Introduction
Diabetic nephropathy (DN) is a progressive kidney
disease and a major debilitating complication of type 1 and type 2
diabetes that can lead to chronic kidney disease (CKD) and related
cardiovascular disorders. Proteinuria is an early clinical
manifestation of diabetic nephropathy, resulting in rapid
progression of renal disease with initial development of
pathological features of glomerulosclerosis and tubulointerstitial
fibrosis (1). Thus, it is crucial
to develop methods to arrest or retard glomerulosclerosis and
tubulointerstitial fibrosis.
Epithelial-mesenchymal transition (EMT) plays a
pivotal role in organ fibrosis, including that of kidney (2,3). It
has been reported that a large proportion of the interstitial
fibroblasts in fibrotic kidneys originate from proximal tubular
cells (4). EMT involves a series of
changes through which epithelial cells lose their epithelial
characteristics and acquire properties typical of mesenchymal
cells. EMT facilitates cell movement and the generation of new
tissue types during development and contributes to the pathogenesis
of disease. Transduction of EMT of tubular cells into α-smooth
muscle actin (α-SMA)-expressing myofibroblasts is a central
mechanism in tubulointerstitial fibrosis. The expression of α-SMA
occurs only in the vascular smooth muscle cells of the normal
kidney. The presence of α-SMA in mesangial, renal tubular
epithelial and other inherent cells, would indicate that EMT has
occurred in the site of the lesion. Synthesis and secretion of ECM
with myfibroblastic characteristics are initiated via inherent
cells (4).
Angiotension-converting enzyme inhibitors (ACEIs)
are used to retard the progression of fibrosis in diabetic
nephropathy and renal failure. Data from the
Angiotensin-Converting-Enzyme Inhibition in Progressive Renal
Insufficiency (AIPRI) trial and The Raminipril Efficacy In
Nephropathy (REIN) study showed that ACEIs are capable of
significantly retarding the rate of decline in renal function
(5,6). A review based on a series of studies
including the REIN study, concluded that urinary protein is one of
the main mediators of glomerular damage to the tubulointerstitium,
and that ACEIs can confer renoprotection via a reduction in urinary
protein excretion in progressive kidney diseases (7). ACEIs have also been shown to slow the
progression of nephropathy in type 1 diabetes patients, although
the effects of ACE inhibition remain inconclusive, possibly due to
its heterogeneous nature (8).
In the present study, we evaluated the efficiency of
benazepril, a type of ACEI, mitigating EMT in a streptozocin
(STZ)-induced DM/N rat model, and clarified the exact mechanism of
benazepril in protecting the kidney.
Materials and methods
Experimental animals
In total, 30 healthy adult male Sprague-Dawley rats
(SD rats, clean grade), weighing 190–200 g were included in the
present study. The rats were purchased from the Beijing Vital River
Laboratory Animal Technology Company and reared in the Qilu
Hospital of Shandong University Experimental Animal Center. The
animals were provided the standard diet and water ad
libitum, and were housed at a temperature of 21±1°C, with
60–70% relative humidity and 12-h light/dark cycle.
Drugs and reagents
Benazepril hydrochloride (Lotensin, Beijing
Novartis, Pharma Ltd., Beijing, China) was equipped with distilled
water to a appropriate concentration prior to application. STZ
(Sigma, St. Louis, MO, USA), mouse anti-rat α-smooth muscle actin
(α-SMA) monoclonal antibody (Abcam, Cambridge, UK), biotin-labeled
goat anti-mouse IgG, goat anti-mouse secondary antibody kit
(Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
and ECL luminescent liquid and PVDF membrane (Millipore
Corporation, Billerica, MA, USA) were prepared.
Establishment of STZ-induced DN rat
model
All 30 rats were fed for one week and the experiment
began after 12 h of fasting. Eight rats were randomly selected as
the control group (N group), and the remaining 22 rats were
injected with STZ with 60 mg/kg once in the lower left abdominal
region. Prior to use, STZ was prepared as 1% concentration with
citrate-sodium citrate buffer solution (0.1 mol/l, pH 4.2). The
rats in the N group were injected with an equivalent citrate-sodium
citrate buffer. After 72 h, three consecutive random blood glucose
(BG) samples were obtained from tail vein of rats and measured.
When BG was >16.7 mmol/l, the diabetes model was considered
successful (9).
Randomization and treatment
Twenty-two rats from the successful diabetes model
were randomly divided into the diabetic (DM group, n=11) and
benazepril (B group, n=11) groups. After four weeks, the rats were
gavaged once daily. Rats in the B group received 10 mg/kg/day
benazepril, and rats in the N and DM groups were treated with the
same amount of distilled water. All 22 rats were gavaged for 8
weeks. The animals were provided the standard diet and water ad
libitum without insulin and antidiabetic drugs throughout the
experimental period.
Specimen collection
Random BG obtaine from the tail vein of rats and
body weight (BW) were measured weekly. The rats were placed in
metabolic cages in order to collect 24-h urine volume one day prior
to sacrifice. The urine specimens were centrifuged and placed in a
freezer at −20°C and the urine protein was measured. Prior to
sacrifice, the animals were anesthetized with 4 ml/kg 10% chloral
hydrate by intraperitoneal injection (10). Subsequently, blood was collected
from the inferior vena cava and centrifuged at 4°C to obtain serum
which was stored at −80°C for the measurement of BG, serum
creatinine (SCr), and blood urea nitrogen (BUN). The left ventricle
was injected with pre-chilled 4°C saline, the right atrium was
cleaved and drained, and the kidneys were repeatedly lavaged until
the entire kidney was pale in color prior to stripping and removing
of the capsule. Saline (0.9%) was applied to the kidney without via
syringe. The kidney was dried with filter paper and weighed.
Subsequently, it was cut along the coronal plane, placed in 10%
neutral formalin solution, fixed, and maintained for
histopathological and immunohistochemical examination.
The remaining kidney tissue was placed in liquid
nitrogen, frozen at −80°C in a freezer and subsequently used for
the western blot analysis.
Pathology assessment
Thirty non-overlapping tubulointerstitial visions
(no glomerular and vascular) with PAS staining at high
magnification (x400) were randomly selected to determine the
tubulointerstitial damage index (TII) (11), namely known as a percentage of the
tubulointerstitial damage area to the total same vision area.
Scoring was performed as follows: 0, normal; 1 point: <25%; 2
points: 25–50%; 3 min: >50%.
Immunohistochemical measurement
The expression of α-SMA was measured using an SABC
assay (12). Pathological slices of
renal tissue were dewaxed, heated in a microwave at 92–95°C for 15
min, and fixed with 0.1 M citrate buffer (pH 6.0). Then, 3%
hydrogen peroxide was used to develop biotin and peroxidase
inactivation. Renal tissue was then added to goat serum blocking
solution, incubated for 30 min at 37°C, and mouse anti-rat α-SMA
(1:50) monoclonal antibody was added prior to incubation at 4°C
overnight. The following day, biotinylated secondary antibody and
streptavidin working solution were added dropwise, and DAB staining
was visualized using a Nikon epifluorescence E600 microscope. Renal
tissue slices were restained with hematoxylin, dehydrated and
differentiated using hydrochloric acid alcohol, mounted with
neutral gum. PBS was used as a negative control instead of the
primary antibody. Staining was considered positive when granules
were brown. Immunohistochemical image analysis was performed as
follows: tubulointerstitial-positive regional optical density
[D(λ)] was calculated by image analysis software (Leica Imaging
systems Ltd., Cambridge, UK) in each slice and the mean value was
adopted (13).
Western blot analysis
RIPA lysate (1 ml) was added to kidney tissue (100
mg) and homogenized. The homogenates were centrifuged at 4°C for
9,180 × g for 5 min, and the supernatant was collected to measure
the protein concentration. Then, 50 mg sample was added to the
sample buffer and boiled for 5 min. After SDS-PAGE (12%
electrophoresis gel, 6% stacking gel), the sample was transferred
to a PVDF membrane and incubated for 1.5 h in 5% skimmed milk at
room temperature. Mouse anti-rat α-SMA (1:250) monoclonal antibody
was then added at 4°C after washing the membrane. The following
day, the sample was incubated with HRP-conjugated secondary
antibody (1:10,000) at room temperature, 1 h, after washing the
membrane again. ECL luminescence was performed, and the X-ray film
was developed and fixed following modereate exposure. Image J
analysis system (National Institute of Mental Health, Bethesda, MD,
USA) was used to scan the hybridization signals on optical density.
β-actin was applied as a protein control and the mean value was
measured by comparing the other groups to obtain the relative
amounts.
Statistical analysis
Samples were assessed using SPSS17.0 statistical
software. Measurement data were expressed as mean ± SD. Groups were
compared using ANOVA and pairwise using LSD test. P<0.05 was
considered statistically significant.
Results
General
In the N group, normal state of drinking water, fur
color and mental status was maintained. However, in the DM group,
the model rats exhibited polydipsia, polyphagia and polyuria.
Extension of the course gray fur, weight loss, slow reaction and
other symptoms were also observed in rats of the DM group. Seven
rats died during the course of the experiment, 4 in the DM group
and 3 in the N group.
General index
The level of BG , kidney weight/body weight, 24-h
urine protein, SCr and BUN were significantly higher in the DM
group compared to that in the N group (p<0.01). However, body
weight was significantly lower in the DM group compared to that in
the N group. In addition to BG and body weight, the remaining
indicators were significantly lower in the B group compared to the
DM group (p<0.01) (Table I).
 | Table IGeneral index in each group (mean ±
SD). |
Table I
General index in each group (mean ±
SD).
| Group | n | BG (mmol/l) | BW (g) | KW/BW
(×10−3) | 24-h urine protein
(mg/24 h) | SCr (μmol/l) | BUN (mmol/l) |
|---|
| N | 8 | 7.83±0.45 | 245.37±18.55 | 4.23±0.18 | 9.18±0.81 | 20.73±1.87 | 5.67±1.52 |
| DM | 7 | 26.38±3.41a | 178.67±5.03a | 8.78±0.16a | 7.10±3.58a | 83.89±2.09a | 25.23±3.58a |
| B | 8 | 25.25±2.54 | 176.34±4.46 | 6.12±0.23b | 43.58±3.68b | 54.89.±4.14b | 12.36±2.83b |
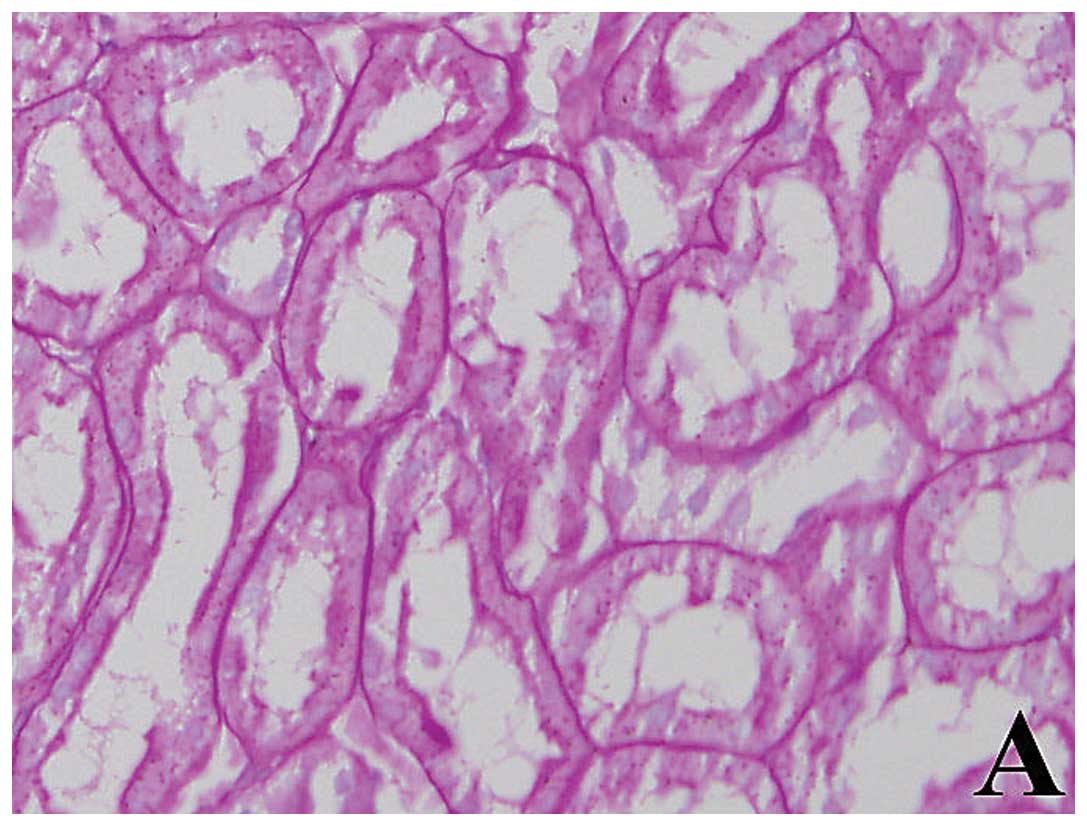
Pathological changes
In the N group, there was clear structure without
any renal tubulointerstitial disease in kidney tissue with PAS
staining by light microscopy. But in the DM group, significant
expansion of tubules, tubular epithelial cell granules
degeneration, basement membrane thickening, and inflammatory cells
infiltration increasing in interstitial were seen in kidney tissue
with PAS staining by light microscopy. The above-mentioned lesions
in group B were significantly reduced compared with the DM group.
Results of the statistical analysis revealed that TII in the DM
group was significantly higher than that in the N and B groups (all
p<0.01) (Fig. 1, Table II).
 | Table IIExpression of TII and α-SMA in renal tubulo-interstitium [D(λ),
(mean ± SD)]. |
Table II
Expression of TII and α-SMA in renal tubulo-interstitium [D(λ),
(mean ± SD)].
| Group | No. | TII | α-SMA |
|---|
| N | 8 | 0±0 | 0.52±0.27 |
| DM | 7 | 2.7±0.5a | 16.23±1.67a |
| B | 8 | 1.5±0.3b | 8.23±0.69b |
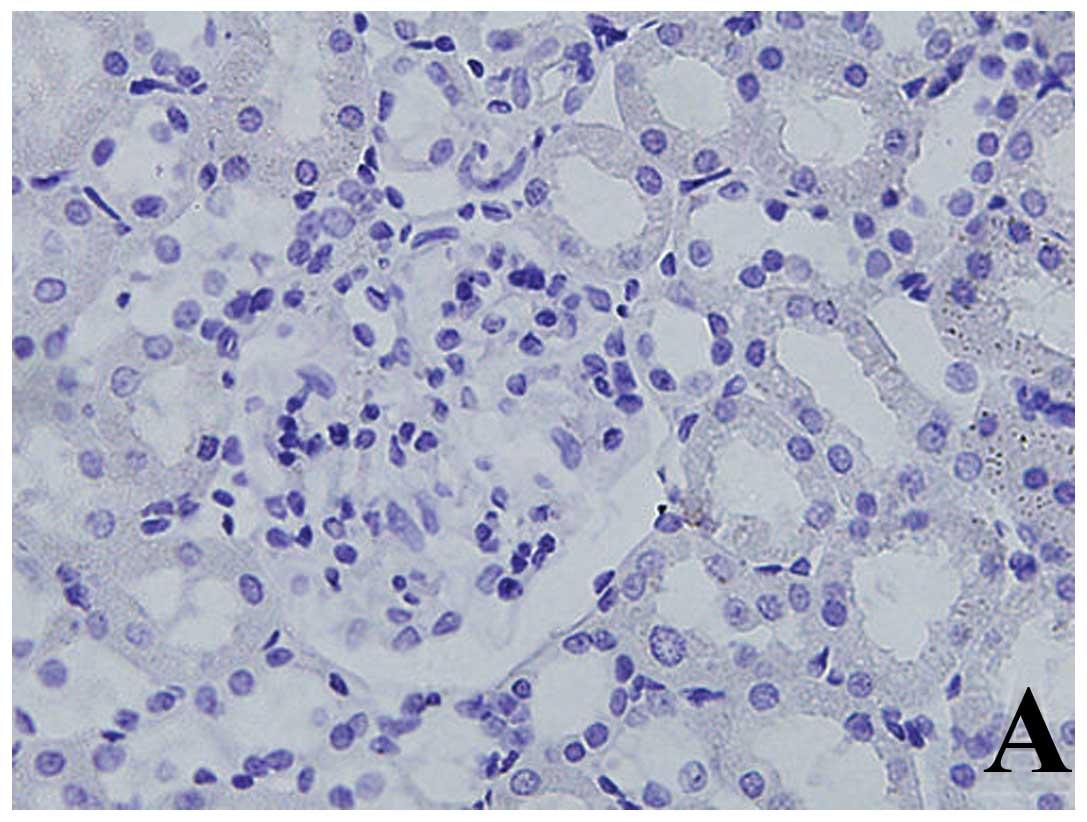
Expression of α-SMA by
immunohistochemistry
In group N, the expression of α-SMA was only
observed on smooth muscle cells of the vessel wall in renal
tubular-interstitium. However, α-SMA was mainly expressed in the
renal tubular epithelial cells and interstitial tubules in addition
to the vessel wall in the DM group, and significantly increased
compared with the N group (p<0.01). Expression of α-SMA was
significantly reduced in the B group compared with the DM group
(p<0.01) (Table II and Fig. 2).
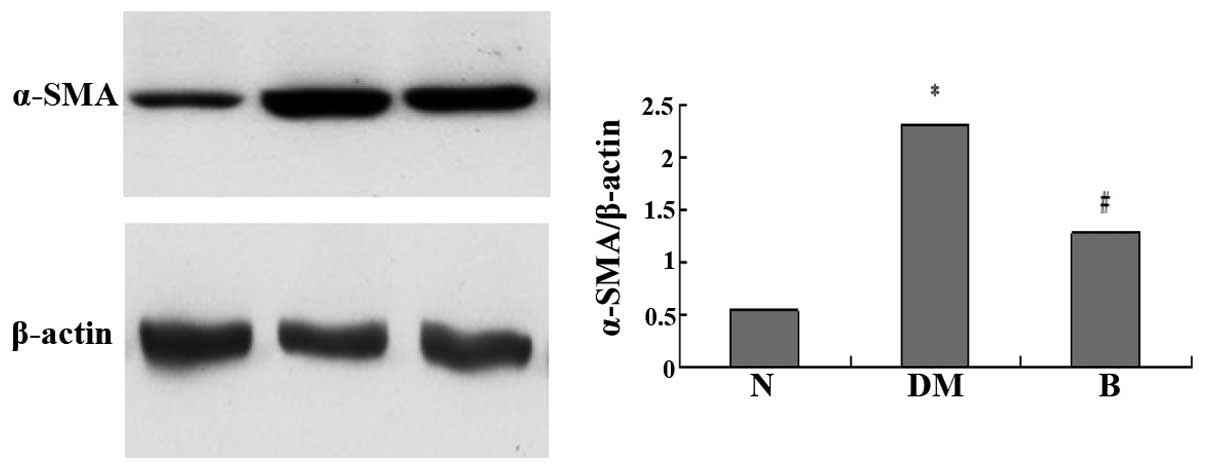
Expression of α-SMA by western
blotting
Analysis of the optical density of bands by western
blotting showed that the expression of α-SMA in renal tissue
increased 3.27-fold in the DM group compared with the N group, and
increased by 1.22-fold in the DM group compared with the B group
(Fig. 3).
Discussion
In the present study, the STZ-induced diabetic rat
model was successfully produced by a single intraperitoneal
injection. At the 12th week of experiment, 24-h urine protein and
SCr of diabetic model rats showed a marked increase. The main
pathological changes of the kidney were glomerular hypertrophy,
mesangial matrix increasing, tubular epithelial granule cells and
degeneration, tubular dilation and irregular thickening of the
basement membrane. Small focal mononuclear-macrophage infiltration
was evident in tubulointerstitium. These changes indicated that the
STZ-induced diabetic rat model was successful.
Most previous studies have focused on glomerular
lesions in diabetic nephropathy (DN), while other studies have
focused on tubulointerstitial injury (14). It has been shown that while the
glomerular filtration membrane changes in DN, tubulointerstitial
lesions have been previously described (15). The results of this study have shown
that tubulointerstitial injury is not entirely dependent on the
glomerular lesions and is itself an independent factor of DN. There
is a close correlation between the serious extent of
tubulointerstitial lesions, degree of urinary protein excretion and
renal function deterioration under high glucose conditions, which
directly affect the prognosis of DN. Therefore, strengthening the
study of renal tubular interstitial lesion of DN is of clinical
significance.
Renal interstitial fibrosis (RIF) is the final
outcome of DN (16). Studies have
shown that EMT is a key link in the process of RIF, and is one of
the initial aspects of occurrence and development of RIF (17). Studies confirmed that in the chronic
renal interstitial fibrosis model, myofibroblast cells from the
transdifferentiation of tubular epithelial cells accounted for 36%
of all myofibroblasts in renal interstitium. Inhibiting or
reversing EMT in delaying the progression of DN is of clinical
significance (4).
α-SMA has been widely used to detect EMT as a marker
protein of myofibroblasts (18).
There are almost no myofibroblasts in normal kidney tissue, and the
expression of α-SMA is only evident in the vascular smooth muscle
cells of kidney. When α-SMA is expressed in glomerular mesangial
and renal tubular epithelial cells, EMT has occurred. These
intrinsic cells began to synthesize and secrete extracellular
matrix (ECM), which possess the characteristics of myofibroblasts
(19). In the present study, the
expression of α-SMA was significantly increased by
immunohistochemistry and western blotting in the 12th week, mainly
in renal tubular epithelial cells, consistent with the
tubulointerstitial damage area in tubulointerstitium of DM model
rats, with TII being significantly increased as compared to the N
group. These results showed that some tubular epithelial cells
already express α-SMA and that EMT has occurred in the early stages
of DN, with myofibroblast phenotypic characteristics.
Renin-angiotensin system (RAS) and its receptor are
active in the diabetic state, and there is an excess of angiotensin
II (Ang II) generation in local tissue of kidney. Ang II leads to
abnormalities of renal blood flow dynamics in diabetic state and
participates in tubulointerstitial fibrosis in a non-hemodynamic
manner (20). By RAS and AT1
receptors, Ang II stimulates renal tubular epithelial cell
hypertrophy, induces the synthesis of TGF-β1, and generates
interstitial fibroblasts and tubular epithelial cells into
myofibroblasts. Consequently, the generation of ECM increases,
degradation decreases and ultimately the result is
tubulointerstitial fibrosis. In addition, Ang II, as an
inflammatory cytokine, can cause an inflammatory initiation factor,
nuclear factor-κB (NF-κB), to activate and shift in the early
stages of DN, leading to ICAM-1, MCP-1, OPN, other cytokines and an
increase in adhesion molecule expression, which recruits a large
number of inflammatory cell infiltration to glomerular and
tubulointerstitium (21).
Expression of TGF-β1, CTGF and other factors increased because of
the role of inflammation in the kidney, and at the same time
fibroblasts proliferate and transdifferentiated into
myofibroblasts. Yang et al also confirmed that Ang II
significantly increased the ability of TNF-β1-induced tubular
epithelial cells to transdifferentiate into myofibroblasts
(22).
As a typical representative of ACEI drugs,
benazepril has been widely used in the treatment of DN due to
decreasing blood pressure and proteinuria. The present study showed
that at end of the 12th week, kidney weight/body weight, 24-h
urinary protein, SCr, BUN and TII were significantly ameliorated in
the B group compared with the DM group, and α-SMA expression was
significantly reduced in the B group compared with the DM group.
The results of present study show that benazepril exerted
protective effects on DN, which may be associated with the
inhibition of excessive α-SMA expression and renal tubular EMT in
diabetic kidney tissue.
In conclusion, the present study has shown that
benazepril ameliorates renal structural and functional damage in
diabetic rats. The mechanism involved may be associated with the
inhibition of renal tubular epithelial cell transdifferentiation,
which can reduce tubulointerstitial fibrosis. However, the detailed
mechanism involved remains to be clarified.
Acknowledgements
This study was supported by the Shandong Province
Outstanding Young Scientist Research Award Fund Project, no.
BS2013YY042.
References
|
1
|
Srivastava SP, Koya D and Kanasaki K:
MicroRNAs in kidney fibrosis and diabetic nephropathy: roles on EMT
and EndMT. Biomed Res Int. 2013:1254692013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lei P, Jiang Z, Zhu H, Li X, Su N and Yu
X: Poly(ADP-ribose) polymerase-1 in high glucose-induced
epithelial-mesenchymal transition during peritoneal fibrosis. Int J
Mol Med. 29:472–478. 2012.PubMed/NCBI
|
|
4
|
Iwano M, Plieth D, Danoff TM, Xue C, Okada
H and Neilson EG: Evidence that fibroblasts derive from epithelium
during tissue fibrosis. J Clin Invest. 110:341–350. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
The GISEN Group. Randomized
placebo-controlled trial of effect of ramipril on decline in
glomerular filtration rate and risk of terminal renal failure in
proteinuric, non-diabetic nephropathy. Lancet. 349:1857–1863. 1997.
View Article : Google Scholar
|
|
6
|
Maschio G, Alberti D, Janin G, Locatelli
F, Mann JF, Motolese M, Ponticelli C, Ritz E and Zucchelli P:
Effect of the angiotensin-converting-enzyme inhibitor benazepril on
the progression of chronic renal insufficiency. The Angiotensin
Converting Enzyme Inhibition in Progressive Renal Insufficiency
Study Group. N Engl J Med. 334:939–945. 1996. View Article : Google Scholar
|
|
7
|
Peng T, Hu Z, Xia Q, Jiang B, Li X and
Yang X: A comparative study of the renoprotective effects of
benidipine and valsartan in primary hypertensive patients with
proteinuria. Arzneimittelforschung. 59:647–650. 2009.PubMed/NCBI
|
|
8
|
Kanno Y, Okada H, Yamaji Y, Nakazato Y and
Suzuki H: Angiotensin-converting-enzyme inhibitors slow renal
decline in IgA nephropathy, independent of tubulointerstitial
fibrosis at presentation. QJM. 98:199–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Wu F, Zheng F and Li H:
Adenovirus-mediated decorin gene transfection has therapeutic
effects in a streptozocin-induced diabetic rat model. Nephron Exp
Nephrol. 116:e11–e21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dada MO, Campbell GT, Horacek MJ and Blake
CA: Intraperitoneal injection of chloral hydrate causes
intra-abdominal adhesions and unilateral testicular atrophy in
golden Syrian hamsters. Life Sci. 51:29–35. 1992. View Article : Google Scholar
|
|
11
|
Magil AB: Tubulointerstitial lesions in
human membranous glomerulonephritis: relationship to proteinuria.
Am J Kidney Dis. 25:375–379. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Y, Yin WL, Ba YF, Tian L, Gu ZQ, Zhang
MS and Zhong CN: Transforming growth factor-1 promotes the
transcriptional activation of plasminogen activator inhibitor type
1 in carcinoma-associated fibroblasts. Mol Med Rep. 6:1001–1005.
2012.PubMed/NCBI
|
|
13
|
Trieschmann M, Heimes B, Hense HW and
Pauleikhoff D: Macular pigment optical density measurement in
autofluorescence imaging: comparison of one- and two-wavelength
methods. Graefes Arch Clin Exp Ophthalmol. 244:1565–1574. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Emmett N, Mann D and Zhao X:
Fenofibrate attenuates tubulointerstitial fibrosis and inflammation
through suppression of nuclear factor-κB and transforming growth
factor-β1/Smad3 indiabetic nephropathy. Exp Biol Med (Maywood).
235:383–391. 2010.PubMed/NCBI
|
|
15
|
Gilbert RE and Cooper ME: The
tubulointerstitium in progressive diabetic kidney disease: more
than an aftermath of glomerular injury? Kidney Int. 56:1627–1637.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sagar SK, Zhang C, Guo Q, Yi R and
Lin-Tang: Role of expression of endothelin-1 and angiotensin II and
hypoxia-inducible factor-1α in the kidney tissues of patients with
diabetic nephropathy. Saudi J Kidney Dis Transpl. 24:959–964.
2013.
|
|
17
|
Loeffler I, Liebisch M and Wolf G:
Collagen VIII influences epithelial phenotypic changes in
experimental diabetic nephropathy. Am J Physiol Renal Physiol.
303:F733–F745. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Czernobilsky B, Gabbiani G, Prus D and
Lifschitz-Mercer B: α-smooth muscle actin-positive myofibroblasts
in endometrial stroma are not a reliable criterion for the
diagnosis of well differentiated endometrioid adenocarcinoma in
small tissue samples. Int J Gynecol Pathol. 20:232–238. 2001.
|
|
19
|
Wei J, Shi Y, Hou Y, Ren Y, Du C, Zhang L,
Li Y and Duan H: Knockdown of thioredoxin-interacting protein
ameliorates high glucose-induced epithelial to mesenchymal
transition in renal tubular epithelial cells. Cell Signal.
25:2788–2796. 2013. View Article : Google Scholar
|
|
20
|
Shi Y, Lo CS, Chenier I, Maachi H, Filep
JG, Ingelfinger JR, Zhang SL and Chan JS: Overexpression of
catalase prevents hypertension and tubulointerstitial fibrosis and
normalization of renal angiotensin-converting enzyme-2 expression
in Akita mice. Am J Physiol Renal Physiol. 304:F1335–F1346. 2013.
View Article : Google Scholar
|
|
21
|
Al-Malki AL, Sayed AA and El Rabey HA:
Proanthocyanidin attenuation of oxidative stress and NF-κB protects
apolipoprotein E-deficient mice against diabetic nephropathy. Evid
Based Complement Alternat Med. 2013:7694092013.
|
|
22
|
Yang J, Dai C and Liu Y: Hepatocyte growth
factor gene therapy and angiotensin II blockade synergistically
attenuate renal interstitial fibrosis in mice. J Am Soc Nephrol.
13:2464–2477. 2002. View Article : Google Scholar : PubMed/NCBI
|