Introduction
Gap junctions are special regions adjoining cell
membranes and are direct pathways for the exchange of signals among
cardiac myocytes. Gap junctions mediate current flow, thereby
coordinating the spread of excitation and subsequent contraction
throughout the myocardium (1). The
electrical conduction velocity in the region of gap junctions is
faster compared to that elsewhere (2). It was previously suggested that
changes in the gap junctions in morbid heart tissue, collectively
referred to as gap junction remodeling, appear to be associated
with the incidence of arrhythmias (3–7).
Heptanol is commonly used as a gap junction
inhibitor in several experiments. Previous studies demonstrated
that regional perfusion with heptanol may decrease the conduction
velocity and induce reentrant arrhythmias (8,9).
However, little is known regarding the effects of heptanol on the
arrhythmias induced by ischemia. The aim of this study was to
investigate the effects of heptanol on ventricular arrhythmias
induced by ischemia and evaluate the changes in connexin 43 (Cx43),
the major gap junction protein, in the ischemic myocardium. As
heptanol may act on sodium and calcium channels (9–11), it
may also affect the action potential. Therefore, the cardiac
electrophysiological properties, such as heart rate (HR), PR
interval, QT interval and monophasic action potential duration at
90% repolarization (MAPD90) were also assessed.
Materials and methods
Animals
A total of 60 adult male Sprague-Dawley (SD) rats,
weighing 0.2–0.3 kg, were provided by the Experimental Animal
Center of Tongji Hospital (Shanghai, China). All the animal
experiments were conducted in compliance with the Guide for the
Care and Use of Laboratory Animals (National Reasearch Council,
1996).
Isolated heart preparation
The SD rats were anesthetized with 1% pentobarbital
(0.5 ml/kg) and heparinized via intraperitoneal injection (50
IU/kg). The hearts were quickly excised, mounted on a Langendorff
apparatus via the aorta and perfused with Krebs-Henseleit (K-H)
buffer (Shanghai Chemical Reagent Co., Shanghai, China) at a
constant perfusion pressure of 90 cmH2O. The K-H
solution consisted of 118.6 mmol/l NaCl, 25 mmol/l
NaHCO3, 4.7 mmol/l KCl, 1.18 mmol/l
KH2SO4, 1.2 mmol/l MgSO4, 2.5
mmol/l CaCl2 and 11.1 mmol/l glucose, was gassed with
95% O2 and 5% CO2 (pH 7.4) and maintained at
37±1°C. Bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO,
USA) was added to the perfusate at a concentration of
6.013×10−4 M (40 mg/l) to improve the stability of the
hearts.
All the measurements were performed after an initial
stabilization period of at least 15 min of perfusion with stable
electrophysiological signals, temperature and coronary flow.
The hearts were randomly divided into 5 groups as
follows: i) control group: the hearts were subjected to normal
perfusion with K-H buffer solution for 45 min; ii) ischemia group:
following perfusion for 15 min with K-H buffer solution, the hearts
were subjected to regional ischemia by ligating the left anterior
descending coronary artery (LAD) close to its origin for 30 min;
iii–v) heptanol groups: the hearts were pretreated with 0.1, 0.3
and 0.5 mM heptanol, respectively, for 15 min prior to the
induction of ischemia. Heptanol was dissolved directly in the
perfusate at different concentrations and perfused into the
isolated hearts.
Electrophysiological measurements
An epicardial electrogram was recorded using two
silver electrodes (with a diameter of 0.3 mm) placed on the surface
of the left and right ventricles. The MAPDs were recorded by
another two electrodes placed on the surface of the left ventricle
near the septal and the aortic cannulae. The epicardial
electrograms and MAPD were amplified and analyzed using Medlab
computer software (Nanjing Madease Science and Technology Co.,
Ltd., Nanjing, China).
The parameters, including HR, PR interval, QT
interval and MAPD, were recorded at baseline (15 min prior to
ischemia; 0 min) and at 10 min (Is10 min), 20 min (Is20 min) and 30
min (Is30 min) after the induction of ischemia. During this period,
VT and VF were recorded. VT was defined as a run of ventricular
beats lasting >1 min.
Immunofluorescence analysis
At the end of the experiment, 5 hearts from each
group were selected and perfused with 1% Evans blue dye. The
non-blue parts were re-stained with triphenyltetrazolium chloride
(TTC; Shanghai Chemical Reagent Co.). The sections that stained
with TTC were identified as ischemic and were fixed in 10% neutral
buffered formalin for immunofluorescence (12,13). A
rabbit polyclonal antibody (Zymed Laboratories, San Francisco, CA,
USA) directed against Cx43 was used as the primary antibody
(dilution, 1:100). FITC-labeled goat anti-rabbit IgG was used as
the secondary antibody (dilution, 1:100). Images were captured with
a TCS SP2 confocal microscope (Leica, Mannheim, Germany) at a
magnification of ×400, using a 40× oil immersion lens. Five areas
were analyzed in each heart, for a total of 25 test areas in each
group.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
RNA samples (2 μg per experiment) were extracted
from the ischemic myocardium. RNA extraction, first-strand
complementary DNA (cDNA) synthesis and DNA amplification were
performed as previously described, with minor modifications
(13). Two pairs of primers
designed with Primer 5.0 software were used to amplify a 588-bp
product of Cx43 and a 770-bp product of β-actin, which was used as
control (Table I). The reaction
system (50 μl) contained 33.75 μl H2O, 5 μl 10X buffer,
200 μM dNTP mixture, 1.875 mM MgCl2, 0.5 μl Taq
DNA polymerase, 2 μl of the pair primers and 4 μl cDNA. The PCR
samples were subjected to initial denaturation for 2 min at 95°C,
30 cycles of 30 sec at 95°C and 30 sec at 60°C, followed by a final
extension at 72°C for 5 min. These procedures were completed in a
PTC-100 automated thermocycler (MJ Research, Watertown, MA,
USA).
 | Table IOligonucleotide primers used for
RT-PCR analysis. |
Table I
Oligonucleotide primers used for
RT-PCR analysis.
| Target | Primer sequence
(5′→3′) | Size (bp) |
|---|
| Cx43 | F: TTG TTT CTG TCA
CCA GTA AC | 588 |
| R: GAT GAG GAA GGA
AGA GAA GC | |
| β-actin | F: CGT GGC GTT TAC
GAA GAT | 770 |
| R: ACC CAG ATC ATG
TTT GAG ACC | |
The RT-PCR products were visualized on 1.5% agarose
gels electrophoresed in 1X Tris-acetate-EDTA buffer. After 25 min,
the gels were placed in a solution containing 0.5 μg/ml ethidium
bromide and then into a UV transilluminator (Shanghai Qin Xiang
Scientific Instrument Co., Ltd., Shanghai, China). The results of
the immunofluorescence and RT-PCR analyses were assessed with Leica
Qwin image software (Leica Microsystems).
Statistical analyses
The values are expressed as means ± SE. The
myocardial electrical characteristics and Cx43 protein and mRNA
expression were compared among groups by the analysis of repeated
measures. The occurance of ventricular arrhythmias among groups was
assessed by the Fisher’s exact test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Experimental hearts
The total number of rats used in this experiment was
60. Of the 60 hearts, 8 were discarded due to ligation failure. The
hearts that completed the entire protocol (n=52) included 11 hearts
in the ischemia group, 10 hearts in the 0.1 mM heptanol group, 10
hearts in the 0.3 mM heptanol group, 9 hearts in the 0.5 mM
heptanol group and 12 hearts in the control group without
ligation.
Electrophysiological parameters
The electrophysiological parameters are presented in
Table II. Ischemia was shown to
decrease the QT interval and MAPD90 and prolong the PR interval;
however, it did not affect HR. Heptanol decreased HR following LAD
ligation (Fig. 1A), whereas it
prolonged the PR interval, QT interval and MAPD90 following LAD
ligation, particularly at the concentration of 0.5 mM (Fig. 1B–D).
 | Table IIElectrophysiological parameters in the
ischemic group. |
Table II
Electrophysiological parameters in the
ischemic group.
| | Time points
(min) |
|---|
| |
|
|---|
| Parameters | Groups | 0 | Is10a | Is20b | Is30c |
|---|
| HR | Control | 238±54 | 233±41 | 218±35 | 210±53 |
| Ischemic | 225±42 | 231±51 | 211±84 | 181±84 |
| PR interval | Control | 36.4±5.2 | 40.2±5.3 | 35.9±4.3 | 41.2±6.3 |
| Ischemic | 33.3±5.9 | 35.5±8.4 | 67.1±12.3d | 76.6±10.3d |
| QT interval | Control | 220±48 | 200±58 | 198±33 | 201±57 |
| Ischemic | 224±45 | 221±68 | 92±25d | 101±33d |
| MAPD90 | Control | 89.3±25.3 | 88.5±20.3 | 75.6±20.8 | 77.6±25.3 |
| Ischemic | 86.7±23.2 | 78.4±18.9 | 49.6±18.9d | 55.2±12.3d |
Incidence of VT and VF
Heptanol decreased the percentage of ventricular
arrhythmias induced by ischemia. The percentage of ventricular
arrhythmias was 45% in the ischemia group, 10% in the 0.1 mM group
and 0% in the 0.3 and 0.5 mM groups (P<0.05) (Table III).
 | Table IIIIncidence of ventricular arrhythmia
induced by regional ischemia. |
Table III
Incidence of ventricular arrhythmia
induced by regional ischemia.
| Groups | No. | VT and VF
incidence | % |
|---|
| Ischemic | 11 | 5 | 45 |
| Heptanol |
| 0.1 mM | 10 | 1 | 10a |
| 0.3 mM | 10 | 0 | 0a |
| 0.5 mM | 9 | 0 | 0a |
Immunofluorescence staining results
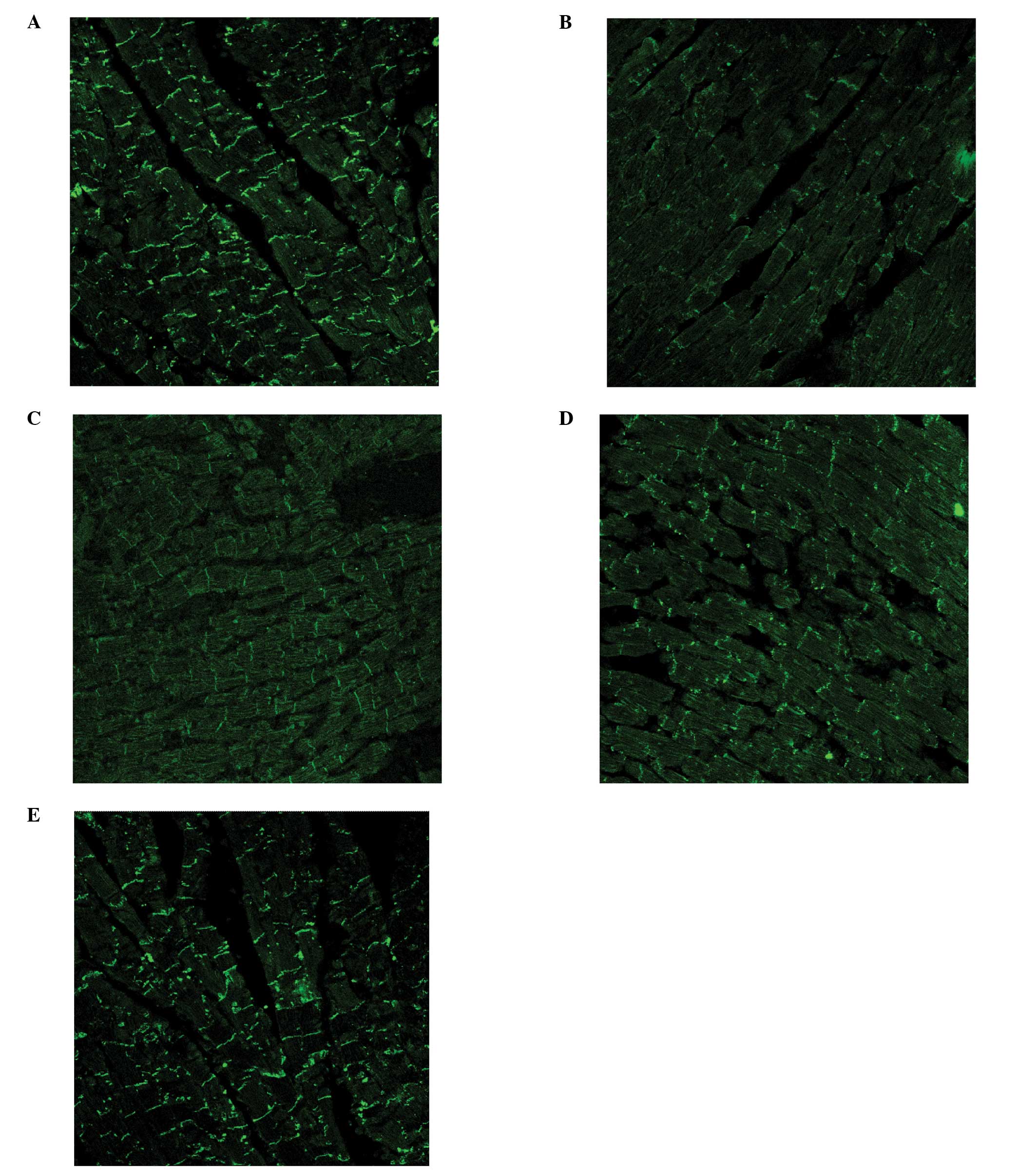
The level of the Cx43 protein, as evaluated by
immunofluorescence microscopy, was found to be lower in the
ischemic myocardium compared to that in normal myocardium (Fig. 2A and B). Heptanol was able to partly
reverse this downregulation induced by ischemia, with the level of
the Cx43 protein being 1,706±397 μM2 in the control
group, 561±147 μM2 in the ischemic group, 1,027±215
μM2 in the 0.1 mM group, 1,112±301 μM2 in the
0.3 mM group and 1,179±425 μM2 in the 0.5 mM group
(P<0.05). There was no significant difference among the treated
groups (Fig. 2C–E).
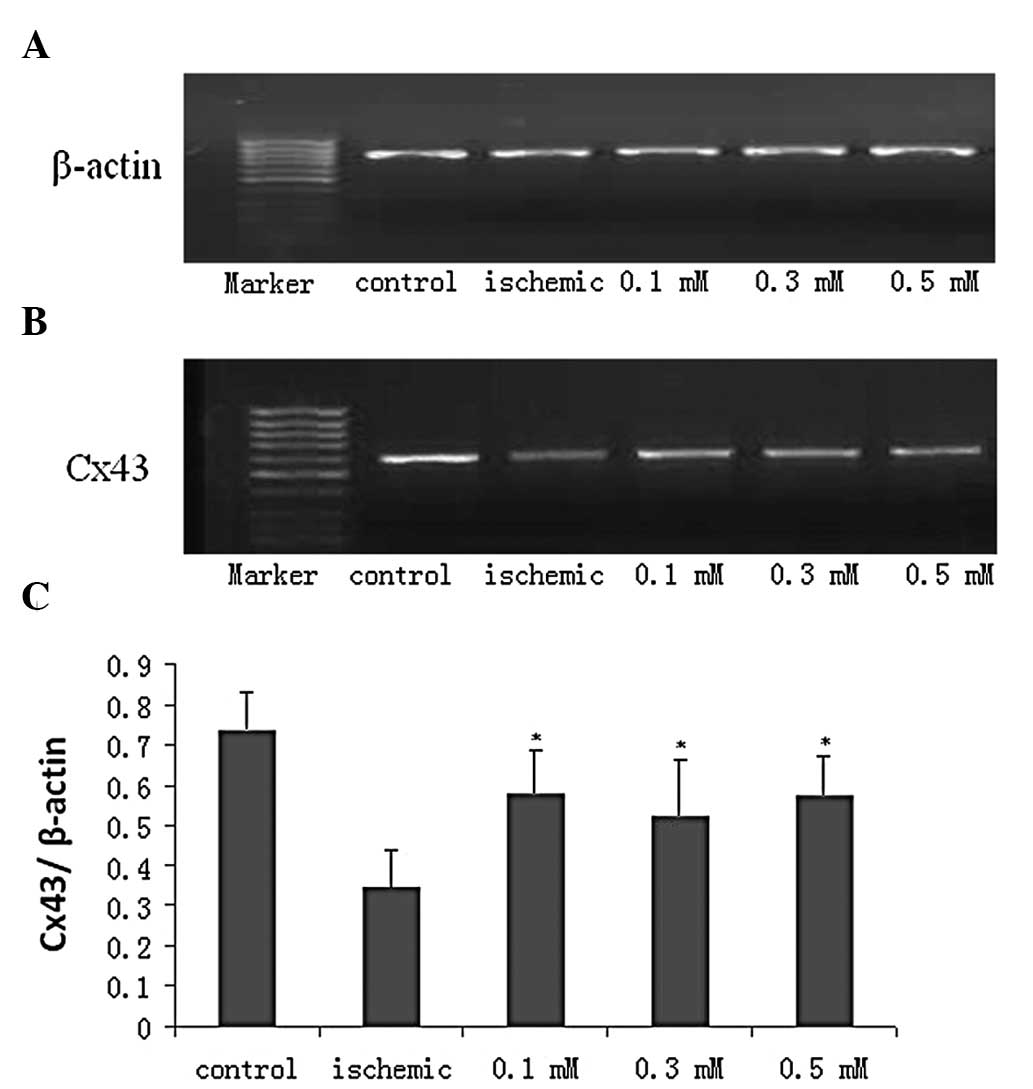
mRNA expression of Cx43
The results of RT-PCR were expressed by the ratio of
Cx43 to β-actin. The mRNA level of Cx43 was found to be lower in
the ischemia group compared to that in the control group and
heptanol was able to partly reverse this downregulation (Fig. 3A–C).
Discussion
In this study, we observed that VT and VF occurred
in almost half of the cases in the ischemic group and in only one
of the 29 cases in the treated groups, suggesting that heptanol
significantly decreased the incidence of VT and VF induced by
regional ischemia. Previous studies demonstrated that the slower
electrical conduction in the ischemic myocardium may lead to
reentrant arrhythmia (14,15). Heptanol was shown to reduce the
electrical conduction velocity by decreasing the function of gap
junctions in all the regions of the myocardium (16). The difference in the reduction of
the velocity between normal and ischemic regions in the heart may
decrease the incidence of reentrant arrhythmia.
Heptanol was shown to depress the inward
Na+ current (17), which
may explain the prolongation of the QT interval and MAPD90. In the
ischemic myocardium, the shorter repolarization duration may lead
to the development of reentrant arrhythmia (14). Thus, the decrease in repolarization
dispersion between the ischemic and the normal myocardium caused by
heptanol may be responsible for the decreased occurrence of
ventricular arrhythmia.
Additionally, heptanol reduced the HR and PR
interval, suggesting that it may affect the function of the sinus
and atrioventricular nodes.
We observed that the expression of Cx43 in the
ischemic myocardium was lower compared to that in the normal
myocardium, indicating that ischemia may damage gap junctions
(18,19). A number of factors may be involved
in this change, such as the decrease in the pH and the accumulation
of free radicals and lipid metabolites. The reduction of gap
junctions in the ischemic myocardium eventually results in a
decrease in the conduction velocity. The difference in conduction
between the ischemic and the normal myocardium may be an important
factor leading to the development of reentrant arrhythmias. In the
present experiment, heptanol was able to partly reverse the
reduction of Cx43 and may participate in the prevention of ischemic
arrhythmias. The result of the RT-PCR revealed that the
upregulation of Cx43 by heptanol occurred at the mRNA level.
Heptanol is liposoluble; thus, it may cross the cell membrane to
regulate the transcription of Cx43.
In conclusion, the gap junction inhibitor heptanol
decreases the incidence of VT and VF induced by regional ischemia
through altering the myocardial electrophysiological properties and
the transcription of Cx43.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81300150).
References
|
1
|
Qi X, Varma P, Newman D and Dorian P: Gap
junction blockers decrease defibrillation thresholds without
changes in ventricular refractoriness in isolated rabbit hearts.
Circulation. 104:1544–1549. 2001. View Article : Google Scholar
|
|
2
|
Moreno AP, Rook MB, Fishman GI and Spray
DC: Gap junction channels: distinct voltage-sensitive and
-insensitive conductance states. Biophys J. 67:113–119. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu ZB and Sheng JJ: Remodeling of cardiac
gap junctions and arrhythmias. Acta Physiologica Sinica.
63:586–592. 2011.(In Chinese).
|
|
4
|
Delmar M and Makita N: Cardiac connexins,
mutations and arrhythmias. Curr Opin Cardiol. 27:236–241. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palatinus JA, Rhett JM and Gourdie RG: The
connexin43 carboxyl terminus and cardiac gap junction organization.
Biochim Biophys Acta. 1818:1831–1843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Severs NJ, Bruce AF, Dupont E and Rothery
S: Remodelling of gap junctions and connexin expression in diseased
myocardium. Cardiovasc Res. 80:9–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dupont E, Matsushita T, Kaba RA, et al:
Altered connexin expression in human congestive heart failure. J
Mol Cell Cardiol. 33:359–371. 2001. View Article : Google Scholar
|
|
8
|
Ohara T, Qu Z, Lee MH, et al: Increased
vulnerability to inducible atrial fibrillation caused by partial
cellular uncoupling with heptanol. Am J Physiol Heart Circ Physiol.
283:H1116–H1120. 2002.PubMed/NCBI
|
|
9
|
Tse G, Hothi SS, Grace AA and Huang CL:
Ventricular arrhythmogenesis following slowed conduction in
heptanol-treated, Langendorff-perfused mouse hearts. J Physiol Sci.
62:79–92. 2012. View Article : Google Scholar
|
|
10
|
Dorian P, Wang M, David I and Feindel C:
Oral clofilium produces sustained lowering of defibrillation energy
requirements in a canine model. Circulation. 83:614–621. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Labhasetwar V, Underwood T, Heil RW Jr, et
al: Epicardial administration of ibutilide from polyurethane
matrices: effects on defibrillation threshold and
electrophysiologic parameters. J Cardiovasc Pharmacol. 24:826–840.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saffitz JE, Green KG, Kraft WJ, et al:
Effects of diminished expression of connexin 43 on gap junction
number and size in ventricular myocardium. Am J Physiol Heart Circ
Physiol. 278:H1662–H1670. 2000.PubMed/NCBI
|
|
13
|
Kwong KF, Schuessler RB, Green KG, et al:
Differential expression of gap junction proteins in the canine
sinus node. Circ Res. 82:604–612. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Xue Q, Ma J, et al: Effects of
imidapril on heterogeneity of action potential and calcium current
of ventricular myocytes in infarcted rabbits. Acta Pharmacol Sin.
25:1458–1463. 2004.PubMed/NCBI
|
|
15
|
Saffitz JE and Kléber AG: Gap junctions,
slow conduction, and ventricular tachycardia after myocardial
infarction. J Am Coll Cardiol. 60:1111–1113. 2012.PubMed/NCBI
|
|
16
|
Callans DJ, Moore EN and Spear JF: Effect
of coronary perfusion of heptanol on conduction and ventricular
arrhythmias in infarcted canine myocardium. J Cardiovasc
Electrophysiol. 7:1159–1171. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li F, Sugishita K, Su Z, et al: Activation
of connexin-43 hemichannels can elevate [Ca2+]i and
[Na+]i in rabbit ventricular myocytes during metabolic
inhibition. J Mol Cell Cardiol. 33:2145–2155. 2001.PubMed/NCBI
|
|
18
|
Sánchez JA, Rodríguez-Sinovas A,
Fernández-Sanz C, et al: Effects of a reduction in the number of
gap junction channels or in their conductance on
ischemia-reperfusion arrhythmias in isolated mouse hearts. Am J
Physiol Heart Circ Physiol. 301:H2442–2453. 2011.
|
|
19
|
Wit AL and Peters NS: The role of gap
junctions in the arrhythmias of ischemia and infarction. Heart
Rhythm. 9:308–311. 2012. View Article : Google Scholar : PubMed/NCBI
|