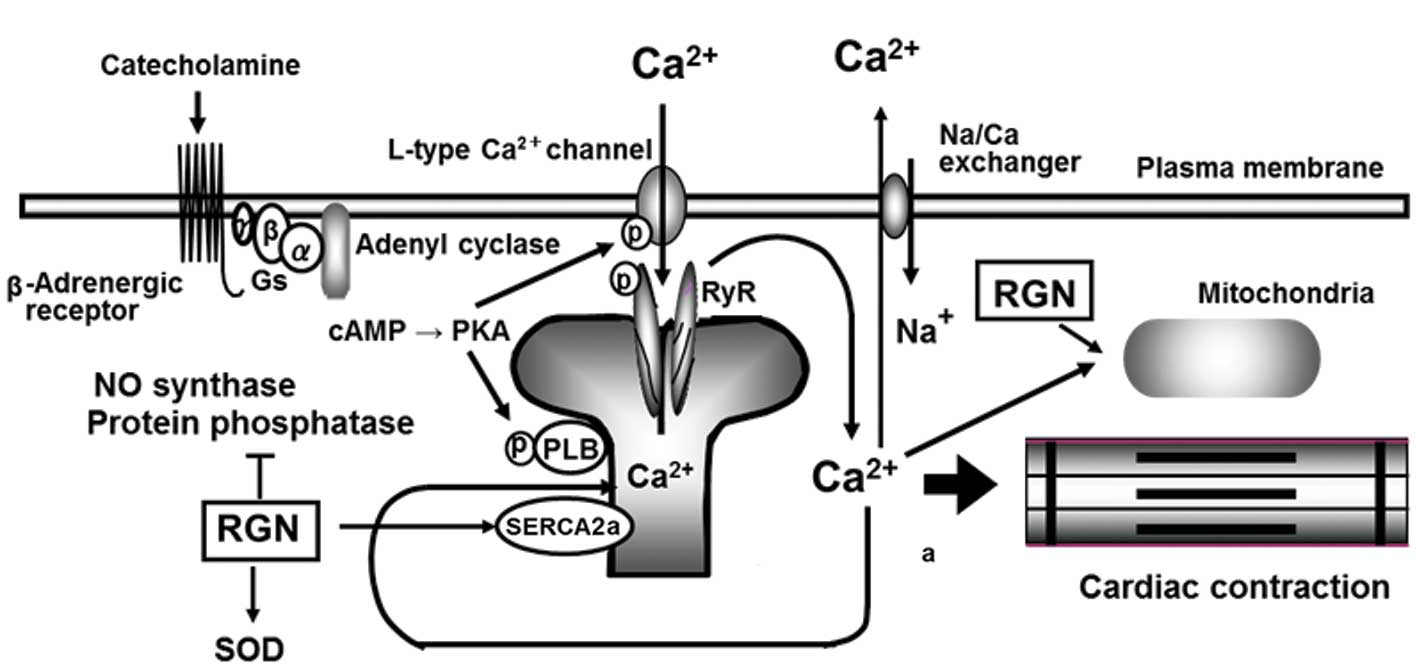
1. Introduction
Regucalcin was first identified in 1978 as a
Ca2+-binding protein that does not contain an EF-hand
motif as a Ca2+-binding domain, which is present in
numerous Ca2+-binding proteins (1). The name ‘regucalcin’ was proposed for
this protein, which was shown to suppress Ca2+-dependent
activation of various enzymes in liver cells (1–4). The
regucalcin gene is localized on the X chromosome (5,6).
Regucalcin was identified in >15 species and is highly conserved
in vertebrate species (7,8). The expression of regucalcin mRNA and
protein is regulated through various hormonal stimuli and
physiological conditions (7–9).
Regucalcin was shown to play a multifunctional role
in cell regulation in the liver and kidney (5,10–13).
Regucalcin plays a pivotal role in maintaining intracellular
Ca2+ homeostasis and suppressing signal transduction,
nuclear Ca2+-dependent protein kinase and protein
phosphatase activity, Ca2+-activated deoxyribonucleic
acid (DNA) fragmentation and DNA and ribonucleic acid synthesis.
Regucalcin was also shown to suppress protein synthesis and
activate proteolysis, suggesting a role in protein turnover. The
overexpression of endogenous regucalcin was demonstrated to
suppress cell proliferation (14)
and apoptosis (15), which is
mediated through various signal transduction pathways, in various
types of cells. Moreover, regucalcin was shown to regulate the gene
expression for a number of proteins, suggesting a role as a novel
transcription factor (16).
Moreover, there is growing evidence that regucalcin
is involved in the regulation of heart cell function. The
Ca2+ current is one of the most important components in
cardiac excitation-contraction coupling. This coupling mechanism is
based on the regulation of intracellular Ca2+
concentration by the Ca2+ pump in the sarcoplasmic
reticulum of heart muscle cells (17–19).
Regucalcin is expressed in the heart and was found to increase rat
heart sarcoplasmic reticulum Ca2+-ATPase (SERCA2a)
activity and ATP-dependent Ca2+ uptake and mitochondrial
Ca2+-ATPase activity (20,21).
Regucalcin was also shown to regulate the activities of various
enzymes, which are associated with Ca2+ signaling in the
heart cytoplasm. Therefore, regucalcin may be a key molecule in
heart muscle cell regulation. The aim of this review was to discuss
the regulatory role of regucalcin in heart Ca2+
signaling (Fig. 1), with insight
into cardiac failure.
2. Expression of regucalcin in the
heart
The expression of regucalcin in rat hearts was
initially demonstrated by immunohistocemical analysis (22). Regucalcin mRNA is expressed in rat
heart muscle and regucalcin is present in the cytoplasm but not the
microsomes of rat heart cells (20,23).
Regucalcin concentration in the heart muscle tissues was estimated
to be ~3.86×10−8 M (23). Regucalcin gene expression may be
enhanced through various transcriptional factors. Nuclear factor
I-A1 (NFI-A1), a transcription factor, was found to be expressed in
rat hearts (24) and was shown to
specifically bind to the TTGGC motif in the regucalcin gene
promoter region (24). In addition,
RGPR-p117, a novel transcription factor that binds to the
regucalcin gene promoter region (25), was found to be expressed in rat
hearts (9,25).
Regucalcin mRNA expression may be altered under
various pathophysiological conditions. It was previously
demonstrated that regucalcin mRNA and protein levels in the hearts
of male and female rats decreased with increasing age, as they were
found to be lower in 50-week-old compared to those in 5-week-old
rats (26). The effect of
1,1-diphenyl-2-picrylhydrazyl (DPPH), a compound that produces free
radicals, on regucalcin mRNA expression in the hearts of 5-week-old
female rats was previously investigated (26). Heart regucalcin mRNA levels were
found to be reduced at 60 or 180 min following a single
intraperitoneal administration of DPPH (5 mg/kg body weight),
suggesting that free radical stress exerts a suppressive effect on
gene expression. DPPH is potently toxic and normal (wild-type)
female rats died within ~300 min following a single intraperitoneal
administration of DPPH (5 mg/kg body weight), whereas regucalcin
transgenic (TG) female rats died within ~150 minutes after the
administration (25). The heart
content of regucalcin protein in DPPH-administered rats was shown
to be higher in regucalcin TG compared to that in wild-type rats
(25). The death of regucalcin TG
rats may be accelerated following the administration of free
radical-generating compounds. The overexpression of endogenous
regucalcin may not contribute to the suppression of free radical
stress, as regucalcin was not found to be a free radical scavenger
in rats.
The presence of regucalcin in normal vs. dystrophic
fibres was demonstrated using comparative mass spectrometry-based
proteomics screening (27).
Following separation by two-dimensional gel electrophoresis, the
spot pattern of the normal vs. the X-linked muscular dystrophy
(mdx) diaphragm muscle proteome was evaluated by densitometry
(27). The expression levels of 20
major protein spots were shown to be altered and their identity was
determined by mass spectrometry (27). A 2-fold reduction of regucalcin in
the mdx diaphragm, as well as in dystrophic limb and heart muscle,
was confirmed by immunoblotting in young as well as aged mdx mice
(27). The results from the
proteomics analysis of the dystrophic diaphragm support the
hypothesis that abnormal Ca2+-handling is involved in
X-linked muscular dystrophy (27).
A decrease in regucalcin levels may be implicated in insufficient
maintenance of Ca2+ homeostasis and dysregulation of
Ca2+-dependent enzymes, resulting in the disturbed
intracellular signaling mechanisms that characterize
dystrophinopathies (27).
3. Regucalcin regulates intracellular
Ca2+ homeostasis in the heart
The mechanism of cardiac excitation-contraction
coupling is based on the regulation of intracellular
Ca2+ concentration by the Ca2+ pump in the
sarcoplasmic reticulum of heart muscle cells (17–19).
The regulatory effect of regucalcin on Ca2+ pump
activity in the microsomes (sarcoplasmic reticulum) of rat heart
muscle was previously investigated (20). The activity of SERCA2a was found to
be increased in the presence of regucalcin
(10−10-10−8 M) at physiological levels in the
enzyme reaction mixture (20).
However, this increase was not observed in the presence of
thapsigargin (10−5 M), a specific inhibitor of the
microsomal Ca2+-ATPase (28), indicating that regucalcin activates
Ca2+-ATPase in the sarcoplasmic reticulum.
Regucalcin (10−10-10−8 M) was
shown to stimulate ATP-dependent 45Ca2+
uptake by the microsomes (20). The
stimulatory effect of regucalcin on SERCA2a activity was completely
inhibited in the presence of digitonin, which exerts a solubilizing
effect on membranous lipid, or N-ethylmaleimide (NEM), a
modifying reagent of sulfhydryl (SH) groups (20). Dithiothreitol (DTT), a protecting
reagent of SH groups, markedly increased Ca2+-ATPase
activity. In the presence of DTT (5 mmol/l), regucalcin was not
able to enhance SERCA2a activity (20). The abovementioned findings suggest
that regucalcin binds to the lipids at the site close to the
Ca2+-ATPase in the heart microsomes, acts on the SH
groups, which may be the active site of the enzyme, and stimulates
Ca2+-dependent phosphorylation of the
Ca2+-ATPase (20). The
stimulatory effect of regucalcin on Ca2+-ATPase activity
was completely inhibited following the addition of vanadate (1
mmol/l), an inhibitor of enzyme phosphorylation (20). In addition, the effect of regucalcin
on Ca2+-ATPase activity was not modulated in the
presence of dibutyryl cyclic AMP (cAMP), inositol
1,4,5-trisphosphate, or calmodulin, which is an intracellular
signaling factor (20). Thus,
regucalcin was found to increase Ca2+-ATPase activity
and ATP-dependent Ca2+ uptake in rat heart microsomes,
which regulates intracellular Ca2+ concentration during
cardiac excitation-contraction coupling, suggesting a pivotal role
for regucalcin in the regulation of heart muscle function.
Phospholamban was shown to regulate SERCA2a activity
in heart muscle (29).
Ca2+-ATPase is activated through cAMP-dependent
phosphorylation of phospholamban following hormonal stimulation.
The function of the endogenous activator protein of SERCA2a has not
been clearly determined. Regucalcin, which is present in the
cytoplasm of heart muscle cells, may play an important role as an
endogenous activator in the regulation of SERCA2a activity in rat
heart muscle (20). Regucalcin may
also play a physiological role in the regulation of cardiac
excitation-contraction coupling.
Augmentation of regucalcin in regucalcin TG male
rats was shown to enhance SERCA2a activity in the heart (30). Western blot analysis revealed a
significant increase of regucalcin protein in the cytoplasm of
regucalcin TG female rat heart cells, compared to that in wild-type
female rats (30). Heart muscle
SERCA2a activity was enhanced in TG rats in vivo and the
changes in the enzyme activity in TG rats were completely abolished
in the presence of anti-regucalcin monoclonal antibody (100 ng/ml)
in the enzyme reaction mixture (30). Thus, endogenous regucalcin plays a
role as an activator in the regulation of heart SERCA2a.
The role of regucalcin in the regulation of
Ca2+-ATPase activity in rat heart mitochondria was also
demonstrated (21). The
mitochondrial Ca2+-ATPase activity was increased with
increasing concentrations of CaCl2 (2.5–50 μM) (21). An increase in the enzyme activity
was saturated at 50 μM CaCl2. The addition of regucalcin
(10−11-10−8 M) to the enzyme reaction mixture
led to an increase in Ca2+-ATPase activity in heart
mitochondria in the presence of 50 μM CaCl2 (21). Regucalcin exerted no effects on
mitochondrial Mg2+-ATPase activity. Furthermore,
regucalcin did not exert a significant effect on
Ca2+-ATPase activity in the presence of digitonin, which
was shown to solubilize membranous lipids (21). The stimulatory effect of regucalcin
on mitochondrial Ca2+-ATPase activity was not observed
in the presence of ruthenium red or lanthanum chloride, which are
inhibitors of the mitochondrial Ca2+ uniporter (21). The stimulatory effect of regucalcin
on mitochondrial Ca2+-ATPase activity was not observed
in the presence of calmodulin or dibutyryl cAMP, which is an
intracellular signaling factor that causes an increase in enzyme
activity (21). Of note,
mitochondrial regucalcin localization was found to be increased in
the hearts of regucalcin TG rats compared to that in wild-type
rats, as determined by western blot analysis.
Ca2+-ATPase activity was also increased in the heart
mitochondria of regucalcin TG rats (21). The abovementioned findings
demonstrate that regucalcin exerts an activating effect on
Ca2+-ATPase in rat heart mitochondria.
Regucalcin was previously shown to reduce agonist
(histamine)-induced Ca2+ transients in
regucalcin-transfected COS-7 cells and increase their
Ca2+ storage capacity (31). These observations may be explained
by the increased mRNA and protein expression levels of SERCA2a in
regucalcin-transfected cells (31).
Therefore, the downregulation of regucalcin expression may
contribute to the characteristics of disturbed regulation of
age-dependent Ca2+ homeostasis by decreasing SERCA2a
levels (31).
4. Regucalcin regulates Ca2+
signaling-dependent enzyme activity
Protein phosphorylation-dephosphorylation is a
universal mechanism by which numerous cellular events are regulated
(32). There are a number of
phosphatases that, similar to kinases, are elaborately and
rigorously controlled (32).
Protein phosphatases are involved in intracellular signal
transduction due to hormonal stimulation.
Ca2+/calmodulin-dependent protein phosphatase
(calcineurin), a calmodulin-binding protein, was shown to possess a
Ca2+-dependent and calmodulin-stimulated protein
phosphatase activity (33,34). Cardiac hypertrophy is induced by
calcineurin, which dephosphorylates the transcription factor NF-A3,
enabling it to translocate to the nucleus (34). In addition, TG mice, which express
activated forms of calcineurin or NF-AT3 in the heart, may develop
cardiac hypertrophy and heart failure that mimic human heart
disease (34), suggesting the
existence of a novel hypertrophic signaling pathway. Thus, protein
phosphatases play an important role in intracellular signal
transduction due to hormonal stimulation in heart cells.
The role of regucalcin in the regulation of protein
phosphatase activity in the heart muscle cytosol was demonstrated
using regucalcin TG rats (35).
Protein phosphatase activity was assayed in a reaction mixture
containing the cytosolic protein in the presence of
phosphotyrosine, phosphoserine and phosphothreonine (35). The addition of CaCl2 (10
and 20 μM) to the enzyme reaction mixture led to an increase in
protein phosphatase activity towards three phosphoaminoacids
(35). This increase was enhanced
following the addition of calmodulin. The addition of regucalcin
(10−9 and 10−8 M) was found to inhibit
protein phosphatase activity towards three phosphoaminoacids in the
presence of ethylene
glycol-bis(2-amino-ethyl)-N,N,N′,N′-tetraacetic acid (EGTA)
(35). The inhibitory effect of
regucalcin was also observed in the presence or absence of
CaCl2 (10 μM). Thus, regucalcin was found to inhibit the
activity of various protein phosphatasees, dependently or
independently of Ca2+.
Regucalcin TG female rats were shown to markedly
express endogenous regucalcin protein in the heart cytoplasm
compared to wild-type female rats (35). Protein phosphatase activity towards
three phosphoaminoacids was significantly decreased in the heart
cytoplasm of TG rats (35). The
effect of Ca2+ addition on increasing protein
phosphatase activity towards three phosphoaminoacids was not
observed in the heart cytoplasm of TG rats (35), supporting the hypothesis that
endogenous regucalcin plays a suppressive role in the regulation of
protein phosphatase activity in rat heart cytoplasm. Thus,
regucalcin was shown to suppress Ca2+-dependent protein
tyrosine phosphatase and calcineurin activity in the heart
cytoplasm of rats (35). The
overexpression of regucalcin, which exerts suppressive effects on
calcineurin activity, may play a pathophysiological role in the
prevention of the development of cardiac hypertrophy and heart
failure.
The role of regucalcin in the regulation of protein
kinases in the heart remains to be elucidated. Regucalcin was shown
to suppress Ca2+/calmodulin-dependent protein kinase and
protein kinase C in the liver, kidney and brain (12–14).
Moreover, it was demonstrated that regucalcin plays
a role in the regulation of nitric oxide (NO) synthase activity in
the cytosol of rat heart muscle (36). The addition of CaCl2
(5–20 μM) to the enzyme reaction mixture containing the heart
cytosolic protein led to an increase in NO synthase activity
(36). The Ca2+ effect
was inhibited by trifluoperazine (TFP), an antagonist of
calmodulin, indicating the presence of
Ca2+/calmodulin-dependent NO synthase activity in rat
heart muscle cytosol (36). The
activity of NO synthase was decreased following the addition of
regucalcin (10−9 or 10−8 M) (36). This effect was also observed in the
presence of CaCl2, TFP or EGTA, a chelator of
Ca2+. The downregulating effect of regucalcin on NO
synthase activity was not observed in the presence of
Nω-nitro-L-arginine methyl ester, an inhibitor of
the enzyme (36). The presence of
anti-regucalcin monoclonal antibody (25 or 50 ng/ml) in the enzyme
reaction mixture led to a significant increase in NO synthase
activity and this effect was completely abolished by the addition
of regucalcin. Therefore, endogenous regucalcin in the heart
cytoplasm may act as a suppressor protein in the regulation of NO
synthase activity.
Of note, NO synthase activity was not altered in the
heart muscle cytoplasm obtained from regucalcin TG rats, which
overexpress endogenous regucalcin compared to wild-type rats
(36). However, the stimulatory
effect of Ca2+ (10 μM) addition on NO synthase activity
was weakened in the heart muscle cytoplasm obtained from regucalcin
TG rats (36). This finding
supports the hypothesis that endogenous regucalcin may exert a
suppressive effect on NO synthase activity in the heart muscle
cytoplasm of rats.
The physiological significance of regucalcin
inhibition on NO synthase in heart muscle cytoplasm is unknown.
However, regucalcin may participate in the regulation of NO
production in heart muscle cells. NO acts as a messenger or
modulator molecule in heart muscle. NO production may be stimulated
through Ca2+ signaling due to hormonal stimulation in
heart muscle cells. Regucalcin may exert a suppressive effect on
the overproduction of NO due to inhibiting NO synthase in heart
muscle cells.
5. Other role of regucalcin in heart cell
regulation
Superoxide dismutase (SOD) plays a role in the
prevention of cell death and apoptosis in the heart (37). A decrease in manganese SOD activity
is associated with increased mitochondrial oxidative damage, as
demonstrated by the decrease in the activities of iron SH proteins
sensitive to oxygen stress (37).
Cu/Zn-SOD was shown to act as a protector against
dexorubicin-induced cardiotoxicity in mice (38). Furthermore, NO is involved in the
control of myocardial O2 consumption in rats (39). Regucalcin was found to increase SOD
activity in rat heart cytoplasm (40).
The addition of regucalcin
(10−10-10−8 M) at a physiological
concentration to the enzyme reaction mixture containing the heart
cytoplasm obtained from wild-type rats led to an increase in SOD
activity, indicating that regucalcin directly activates this enzyme
(40). The stimulatory effect of
regucalcin on SOD activity was not observed in the presence of DTT,
a protecting reagent for SH groups, or NEM, a modifying reagent for
SH groups, in the reaction mixture, indicating that regucalcin does
not affect the SH groups (40). The
addition of zinc sulfate to the reaction mixture did not lead to a
significant change in SOD activity, whereas the enzyme activity was
markedly decreased in the presence of cupric sulfate (40). The activating effect of regucalcin
on SOD was observed in the presence of zinc, whereas it was not
observed in the presence of copper (40). Moreover, SOD activity was enhanced
in the heart cytoplasm of regucalcin TG rats compared to the
wild-type rats (40). The
abovementioned findings demonstrate that regucalcin increases SOD
activity in the heart cytosol of rats and this effect is not
associated with the enzyme SH groups.
Regucalcin was found to increase SOD activity in rat
heart cytoplasm. Regucalcin exerts an inhibitory effect on NO
synthase activity in the heart cytosol (36). The production of superoxide radicals
is known to be the cause of cardiac damage. Regucalcin may
participate in the regulation of the production of superoxide
radicals in rat heart muscle cells.
Ageing is an important risk factor of cardiovascular
diseases, including heart failure. The role of regucalcin in
cardiac remodelling was previously reported (41). Regucalcin-knockout and wild-type
mice were subjected to continuous angiotensin II infusion. This
treatment caused more prominent cardiac hypertrophy and myocardial
fibrosis in regucalcin-knockout compared to those observed in
wild-type mice (41).
Regucalcin-knockout mice exhibited increased generation of reactive
oxygen species, increased number of deoxynucleotidyl
transferase-mediated dUTP nick end-labelling positive nuclei,
activation of caspase-3, increases in the BAX:Bcl-2 ratio and
phosphorylation of c-Jun N-terminal kinase (41). Thus, regucalcin deficiency may
exacerbate angiotensin II-induced cardiac hypertrophy, dysfunction
and remodelling. Regucalcin may play a cardioprotective role in
cardiac remodelling in response to angiotensin II, due to its
antioxidative and anti-apoptotic properties.
6. Prospect
Regucalcin plays a pivotal role as a suppressor
protein in Ca2+-related signal transduction in various
types of cells and tissues, including the liver and kidney
(10–12). Moreover, regucalcin was demonstrated
to regulate intracellular Ca2+ homeostasis due to
activating the Ca2+-ATPase in the sarcoplasmic reticulum
and mitochondria of rat heart cells. Regucalcin suppresses
Ca2+/calmodulin-dependent enzymes, including protein
phosphatase and NO synthase, which are associated with
Ca2+ signaling. Ca2+ signaling is one of the
most important components in cardiac excitation-contraction
coupling. This coupling system may be regulated through regucalcin.
Regucalcin may play a physiological role in the regulation of
Ca2+-related heart cell functions. Whether regucalcin is
associated with other protein molecules that are involved in
cardiac excitation-contraction coupling in heart cells, remains to
be elucidated.
Moreover, regucalcin was found to play a pivotal
role as a suppressor of NO synthase and an activator of SOD in the
heart cytoplasm. The overproduction of NO may lead to heart cell
damage. SOD plays a pivotal role in the suppression of free radical
production that leads to heart failure. Regucalcin may play a
physiological role by exerting protective effects against heart
failure, through the activation of SOD or the suppression of NO
overproduction in heart cells. The pathophysiological role of
regucalcin in heart dysfunction remains to be fully elucidated.
However, the currently available evidence indicate that regucalcin
may be a target molecule in heart disease.
Acknowledgements
The studies on regucalcin by the author were
supported by Grants-in-Aid for Scientific Research (C) (nos.
63571053, 02671006, 04671362, 06672193, 08672522, 10672048,
13672292 and 17590063) from the Ministry of Education, Science,
Sports and Culture, Japan. Furthermore, the author (M.Y.) was
awarded the Bounty of Encouragement Foundation in Pharmaceutical
Research, Japan, as well as the Bounty of the Yamanouchi Foundation
for Research on Metabolic Disorders, Japan. This study was also
supported by the Foundation for Biomedical Research on
Regucalcin.
References
|
1
|
Yamaguchi M and Yamamoto T: Purification
of calcium binding substance from soluble fraction of normal rat
liver. Chem Pharm Bull (Tokyo). 26:1915–1918. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamaguchi M and Mori S: Effect of
Ca2+and Zn2+on 5′-nucleotidase activity in
rat liver plasma membranes: hepatic calcium-binding protein
(regucalcin) reverses the Ca2+effect. Chem Pharm Bull
(Tokyo). 36:321–325. 1988.
|
|
3
|
Yamaguchi M: A novel
Ca2+-binding protein regucalcin and calcium inhibition.
Regulatory role in liver cell function. Calcium Inhibition. Kohama
K: Japan Sci Soc Press, Tokyo and CRC Press; Boca Raton: pp. 19–41.
1992
|
|
4
|
Shimokawa N and Yamaguchi M: Molecular
cloning and sequencing of the cDNA coding for a calcium-binding
protein regucalcin from rat liver. FEBS Lett. 327:251–255. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimokawa N, Matsuda Y and Yamaguchi M:
Genomic cloning and chromosomal assignment of rat regucalcin gene.
Mol Cell Biochem. 151:157–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiselton DL, McDowall J, Brandau O,
Ramser J, d’Esposito F, Bhattacharya SS, Ross MT, Hardcastle AJ and
Meindl A: An integrated, functionally annotated gene map of the
DXS8026-ELK1 interval on human Xp11.3-Xp11.23: Potential hotspot
for neurogenetic disorders. Genomics. 79:560–572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Misawa H and Yamaguchi M: The gene of
Ca2+-binding protein regucalcin is highly conserved in
vertebrate species. Int J Mol Med. 6:191–196. 2000.
|
|
8
|
Yamaguchi M: The transcriptional
regulation of regucalcin gene expression. Mol Cell Biochem.
346:147–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamaguchi M: Novel protein RGPR-p117: its
role as the regucalcin gene transcription factor. Mol Cell Biochem.
327:53–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamaguchi M: Role of regucalcin in calcium
signaling. Life Sci. 66:1769–1780. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaguchi M: Role of regucalcin in
maintaining cell homeostasis and function (Review). Int J Mol Med.
15:371–389. 2005.PubMed/NCBI
|
|
12
|
Yamaguchi M: Regucalcin and cell
regulation: role as a supressor protein in signal transduction. Mol
Cell Biochem. 353:101–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamaguchi M: Suppressive role of
regucalcin in liver cell proliferation: involvement in
carcinogenesis. Cell Prolif. 46:243–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamaguchi M: The anti-apoptotic effect of
regucalcin is mediated through multisignaling pathways. Apoptosis.
18:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi M: Regucalcin: Genomics, Cell
Regulation and Diseases. Nova Science Publishers, Inc; New York,
NY: 2012
|
|
16
|
Yamaguchi M: Role of regucalcin in cell
nuclear regulation: involvement as a transcription factor. Cell
Tissue Res. 354:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Langer GA: Calcium and the heart: exchange
at the tissue, cell and organelle levels. FASEB J. 6:893–902.
1992.PubMed/NCBI
|
|
18
|
Thastrup O, Culler PJ, Drobak BK, Hanley
MR and Dawson AP: Thapsigargin, a tumor promoter, discharges
intracellular Ca2+stores by specific inhibition of the
endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci
USA. 87:2466–2470. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tada M and Kadoma M: Regulation of the
Ca2+pump ATPase by cAMP-dependent phosphorylation of
phospholamban. Bioessays. 10:157–163. 1989.
|
|
20
|
Yamaguchi M and Nakajima R: Role of
regucalcin as an activator of sarcoplasmic reticulum
Ca2+-ATPase activity in rat heart muscle. J Cell
Biochem. 86:184–193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akhter T, Sawada N and Yamaguchi M:
Regucalcin increases Ca2+-ATPase activity in the heart
mitochondria of normal and regucalcin transgenic rats. Int J Mol
Med. 18:171–176. 2006.
|
|
22
|
Yamaguchi M, Isogai M, Kato S and Mori S:
Immunohistochemical demonstration of calcium-binding protein
regucalcin in the tissues of rats: the protein localizes in liver
and brain. Chem Pharm Bull (Tokyo). 39:1601–1603. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamaguchi M and Isogai M: Tissue
concentration of calcium-binding protein regucalcin in rats by
enzyme-linked immunoadsorbent assay. Mol Cell Biochem. 122:65–68.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Misawa H and Yamaguchi M: Indentification
of transcription factor in the promoter region of rat regucalcin
gene: binding of nuclear factor I-A1 to TTGGC motif. J Cell
Biochem. 84:795–802. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Misawa H and Yamaguchi M: Molecular
cloning and sequencing of the cDNA coding for a novel regucalcin
gene promoter region-related protein in rat, mouse and human liver.
Int J Mol Med. 8:513–520. 2001.PubMed/NCBI
|
|
26
|
Akhter T, Nakagawa T, Kobayashi A and
Yamaguchi M: Suppression of regucalcin mRNA expression in the
hearts of rats administered with free radical compound: The
administreation-induced death is accelerated in regucalcin
transgenic rats. Int J Mol Med. 19:653–658. 2007.PubMed/NCBI
|
|
27
|
Doran P, Dowling P, Donoghue P, Buffini M
and Ohlendieck K: Reduced expression of regucalcin in young and
aged mdx diaphragm indicates abnormal cytosolic calcium handling in
dystrophin-deficient muscle. Biochim Biophys Acta. 1764:773–785.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng H, Smith GL, Hancox JC and Orchard
CH: Inhibition of spontaneous activity of rabbit atrioventricular
node cells by KB-R7943 and inhibitors of sarcoplasmic reticulum
Ca2+ATPase. Cell Calcium. 49:56–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marks AR: Calcium cycling proteins and
heart failure: mechanisms and therapeutics. J Clin Invest.
123:46–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamaguchi M, Morooka Y, Misawa H,
Tsurusaki Y and Nakajima R: Role of endogenous regucalcin in
transgenic rats: suppression of kidney cortex cytosolic protein
phosphatase activity and enhancement of heart muscle microsomal
Ca2+-ATPase activity. J Cell Biochem. 86:520–529. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lai P, Yip NC and Michelangeli F:
Regucalcin (RGN/SMP30) alters agonist- and thapsigargin-induced
cytosolic [Ca2+] transients in cells by increasing SERCA
Ca2+ATPase levels. FEBS Lett. 585:2291–2294.
2011.PubMed/NCBI
|
|
32
|
Hunter T: Protein kinases and
phosphatases: the Yin and Yang of protein phosphorylation and
signaling. Cell. 80:225–236. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pallen CJ and Wang JH:
Calmodulin-stimulated dephosphorylation of p-nitrophenyl phosphate
and free phosphotyrosine by calcineurin. J Biol Chem.
258:8550–8553. 1983.PubMed/NCBI
|
|
34
|
Molkentin JD, Lu JR, Antos CL, Markham B,
Richardson J, Robbins J, Grant SR and Olson EN: A
calcineurin-dependent transcriptional pathway for cardiac
hypertrophy. Cell. 93:215–228. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ichikawa E, Tsurusaki Y and Yamaguchi M:
Suppressive effect of regucalcin on protein phosphatase activity in
the heart cytosol of normal and regucalcin transgenic rats. Int J
Mol Med. 13:289–293. 2004.PubMed/NCBI
|
|
36
|
Ma ZJ and Yamaguchi M: Suppressive role of
endogenous regucalcin in the regulation of nitric oxide synthase
activity in heart muscle cytosol of normal and regucalcin
transgenic rats. Int J Mol Med. 10:761–766. 2002.PubMed/NCBI
|
|
37
|
Van Remmen H, Williams MD, Guo Z, Estlack
L, Yang H, Carlson EJ, Epstein CJ, Huang TT and Richardson A:
Knockout mice heterozygous for Sod2 show alterations in cardiac
mitochondrial function and apoptosis. Am J Physol Heart Circ
Physiol. 281:H1422–H1432. 2001.PubMed/NCBI
|
|
38
|
den Hartog GJ, Haenen GR, Boven E, van der
Vijgh WJ and Bast A: Lecithinized copper, zinc-superoxide dismutase
as a protector against dexorubicin-induced cardiotoxicity in mice.
Toxicol Appl Pharmacol. 194:180–188. 2004.PubMed/NCBI
|
|
39
|
Adler A, Messina E, Sherman B, Wang Z,
Huang H, Linke A and Hintze TH: NAD(P)H oxidase-generated
superoxide anion accounts for reduced control of myocardial
O2consumption by NO in old Fisher 344 rats. Am J Physiol
Heart Circ Physiol. 285:H1015–H1022. 2003.PubMed/NCBI
|
|
40
|
Ichikawa E and Yamaguchi M: Regucalcin
increases superoxide dismutase activity in the heart cytosol of
normal and regucalcin transgenic rats. Int J Mol Med. 14:691–695.
2004.PubMed/NCBI
|
|
41
|
Misaka T, Suzuki S, Miyata M, Kobayashi A,
Shishido T, Ishigami A, Saitoh S, Hirose M, Kubota I and Takeishi
Y: Deficiency of senescence marker protein 30 exacerbates
angiotensin II-induced cardiac remodelling. Cardiovasc Res.
99:461–470. 2013. View Article : Google Scholar : PubMed/NCBI
|