Introduction
Arachis hypogaea L. stem and leaf extract
(AHSLE) is a type of sleep aid used in China (1–3).
γ-aminobutyric acid (GABA) receptors are known to play an important
role in the modulation of barbiturate-induced sleep through
interaction with GABAergic systems (4,5). We
previously reported (6) that AHSLE
may lead to a significant decrease of glutamate/GABA ratio in
corresponding brain areas in freely behaving rats. These findings
raised the questions of whether AHSLE results in external
manifestations of rat sleep behavior and whether AHSLE modulates
barbiturate-induced sleep through GABAergic channels.
In this study, in order to evaluate the sedative
effects of AHSLE on rats and investigate its possible mechanisms of
action, male Sprague-Dawley (SD) rats were employed and sleep was
induced by pentobarbital. Sleep time and sleep latency were
recorded following AHSLE administration. Muscimol, a GABA type A
(GABAA) receptor agonist, and flumazenil, a
GABAA receptor antagonist, were used as positive
controls, respectively.
Materials and methods
Plant, AHSLE and reagents
The Arachis hypogaea L. plants were collected
from the shores of lake Yezi (Wuhan, Hubei, China) in August, 2011.
The plant was authenticated by the Agriculture and Forestry
Research Center of Tsukuba University, Japan. AHSLE powder was
obtained as previously described (6,7),
comprising 1.92% protein and 65.31% carbohydrate. All the reagents
used were of the highest available purity. Pentobarbital sodium was
purchased from Rejuvenation Pharmaceutical Co., Ltd., (Fuzhou,
China). Muscimol was purchased from Sigma (St. Louis, MO, USA).
Flumazenil and other chemicals used were purchased from Wako Pure
Chemical Industries, Ltd., (Osaka, Japan).
Animals
Male SD rats, aged 8 weeks and weighing 270±30 g,
were provided by the Laboratory Animal Resource Center, University
of Tsukuba, Japan. The animals were housed at ambient conditions of
25°C, with 12-h light/dark cycles (light on at 08:00, light off at
20:00) and were given ad libitum access to food and water.
All the animal experiments were conducted humanely, following
approval from the Institutional Animal Experiment Committee of
Tsukuba University, Japan and in accordance with the regulations
for Animal Experiments and Fundamental Guidelines under the
jurisdiction of the Japanese Ministries of Education, Culture,
Sports, Science and Technology.
Experimental protocols
The experiments were performed between 20:00 and
24:00. Rats (n=48) were employed and habituated in an animal lab
for at least 7 days, then randomly divided into groups. In the 4
experiments, the number of rats in each group was 12, 9–12, 12 and
8, respectively. All the rats were reused after 2 weeks. The
animals were fasted for 24 h prior to the experiment. AHSLE in
physiological saline and flumazenil in 10% dimethyl sulfoxide were
administered orally to the rats, whereas muscimol was administered
intraperitoneally (i.p.). At 30 min following the administration of
muscimol, flumazenil or AHSLE, pentobarbital sodium in
physiological saline was administered i.p. to each rat to induce
sleep. The animals that remained immobile for >1 min were
considered to be asleep. The sleep latency was recorded from the
pentobarbital injection to 1 min after the loss of the righting
reflex and the sleep time was recorded from 1 min after the loss of
the righting reflex to recovery (8).
Statistical analysis
The obtained data were analyzed using two-tailed
Student’s t-test (version 2003, Microsoft excel) for comparisons
and the results are expressed as means ± SD. P<0.05 was
considered to indicate statistically significant differences.
Results
Sedative effects of pentobarbital, AHSLE
and muscimol
The results (Table
I) revealed that pentobarbital induced sleep at a dosage of 50
mg/kg. Muscimol was unsuccessful in inducing sleep. AHSLE alone,
similar to muscimol, was unable to induce sleep directly, even at a
high dose (500 mg/kg).
 | Table IEffects of pentobarbital, AHLAE and
muscimol on rats. |
Table I
Effects of pentobarbital, AHLAE and
muscimol on rats.
| Groups | Dose (mg/kg) | No. falling
asleep/total | Sleep latency
(min) | Sleep time (min) |
|---|
| Pentobarbital | 30 | 5/12 | 31.67±1.15 | 41.33±1.53 |
| Pentobarbital | 50 | 12/12 | 15.33±2.52 | 48.33±9.07 |
| AHSLE | 500 | 0/12 | 0 | 0 |
| Muscimol | 10 | 0/12 | 0 | 0 |
Effects of AHSLE on sleep in
pentobarbital-treated rats
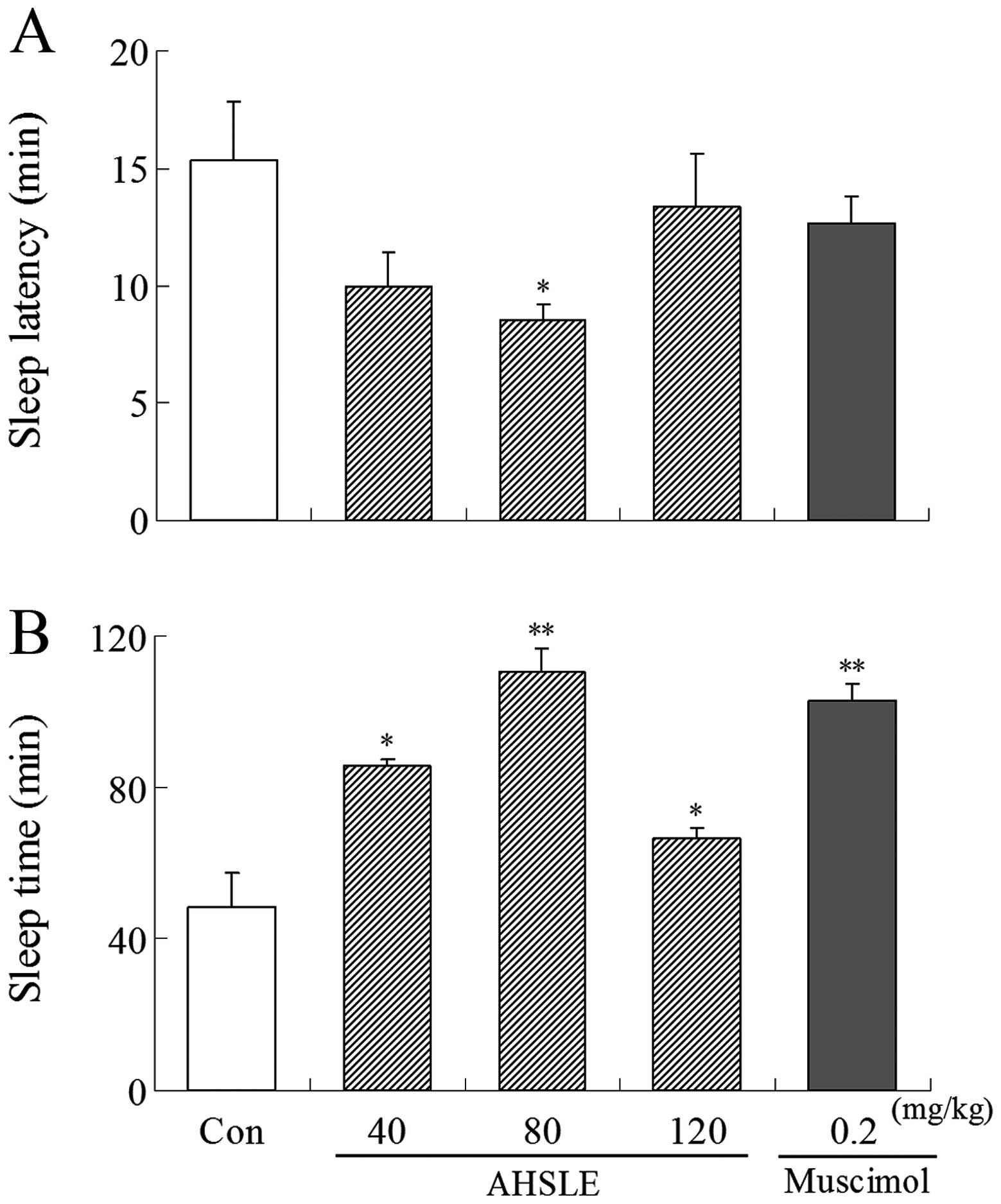
Following administration of muscimol or AHSLE,
pentobarbital (50 mg/kg) was administered i.p. to the rats. AHSLE
decreased sleep latency and prolonged sleep time in rats,
particularly at a dose of 80 mg/kg, following sleep induction by
pentobarbital. Muscimol (0.2 mg/kg) as a positive control also
induced a decrease in sleep latency and a prolongation of the total
sleep time (Fig. 1).
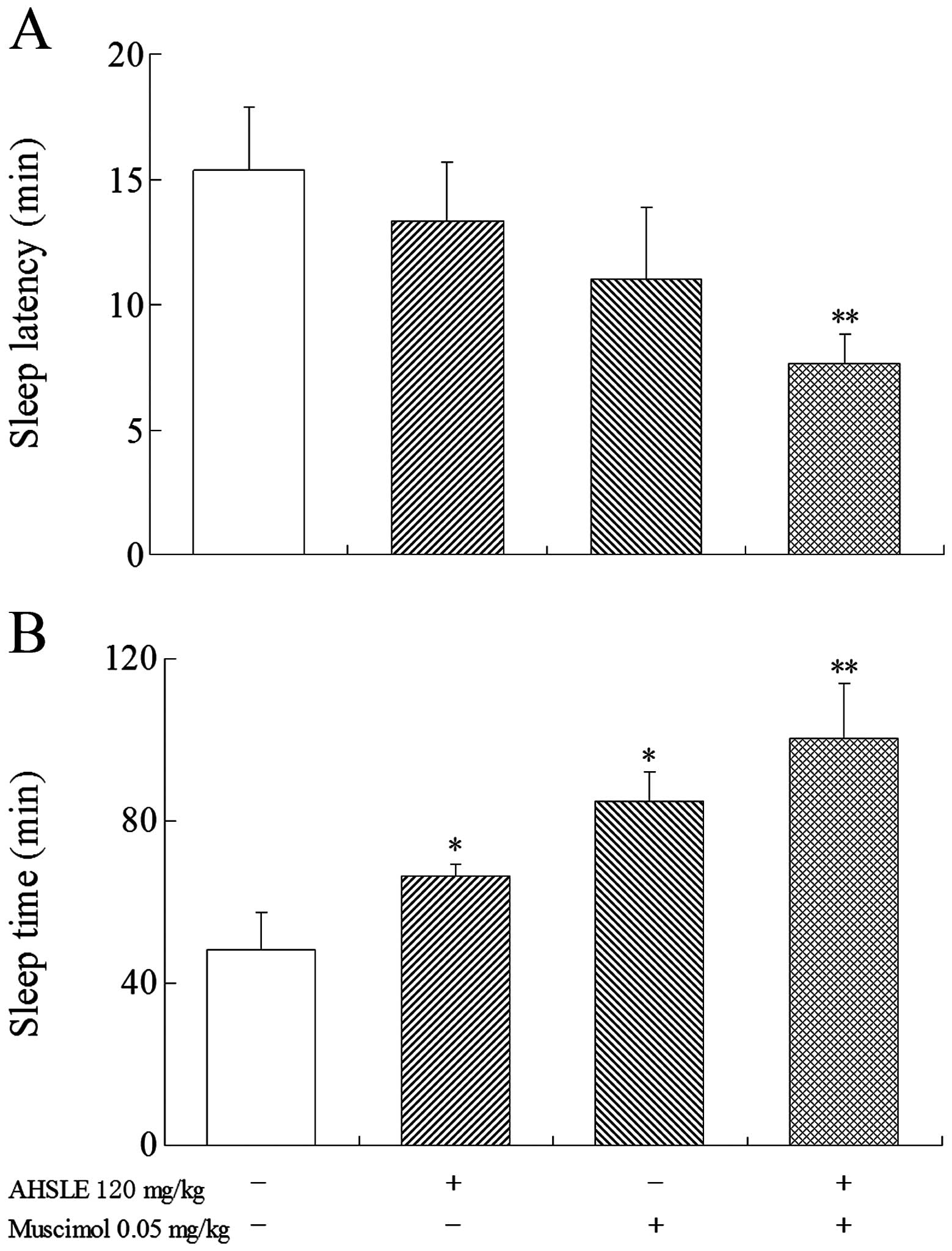
Effects of AHSLE with muscimol on sleep
in pentobarbital-treated rats
Prior to the administration of pentobarbital (50
mg/kg), low doses of muscimol (0.05 mg/kg) were used in the AHSLE
(120 mg/kg)-treated groups (Fig. 2)
to observe the direct association between the effects of muscimol
and those of AHSLE. The results demonstrated that the
pentobarbital-treated rats in the AHSLE+muscimol group exhibited
significantly (P<0.01) increased sleep time and reduced sleep
latency.
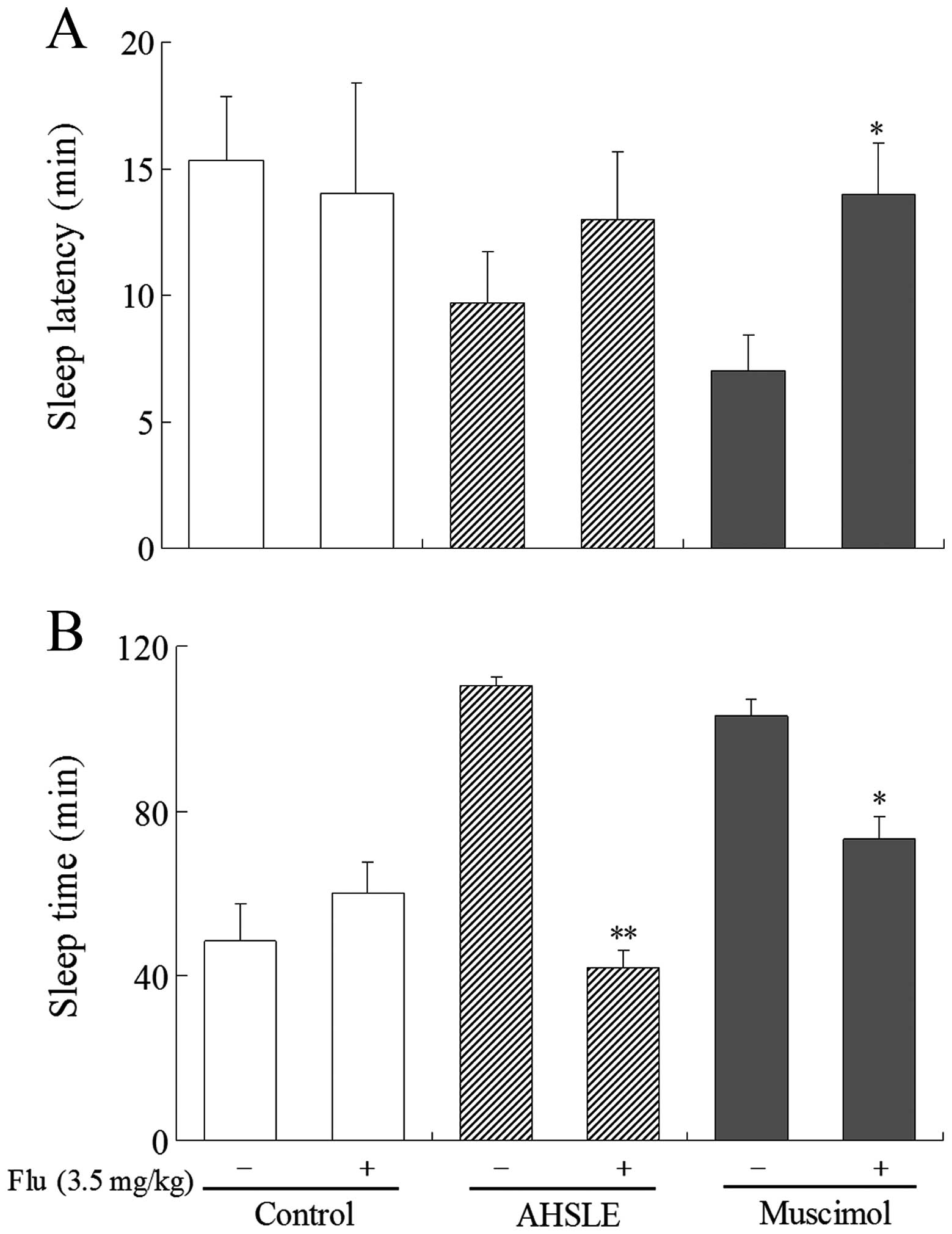
Effects of AHSLE with flumazenil on sleep
in pentobarbital-treated rats
In pentobarbital (50 mg/kg)-treated rats, AHSLE
decreased sleep latency and also significantly prolonged sleep time
at doses of 80 mg/kg (Fig. 1A and
B). These actions were significantly antagonized by flumazenil,
a benzodiazepine receptor antagonist, at a dose of 3.5 mg/kg
(Fig. 3A and B). This antagonistic
effect of flumazenil was also observed in rats that were
administered muscimol.
Discussion
GABA is a type of inhibitory neurotransmitter amino
acid in the brain, is primarily formed from glutamate via the
action of glutamate decarboxylase (9) and may be used as a parameter to
characterize the sleep-wake cycle (10). Pentobarbital is known to potentiate
the effects of GABA through acting on the receptor sites of the
GABA/benzodiazepine receptor-ionophore complex (11). The sedative effects may be assessed
by decreases in the pentobarbital-induced sleep time and are
possibly mediated through GABAergic systems (12). In this study, we aimed to determine
whether sleep enhancement following AHSLE administration is
mediated via the GABAergic systems.
The effects of different doses of AHSLE on rats with
or without pentobarbital treatment were investigated. We observed
that AHSLE was able to prolong pentobarbital-induced sleep at a
lower dose (40 mg/kg); however, it was unable to induce sleep
alone, even at a significantly higher dosage (500 mg/kg), which was
similar to the effects of the GABAA receptor agonist
muscimol. Moreover, AHSLE acted synergistically with muscimol in
pentobarbital-treated rats. It was indicated that GABAA
receptor channels may be involved in the AHSLE sedative effect
pathways.
Previous studies demonstrated that flumazenil
decreases sleep time and increases waking and sleep latency through
suppressing the effects of GABAA receptors (13,14).
To further investigate the mechanisms underlying the potentiation
of sedation caused by AHSLE, the effects of flumazenil on sleep
induced by AHSLE in pentobarbital-treated rats were investigated.
Flumazenil exerted no obvious effect when used alone, whereas it
exhibited a significant antagonistic effect to that of AHSLE in
pentobarbital-treated rats. These results demonstrated that the
synergistic effects of AHSLE with pentobarbital or muscimol may
antagonize the effects of flumazenil, a GABAA receptor
antagonist. These findings also suggested that the activation of
GABAergic systems induced by AHSLE may potentiate the activity of
sedative agents.
We analyzed the essential oil components of AHSLE
according to previous studies (15,16)
and found that it contained linalool (7), which may contribute to the sedative
effects of AHSLE. The role of linalool requires validation by
further experiments.
In conclusion, our results indicated that AHSLE
enhanced the sedative effects in pentobarbital-treated rats and
these effects were possibly mediated through GABA-gated channels
and benzodiazepine receptors. AHSLE may be a candidate agent for
the management of sleep disorders. However, the pharmacological
effects of AHSLE derivatives, such as linalool, require further
investigations.
References
|
1
|
Wang QC, Xu J and Shi M: Clinical efficacy
of Groundnut leaves on insomnia treatments. Shanghai J Trad Chin
Med. 5:8–10. 2001.
|
|
2
|
Hu PF, Fan RP, Li YP and Pang CY: Studies
on pharmacological action of Luohuashengzhiye extract. Chin Trad
Pat Med. 23:919–920. 2001.(In Chinese).
|
|
3
|
Wang YF, Li HF, Xu YF, Zhang YL, Xu DS,
Xiao LM and Li XM: Clinical confirmation of preparation from the
branch and leaf of peanut in treating insomnia. Shanghai J Trad
Chin Med. 5:11–14. 2001.(In Chinese).
|
|
4
|
Doghramji PP: Trends in the pharmacologic
management of insomnia. J Clin Psychiatry. 67:5–8. 2006.
|
|
5
|
Ma Y, Han H, Eun JS, Kim HC, Hong JT and
Oh KW: Sanjoinine A isolated from Zizyphi Spinosi Semen augments
pentobarbital-induced sleep behaviors through the modification of
GABA-ergic systems. Biol Pharm Bull. 30:1748–1753. 2007. View Article : Google Scholar
|
|
6
|
Zu XY, Zhang ZY, Liu JQ, Hu HH, Xing GQ,
Zhang Y and Guan D: Sedative effects of peanut (Arachis
hypogaea L.) leaf aqueous extracts on brain ATP AMP, adenosine
and glutamate/GABA of rats. J Biomed Sci Eng. 3:268–273. 2010.
|
|
7
|
Zu XY, Zhang ZY, Xiong GQ, Liao T, Qiao Y,
Li YT, Geng SR and Li X: Sedative effects of Arachis
hypogaea L. stem and leaf extracts on sleep-deprived rats. Exp
Ther Med. 6:601–605. 2013.
|
|
8
|
Chu QP, Wang LE, Cui XY, Fu HZ, Lin ZB,
Lin SQ and Zhang YH: Extract of Ganoderma lucidum
potentiates pentobarbital-induced sleep via a GABAergic mechanism.
Pharmacol Biochem Behav. 86:693–698. 2007.
|
|
9
|
Bown AW and Shelp BJ: The metabolism and
functions of (gamma)-aminobutyric acid. Plant Physiol. 115:1–5.
1997.PubMed/NCBI
|
|
10
|
Schousboe A, Westergaard N, Sonnewald U,
Petersen SB, Yu AC and Hertz L: Regulatory role of astrocytes for
neuronal biosynthesis and homeostasis of glutamate and GABA. Prog
Brain Res. 94:199–211. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Sousa FC, Pereira BA, Lima VT, Lacerda
CD, Melo CT, Barbosa-Filho JM, Vasconcelos SM and Viana GS: Central
nervous system activity of yangambin from Ocotea duckei
Vattimo (Lauraceae) in mice. Phytother Res. 19:282–286.
2005.PubMed/NCBI
|
|
12
|
Ebert B, Thompson SA, Saounatsou K,
McKernan R, Krogsgaard-Larsen P and Wafford KA: Differences in
agonist/antagonist binding affinity and receptor transduction using
recombinant human gamma-aminobutyric acid type A receptors. Mol
Pharmacol. 52:1150–1156. 1997.
|
|
13
|
Gaillard JM and Blois R: Differential
effects of flunitrazepam on human sleep in combination with
flumazenil. Sleep. 12:120–132. 1989.PubMed/NCBI
|
|
14
|
Borbély AA, Akerstedt T, Benoit O,
Holsboer F and Oswald I: Hypnotics and sleep physiology: a
consensus report. European Sleep Research Society, Committee on
Hypnotics and Sleep Physiology. Eur Arch Psychiatry Clin Neurosci.
241:13–21. 1991.PubMed/NCBI
|
|
15
|
Guzman-Gutierrez SL, Gomez-Cansino R,
Garcia-Zebadua JC, Jimenez-Perez NC and Reyes-Chilpa R:
Antidepressant activity of Litsea glaucescens essential oil:
identification of β-pinene and linalool as active principles. J
Ethnopharmacol. 143:673–679. 2012.
|
|
16
|
Jalali Heravi M and Sereshti H:
Determination of essential oil components of Artemisia
haussknechtii Boiss. using simultaneous
hydrodistillation-static headspace liquid phase microextraction-gas
chromatography mass spectrometry. J Chromatogr A. 1160:81–89.
2007.PubMed/NCBI
|