EMT-associated signaling
pathways, such as Twist1, Zeb1, Zeb2, SNAIL1 and SNAIL2/Slug, has
been associated with a decreased risk of metastatic relapse
(10). However, the molecular
mechanisms by which CTCs maintain the EMT state have not been
elucidated.
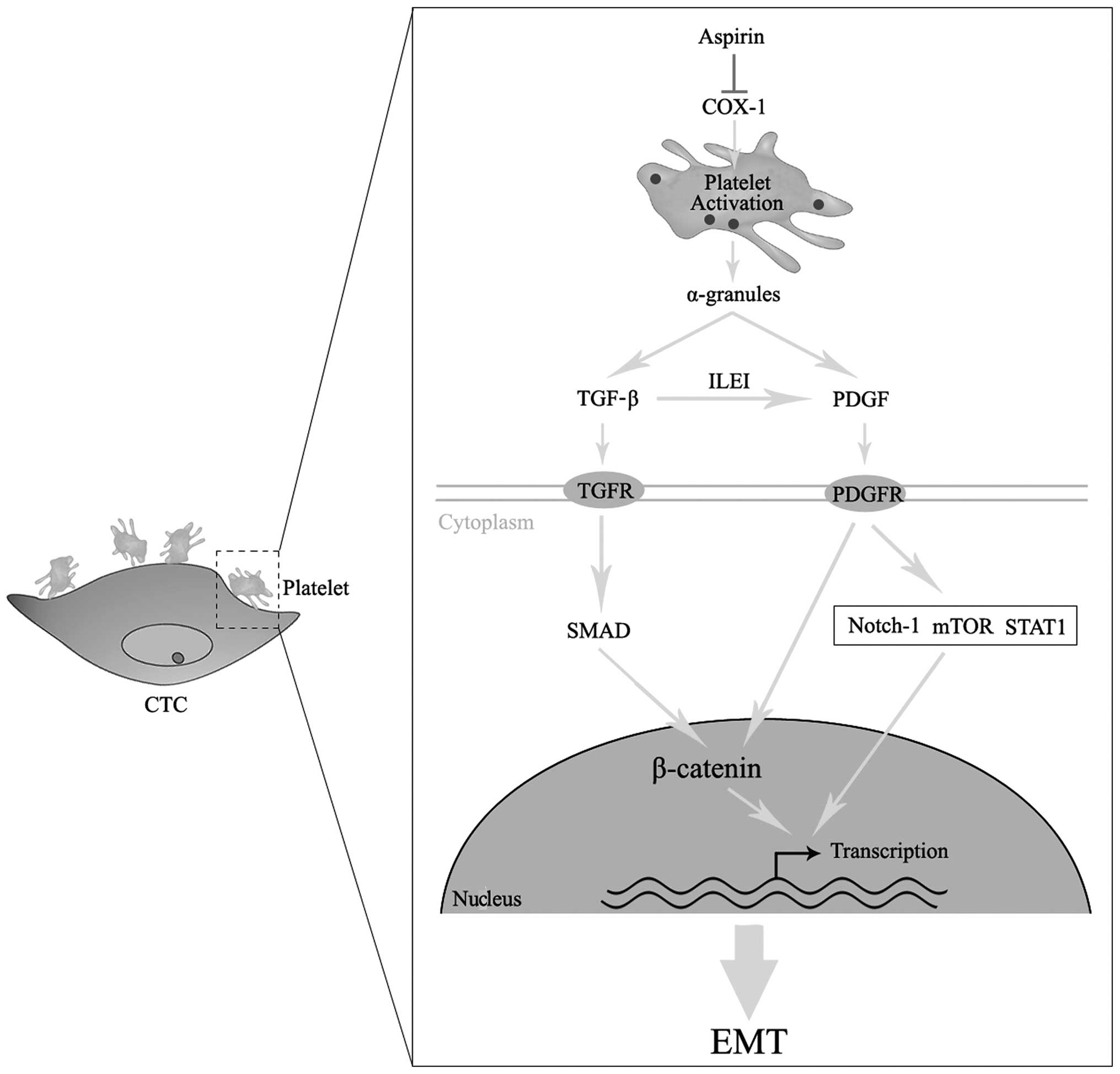
3. Platelets promote EMT of CTCs
Thrombocytosis is observed in several metastatic
cancers and correlates with a worse prognosis, indicating that
platelets play a significant role in cancer metastasis (11). In addition to their well-established
role in protecting CTCs against mechanical and immune assaults in
the circulation, platelets were recently shown to induce EMT in
CTCs (12). In addition, platelets
are activated through direct interactions with CTCs and secrete
α-granules, which contain TGF-β and platelet-derived growth factor
(PDGF) at concentrations several-fold higher compared to that in
most cell types (13). Treatment
with platelets induces increased phosphorylation of the TGF-β
signaling effector Smad2 and Smad-binding element-dependent
transcription (12).
Platelet-secreted PDGF is another important mediator of EMT.
Overexpression of PDGF-D, a member of the PDGF family, in prostate
cancer cells promotes EMT in vitro and in vivo
through the activation of the mammalian target of rapamycin
downstream targets S6K and 4E-BP1 (14). PDGF-D may also increase the
expression of Notch-1 in pancreatic cancer cells, which is known as
a conserved ligand receptor pathway and an inducer of EMT (15). The extensive crosstalk between
PDGF-D and multiple signaling pathways, such as nuclear factor
κ-light-chain-enhancer of activated B cells, chemokine (C-X-C
motif) receptor 4 and B-cell lymphoma 2 pathways, suggest that
efficient inhibition of PDGF during EMT may prevent the progression
of metastasis (16–18). Another study indicates that
autocrine platelet-derived growth factor receptor (PDGFR) signaling
may contribute to the maintenance of EMT, possibly through
activation of the signal transducer and activator of transcription
(STAT) 1 (19).
In addition to platelet-derived PDGF, a previous
study revealed that TGF-β signaling may increase the expression of
PDGF in cancer cells, which acts in a sequential auto- or paracrine
manner to promote sustained EMT (20). The components of the PDGF signaling
pathway were found to upregulated during TGF-β-induced EMT in
breast cancer (21). The
TGF-β-inducible secretion of interleukin-like EMT-inducer may
upregulate the expression of PDGF and PDGFR, leading to signaling
via β-catenin and STAT3 to establish EMT (22). TGF-β-induced PDGF activates
phosphatidylinositol-3 kinase and, furthermore, increases the
accumulation of nuclear β-catenin (23). In gliomas, high TGF-β signaling is
associated with a poor prognosis and promotes glioma cell
proliferation by activating PDGF-B/PDGFR signaling (24). Based on the abovementioned findings,
we may reasonably deduce that cytokines released by activated
platelets contribute to the EMT of CTCs.
4. Chemotherapeutic effects of aspirin
Accumulating evidence from observational studies in
humans indicates that aspirin reduces the incidence of colorectal
cancer and increases the overall survival of cancer patients after
a delay of 8–10 years (25–27). One hypothesis argues that aspirin
inhibits the malignant transformation from adenoma to
adenocarcinoma and this process may take a long time. However,
recently published meta-analyses of the results from randomized
trials provided evidence that daily aspirin treatment at doses of
≥75 mg reduced all-cancer mortality after only 5 years (27,28).
Those results can hardly be interpreted by aspirin only affecting
carcinogenesis or early cancer growth. Aspirin was recently shown
to improve the prognosis of metastatic cancer patients with unknown
primary site (28). In a separate
analysis of five randomized trials in the UK on daily aspirin use
at ≥75 mg, the risk of cancer with distant metastases was also
reduced (29). These accumulating
data suggest that aspirin may act as an inhibitor of cancer
metastasis. The molecular mechanism that defines aspirin and other
non-steroidal anti-inflammatory drugs as a class, is their ability
to block the prostaglandin H or the cyclooxygenase (COX) pathway.
Inhibition of COX activity decreases the formation of prostanoids,
including PGD2, PGE2, PGF2α, PGI2 and thromboxane (TXA) 2 (30). TXA2 is a major metabolite in
platelets that promotes their activation and aggregation and, in
turn, release of their α-granules. COX-1 is the only isoform
present in mature platelets. Aspirin irreversibly inactivates COX-1
through selective acetylation of a critical serine residue within
the COX-channel (Ser529). Therefore, the chemotherapeutic effects
of aspirin on the metastatic process may depend on the inhibition
of platelet-related COX-1 signaling pathway.
5. Hypothesis and implications
Based on abovementioned data, we hypothesized that
the downregulation of the platelet-related COX-1 pathway may
contribute to the antimetastatic effects of aspirin through
inhibiting the EMT of CTCs (Fig.
1). The platelet-tumor cell interactions are transient and
occur only within the first 24 h (31). Activated platelets may provide a
pulse of TGF-β and PDGF, which in turn promotes CTCs to undergo
EMT. The recovery of COX-1 activity after treatment with aspirin
requires de novo synthesis of this enzyme. Platelets lack a
nucleus, thus low-dose aspirin (75–162.5 mg) treatment may exert a
long-lasting effect on the inhibition of COX-1-related EMT. As the
dissemination of CTCs may occur during the early stages of cancer,
preventive aspirin use may provide significant therapeutic
benefits.
The most frequently reported severe adverse event
associated with regular aspirin use is gastrointestinal bleeding.
Previous studies reported that the incidence of this adverse event
is largely dose-related, with the risk of bleeding being generally
higher with standard-dose (300–325 mg) compared to that with
low-dose aspirin (75–162.5 mg) (32–34).
Therefore, the benefits of long-term use of low-dose aspirin for
the prevention of cancer metastasis may outweigh the consequences
associated with the increased risk of bleeding.
Cancer metastasis is commonly encountered and is
associated with severe clinical consequences that arise from the
formation of CTCs. However, the currently available treatments are
insufficient for the effective management of these disorders.
Therefore, the characterization of the biological behavior of CTCs
is crucial in manipulating this process therapeutically. Aspirin
may represent an anticancer drug for modulating the
platelet-related EMT of CTCs. Should our hypothesis be confirmed,
it may change the way we treat metastatic cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81300347) and the Natural
Science Foundation of Jiangxi Province, China (no.
20132BAB205037).
References
|
1
|
Yu M, Ting DT, Stott SL, et al: RNA
sequencing of pancreatic circulating tumour cells implicates WNT
signalling in metastasis. Nature. 487:510–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar
|
|
3
|
Deng H, Wang HF, Gao YB, Jin XL and Xiao
JC: Hepatic progenitor cell represents a transitioning cell
population between liver epithelium and stroma. Med Hypotheses.
76:809–812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Armstrong AJ, Marengo MS, Oltean S, et al:
Circulating tumor cells from patients with advanced prostate and
breast cancer display both epithelial and mesenchymal markers. Mol
Cancer Res. 9:997–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bednarz N, Eltze E, Semjonow A, et al:
BRCA1 loss preexisting in small subpopulations of prostate cancer
is associated with advanced disease and metastatic spread to lymph
nodes and peripheral blood. Clin Cancer Res. 16:3340–3348. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joosse SA, Hannemann J, Spotter J, et al:
Changes in keratin expression during metastatic progression of
breast cancer: impact on the detection of circulating tumor cells.
Clin Cancer Res. 18:993–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bednarz-Knoll N, Alix-Panabières C and
Pantel K: Plasticity of disseminating cancer cells in patients with
epithelial malignancies. Cancer Metastasis Rev. 31:673–687. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gradilone A, Raimondi C, Nicolazzo C, et
al: Circulating tumour cells lacking cytokeratin in breast cancer:
the importance of being mesenchymal. J Cell Mol Med. 15:1066–1070.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013.PubMed/NCBI
|
|
11
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Assoian RK, Komoriya A, Meyers CA, Miller
DM and Sporn MB: Transforming growth factor-beta in human
platelets. Identification of a major storage site, purification,
and characterization. J Biol Chem. 258:7155–7160. 1983.PubMed/NCBI
|
|
14
|
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View
Article : Google Scholar
|
|
15
|
Bao B, Wang Z, Ali S, et al: Notch-1
induces epithelial-mesenchymal transition consistent with cancer
stem cell phenotype in pancreatic cancer cells. Cancer Lett.
307:26–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kong D, Wang Z, Sarkar SH, et al:
Platelet-derived growth factor-D overexpression contributes to
epithelial-mesenchymal transition of PC3 prostate cancer cells.
Stem Cells. 26:1425–1435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmad A, Wang Z, Kong D, et al:
Platelet-derived growth factor-D contributes to aggressiveness of
breast cancer cells by up-regulating Notch and NF-kappaB signaling
pathways. Breast Cancer Res Treat. 126:15–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Liao S, Huang Y, et al: PDGF-D
improves drug delivery and efficacy via vascular normalization, but
promotes lymphatic metastasis by activating CXCR4 in breast cancer.
Clin Cancer Res. 17:3638–3648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jechlinger M, Sommer A, Moriggl R, et al:
Autocrine PDGFR signaling promotes mammary cancer metastasis. J
Clin Invest. 116:1561–1570. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gotzmann J, Fischer AN, Zojer M, et al: A
crucial function of PDGF in TGF-beta-mediated cancer progression of
hepatocytes. Oncogene. 25:3170–3185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jechlinger M, Grunert S, Tamir IH, et al:
Expression profiling of epithelial plasticity in tumor progression.
Oncogene. 22:7155–7169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lahsnig C, Mikula M, Petz M, et al: ILEI
requires oncogenic Ras for the epithelial to mesenchymal transition
of hepatocytes and liver carcinoma progression. Oncogene.
28:638–650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fischer AN, Fuchs E, Mikula M, Huber H,
Beug H and Mikulits W: PDGF essentially links TGF-beta signaling to
nuclear beta-catenin accumulation in hepatocellular carcinoma
progression. Oncogene. 26:3395–3405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruna A, Darken RS, Rojo F, et al: High
TGFbeta-Smad activity confers poor prognosis in glioma patients and
promotes cell proliferation depending on the methylation of the
PDGF-B gene. Cancer Cell. 11:147–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flossmann E and Rothwell PM; British
Doctors Aspirin Trial and the UK-TIA Aspirin Trial. Effect of
aspirin on long-term risk of colorectal cancer: consistent evidence
from randomised and observational studies. Lancet. 369:1603–1613.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rothwell PM, Wilson M, Elwin CE, et al:
Long-term effect of aspirin on colorectal cancer incidence and
mortality: 20-year follow-up of five randomised trials. Lancet.
376:1741–1750. 2010.PubMed/NCBI
|
|
27
|
Rothwell PM, Fowkes FG, Belch JF, Ogawa H,
Warlow CP and Meade TW: Effect of daily aspirin on long-term risk
of death due to cancer: analysis of individual patient data from
randomised trials. Lancet. 377:31–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rothwell PM, Price JF, Fowkes FG, et al:
Short-term effects of daily aspirin on cancer incidence, mortality,
and non-vascular death: analysis of the time course of risks and
benefits in 51 randomised controlled trials. Lancet. 379:1602–1612.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: a study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patrono C, Garcia Rodriguez LA, Landolfi R
and Baigent C: Low-dose aspirin for the prevention of
atherothrombosis. N Engl J Med. 353:2373–2383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laubli H, Stevenson JL, Varki A, Varki NM
and Borsig L: L-selectin facilitation of metastasis involves
temporal induction of Fut7-dependent ligands at sites of tumor cell
arrest. Cancer Res. 66:1536–1542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Serebruany VL, Steinhubl SR, Berger PB, et
al: Analysis of risk of bleeding complications after different
doses of aspirin in 192,036 patients enrolled in 31 randomized
controlled trials. Am J Cardiol. 95:1218–1222. 2005. View Article : Google Scholar
|
|
33
|
Peters RJ, Mehta SR, Fox KA, et al:
Effects of aspirin dose when used alone or in combination with
clopidogrel in patients with acute coronary syndromes: observations
from the Clopidogrel in Unstable angina to prevent Recurrent Events
(CURE) study. Circulation. 108:1682–1687. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Topol EJ, Easton D, Harrington RA, et al:
Randomized, double-blind, placebo-controlled, international trial
of the oral IIb/IIIa antagonist lotrafiban in coronary and
cerebrovascular disease. Circulation. 108:399–406. 2003. View Article : Google Scholar
|