Introduction
Morus alba is a fast-growing and small-medium
sized mulberry tree. According to the Dong-eui-bo-gam, the oldest
Korean medicinal book, Morus alba leaf (MAL), also known as
Mori folium when used as a herbal medicine, alleviates the symptoms
of beriberi, body swelling, dropsy and diabetes (1). Modern medical studies of the leaves of
the white mulberry have reported anti-atherogenic (2), anti-hypertensive (3,4),
anti-obesity (5), anti-diabetic
(6) and liver protective (7,8)
effects.
The main constituents of MAL are known to consist of
antioxidative and anti-inflamatory flavonols, including quercetin,
astragalin, isoquercitrin and rutin (9–11).
Polyphenols are alcohols containing ≥2 benzene
rings, which each have ≥1 hydroxyl (OH) group attached and can
range from simple molecules (phenolic acids, phenylpropanoids and
flavonoids) to highly polymerized compounds (lignins, melanins and
tannins), with flavonoids representing the most common and widely
distributed subgroup (12). These
compounds have been reported to have antioxidant activity, but
flavonoids in particular exhibit a wide range of biological
effects, including antibacterial, antiviral, anti-inflammatory,
antiallergic, antithrombotic, anticarcinogenic, hepatoprotective
and vasodilatory activities, in addition to their antioxidant
activities (13,14). Indeed, a number of these biological
functions have been attributed to their free radical scavenging and
antioxidant activities (15).
Oxidative damage appears to be associated with the etiology of
cardiovascular disease, diabetes mellitus, gastric ulcers,
arthritis, cancer and inflammation (16,17).
The antioxidant activity of MAL has been previously
reported. A previous study has shown that the butanol extract of
MAL and isoquercitrin is able to scavenge the
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical and inhibited
oxidation of rabbit and human low-density lipoprotein (18). The levels of the main antioxidative
components of MAL, namely rutin, isoquercitrin and astragalin, have
also been reported (10). However,
the antioxidant activity, the polyphenol content and the levels of
the antioxidants in MAL have not been reported according to
collection area.
The present study was designed to investigate the
antioxidant activity and total polyphenol content, including the
main polyphenol constituent content, of MAL according to growing
region.
Materials and methods
Preparation of MAL extracts
MAL was collected from 7 provinces in Korea
(Table I). Commercial MAL was
purchased from Omniherb Co., Ltd., (Yeoungcheon, Korea), JungDo
Co., (Seoul, Korea) and Dongkyung Corporation (Jeungpyung, Korea),
and was authenticated, based on its microscopic and macroscopic
characteristics, by the Classification and Identification Committee
of the Korea Institute of Oriental Medicine (KIOM). The committee
consisted of nine experts in the fields of plant taxonomy, botany,
pharmacognosy and herbology. The voucher specimens were deposited
at the herbarium of Herbal Medicine Resources Group at the
KIOM.
 | Table ICollection area and date of MAL. |
Table I
Collection area and date of MAL.
| Sample | Collection area | Collection date |
|---|
| MAL628A | Mountain in
Daejeon | June 28 |
| MAL628B | Street in
Daejeon | June 28 |
| MAL704 | Mountain in Chungbuk
Chungwon | July 4 |
| MAL712 | Mountain in Kyungbuk
Youngchun | July 12 |
| MAL718 | Mountain in Kangwon
Yanggu | July 18 |
| MAL730 | Street in Jeonnam
Damyang | July 30 |
| MAL801 | Street in Kyungnam
Sanchung | August 1 |
| MAL805 | Street in Chungbuk
Chungju | August 5 |
| MAL903 | Mountain in
Daejeon | September 3 |
| MAL906 | Mountain in Chungnam
Cheonan | September 6 |
The dried and coarsely powdered leaves (each 100 g)
were extracted with methanol (each 2 liters) for 4 h at 60°C. The
extracts were filtered and evaporated until dry under a reduced
pressure at 40°C.
Determination of total polyphenol
content
The total polyphenol content of the samples was
determined by the Folin-Ciocalteu method (19). Appropriate dilutions of samples (2
ml) were oxidized with Folin-Ciocalteu’s reagent (2 ml; Sigma, St.
Louis, MO, USA) for 3 min. The reaction was neutralized with 10%
sodium carbonate solution (2 ml). The contents in the tubes were
then thoroughly mixed and allowed to stand at ambient temperature
for 1 h until the characteristic blue color developed. The
absorbance of the clear supernatant was measured at 700 nm using a
spectrophotometer (LAMBDA 25 UV/Vis Spectrophotometer, PerkinElmer
Inc., Waltham, MA, USA). The total polyphenol content in each
sample was calculated based on a standard curve, which was prepared
using gallic acid (Sigma) and expressed as milligrams of gallic
acid equivalent (GAE) per gram of sample.
Free radical scavenging assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical
scavenging activity was determined, as previously described
(20). A DPPH solution in ethanol
and dimethylsulfoxide (DMSO) was prepared and 900 μl of this
solution was added to 100 μl of each sample dissolved in ethanol
(500 μg/ml). The mixture was agitated and then allowed to stand at
room temperature for 10 min. The absorbance was subsequently
measured at 518 nm using a spectrophotometer (LAMBDA 25 UV/Vis
Spectrophotometer, PerkinElmer Inc.). The percentage of scavenging
activity at different concentrations was determined and compared
with that of L-ascorbic acid (100 μg/ml), which was used as the
standard. The inhibition of the DPPH radical scavenging effect was
calculated as: DPPH radical scavenging effect (%) = (Ao-A)/Ao ×
100, where Ao was the absorbance of the control solution
(containing only DPPH) and A was the absorbance of DPPH in the
sample solution. The determinations were performed in triplicate
for each sample and the values were averaged.
High-performance liquid chromatography
(HPLC) analysis
HPLC-grade reagents, acetonitrile and water were
obtained from J.T. Baker (Phillipsburg, NJ, USA). All other
chemicals were of reagent grade.
The samples were analyzed by reversed-phase HPLC
using an Waters Alliance 2695 HPLC system (Waters Co., Milford, MA,
USA), coupled with a 2996 photodiode array detector. A Phenomenex
Luna C18 column (250×4.6 mm; particle size, 5 μm; Phenomenex,
Torrance, CA, USA) was used, and the mobile phase was composed of
0.1% (v/v) trifluoroacetic aqueous solution (A) and acetonitrile
(B).
The elution conditions for identification of the
main polyphenol constituents were as follows: At 0 min, the mobile
phase consisted of 90% A/10% B and was held for 10 min. From 10–40
min a gradient was applied to 60% A/40% B, which was followed by a
wash with 100% B for 5 min and a 15-min equilibration period at 90%
A/10% B. The elution conditions for simultaneous quantification of
rutin, isoquercitrin and astragalin were as follows: At 0 min, the
mobile phase consisted of 85% A/15% B and was held for 30, which
was followed by a wash with 100% B for 5 min and a 15-min
equilibration period at 85% A/15% B. The separation temperature was
kept at a constant 40°C throughout the analysis, with a flow rate
of 1.0 ml/min and an injection volume of 20 μl.
Identification was based on retention time and UV
spectra by comparison with commercial standards. The identified
components were quantified based on peak areas at 260 nm.
Calibration curves of the standards ranged between 12.5 and 200
μg/ml (5 levels), revealing good linearity, with R2
values exceeding 0.99 (peak areas vs. concentration).
Statistical analysis
All data are presented as the mean ± standard
deviation of at least triplicate measurements. The significance of
differences among treatment means were calculated using the SPSS
package for Windows (version 12.0; SPSS Inc., Chicago, IL, USA)
with a significance level indicated by P<0.05.
Results
Extraction yields
Methanol was selected as the extraction solvent
since it is commonly used for polyphenols and flavonoids. The yield
of methanol extracts obtained from 10 different MAL samples that
were collected from seven provinces in Korea is presented in
Table II. The extraction yields
did not differ greatly in terms of overall mass, and revealed
values ranging between 9.1 (MAL712) and 10.4% (MAL805).
 | Table IIPolyphenol contents and free radical
scavenging effects of MAL collected from various areas. |
Table II
Polyphenol contents and free radical
scavenging effects of MAL collected from various areas.
| Sample | Extraction yield,
% | Free radical
scavenging activity (SC50a), μg/ml | Polyphenol contents,
mg/g |
|---|
| MAL628A | 9.3 | 455±56 | 32.6±1.3 |
| MBL628B | 9.7 | 468±54 | 32.2±1.3 |
| MAL704 | 9.3 | 301±27 | 45.2±1.5 |
| MAL712 | 9.1 | 330±41 | 36.2±1.8 |
| MAL718 | 10.1 | 139±15 | 55.4±2.1 |
| MAL730 | 9.4 | 250±19 | 45.1±1.7 |
| MAL801 | 9.2 | 461±31 | 33.3±1.3 |
| MAL805 | 10.4 | 317±27 | 29.7±1.9 |
| MAL903 | 9.2 | 584±71 | 28.2±1.7 |
| MAL906 | 9.8 | 339±28 | 35.0±1.6 |
Polyphenol contents
The total polyphenol content of 10 MAL extracts are
shown in Table II. Among the
extracts tested, the highest total polyphenol level was observed at
55.4 mg GAE/g extract of MAL718 and the lowest at 28.2 mg GAE/g
extract of MAL903.
The total polyphenol content of MAL628A and MAL628B,
collected from a mountain and a street in Daejeon, respectively,
were extremely similar, with values of 32.6 and 32.2 mg/g,
respectively.
The lowest total polyphenol content was 23.2 mg/g
(MAL903) and the highest total polyphenol content was 55.4 mg/g
(MAL718), with large differences in their inhibition of DPPH
radical scavenging. Meanwhile, MAL903 and MAL628A collected at
varying times from the same tree had a total polyphenol content of
32.6 and 23.2 mg/g, respectively. This indicated certain variations
within the area, but the change was not significantly different
compared with samples from varying locations.
Free radical scavenging activity
DPPH radical scavenging activities of MAL628A and
MAL628B collected from adjacent areas in Daejeon were similar, with
an SC50 of 455 and 468 μg/ml, respectively (Table II). The lowest DPPH radical
scavenging activity (MAL903) was an SC50 of 584 and the
highest DPPH radical scavenging activity (MAL718) was an
SC50 of 139 μg/ml. MAL628A and MAL903 were collected
from the same tree, with a gap of 2 months, with no significant
difference in their SC50 values of 455 and 584 μg/ml,
respectively.
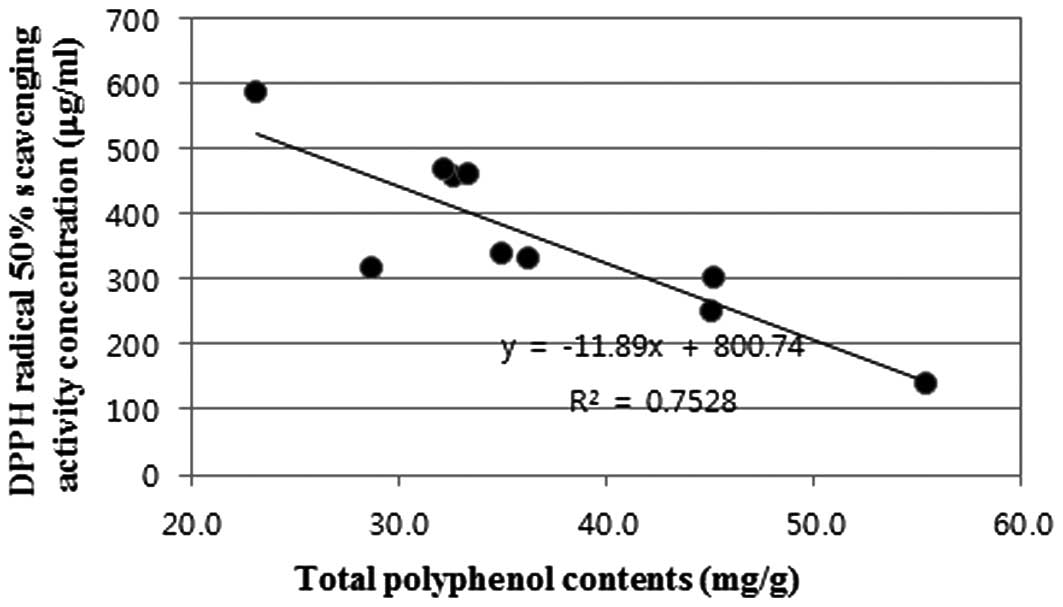
The correlation between total polyphenol contents
and antioxidant activity has been widely studied in a variety of
herbs. As previously reported (21), the present study also showed that
antioxidant activity significantly increases with the presence of a
high total polyphenol content. The present study also showed a good
correlation between radical scavenging activity and the total
polyphenol content of 10 MAL extracts. The polyphenol contents were
correlated with DPPH radical scavenging activity
(R2=0.7528) (Fig.
1).
Identification and quantification of main
polyphenol constituents of MAL extracts
The main polyphenol constituents were identified
based on the ultraviolet (UV)-visible spectrum using a HPLC/diode
array detection (DAD) chromatogram. At the gradient elution
conditions for identification [retention times, ~17.5, 21.0 and
36.2 min; wavelengths, with maximum absorption from UV-visible
spectrum (λmax1 and λmax2), 255.4 and 353.2,
255.4 and 353.2, and 254.2 and 348.4 nm, respectively], the
constituents, rutin, isoquercitrin and astragalin, were an exact
match to the commercial standards (Fig.
2).
Simultaneous quantitative analysis of these three
components in MAL extracts was further developed with good
separation. The flavonol contents were varied as the values for
rutin ranged from 0.68 (MAL906)-12.7 mg/g (MAL718), isoquercitrin
ranged from 0.69 (MAL903)-9.86 mg/g (MAL718), astragalin ranged
from 0.05 (MAL903)-3.55 mg/g (MAL730) and the total ranged from
1.72 (MAL903)-25.82 mg/g (MAL718). The average total flavonol
content was 9.52 mg/g. Of the three components, isoquercitrin
showed the highest average content (5.68) followed by rutin (3.1)
and then astragalin (2.4 mg/g).
Although MAL628A and MAL628B were located in the
same area, within a short distance, the total flavonoid contents of
MAL628A (collected in the street) and MAL628B (collected in the
mountain) were substantially different, recorded as 10.89 and 1.99
mg/g, respectively (Table III).
Meanwhile, the total flavonols of MAL628A and MAL903 collected at
various times from the same tree were not substantially different,
recorded as 1.99 and 1.72 mg/g, respectively.
 | Table IIIContent of flavonols of MAL collected
from various areas. |
Table III
Content of flavonols of MAL collected
from various areas.
| Content, mg/g |
|---|
|
|
|---|
| Extract | Rutin | Isoquercitrin | Astragalin | Total |
|---|
| MAL628A | 0.98±0.04 | 0.84±0.05 | 0.17±0.01 | 1.99 |
| MAL628B | 4.24±0.16 | 4.97±0.18 | 1.68±0.07 | 10.89 |
| MAL704 | 1.03±0.06 | 1.32±0.06 | 1.04±0.04 | 3.39 |
| MAL712 | 4.01±0.13 | 7.75±0.23 | 2.85±0.11 | 14.62 |
| MAL718 | 12.70±0.39 | 9.86±0.35 | 3.26±0.13 | 25.82 |
| MAL730 | 2.08±0.08 | 9.09±0.31 | 3.55±0.14 | 14.71 |
| MAL801 | 1.99±0.08 | 4.32±0.17 | 1.96±0.08 | 8.27 |
| MAL805 | 2.16±0.09 | 5.42±0.19 | 2.62±0.12 | 10.20 |
| MAL903 | 0.98±0.04 | 0.69±0.03 | 0.05±0.00 | 1.72 |
| MAL906 | 0.68±0.03 | 2.10±0.07 | 0.78±0.04 | 3.56 |
| Mean | 3.10 | 5.68 | 2.41 | 9.52 |
Quantification of three major flavonols
in methanolic extract in commercial MAL
Using the established quantitative HPLC analysis
method, the flavonol content of the methanolic extracts from three
different commercial MAL extracts were analyzed. The flavonol
content of the methanolic extracts from MAL purchased at a variety
of markets were also analyzed for comparison with those MAL samples
that were collected in the fields (Table IV). The flavonol contents were also
varied as the values for rutin ranged from 1.27–2.12, isoquercitrin
ranged from 4.66–7.53 and astragalin ranged from 2.57–3.90 mg/g.
The flavonol content of the samples from the various collection
areas were not markedly different.
 | Table IVContent of flavonols of MAL collected
from markets. |
Table IV
Content of flavonols of MAL collected
from markets.
| Content, mg/g |
|---|
|
|
|---|
| Extract | Rutin | Isoquercitrin | Astragalin | Total |
|---|
| Omni herb | 1.27±0.05 | 4.73±0.14 | 2.57±0.13 | 8.57 |
| JungDo herb | 1.86±0.04 | 4.66±0.11 | 3.11±0.09 | 9.63 |
| Dongkyung herb | 2.12±0.07 | 7.53±0.19 | 3.90±0.08 | 13.55 |
| Mean | 1.75 | 5.64 | 3.56 | 10.58 |
Discussion
Flavonols are flavonoids that possess the
3-hydroxyflavone backbone (IUPAC name,
3-hydroxy-2-phenylchromen-4-one).
The flavonols are subclassified into kaempferol,
quercitrin and myricetin, according to the number and position of
the OH groups of the A and B rings. In the present study, rutin
(quercetin-3-O-β-rutinoside), isoquercitrin
(quercetin-3-O-β-D-glucoside) and astragalin
(kaempferol-3-O-β-D-glucoside) were identified as the main
polyphenol constituents in MAL.
Rutin has been reported to have anti-platelet
aggregation (22),
anti-inflammatory (23) and aldose
reductase inhibitory (24) effects.
Isoquercitrin has been reported to have antimicrobial (25) and antioxidant (26) effects. Astragalin has also been
reported to have antioxidant (27)
and anti-inflammatory (28)
effects. The present study also identified quercetin from a minor
peak (data not shown), but its contents in extract samples were far
lower than those of the other compounds of interest, with a maximum
concentration of 0.28 mg/g determined.
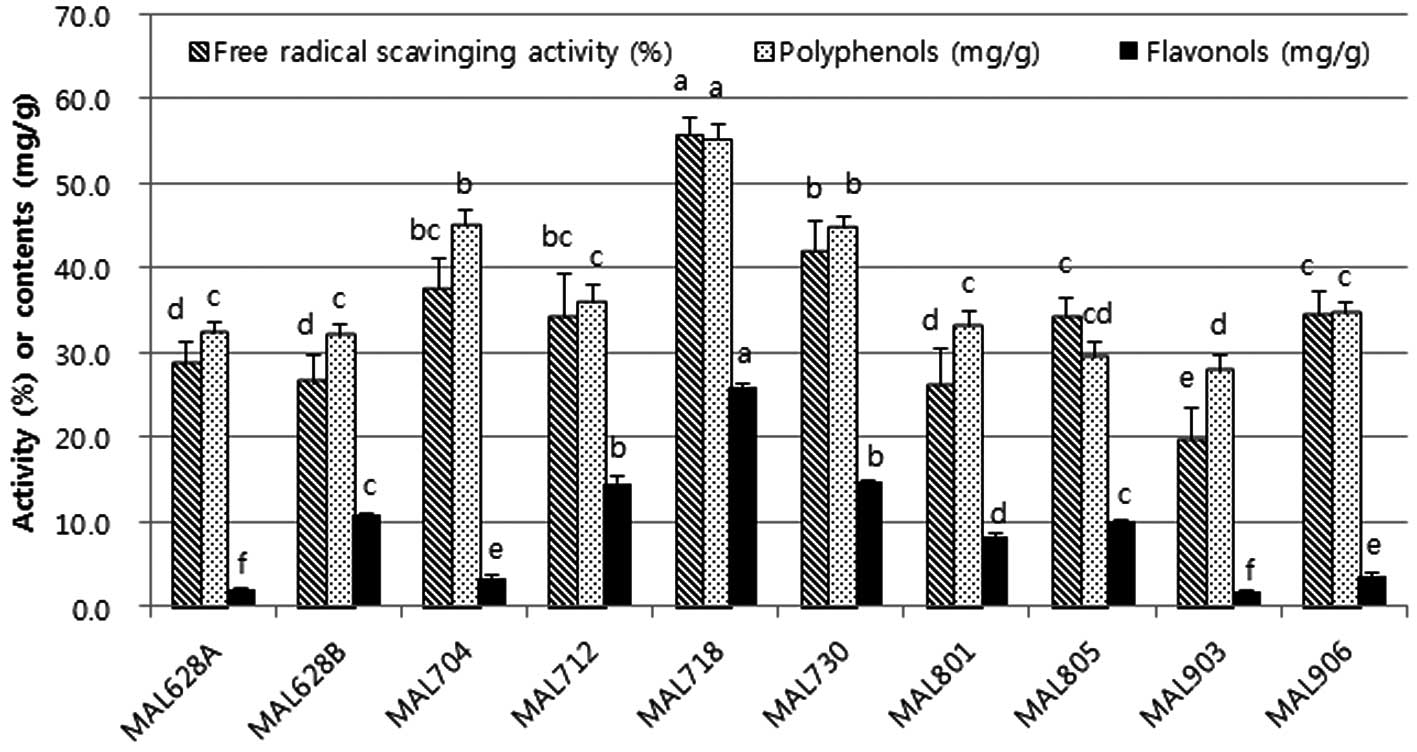
In the present study, the radical scavenging
activity, polyphenol content and flavonol content of MAL were
significantly different according to the growing area (Fig. 3). However, no association could be
determined between the polyphenol content and the geographic
location or altitude. Thus, changes in the polyphenol content and
the scavenging activities may be soil-related conditions rather
than, for example, temperature-, height- or weather-related
conditions, as soil in forests is specific to the particular
environment and has a direct bearing on the composition of the
plants found there.
Significantly, samples collected in the mountain in
Kangwon Yanggu (MAL718) had the highest radical scavenging
activity, polyphenol content and flavonol content. If the factors
affecting polyphenol content were to be identified, this would
greatly advance cultivation technology for mulberry polyphenol
production.
While there were was variation in the three flavonol
compounds (rutin, isoquercitrin and astragalin) of several
locations, the average total flavonol compound content in the
collected samples was similar to the average in samples purchased
on the market. The isoquercitrin content of samples collected from
several locations and the samples purchased from the market were
particularly similar, recorded as 5.68 and 5.64 mg/g, respectively.
Therefore, it may be concluded that the sum of these three flavonol
compounds or isoquercitrin alone is indicated as a good marker for
the quality control of mulberry leaves. The present study also
established a simultaneous analysis method of these three compounds
using HPLC/DAD, which will be useful for quality control.
Acknowledgements
This study was mainly supported by Discovery of
Herbal Medicine for Prevention (K13202), the Korea Institute of
Oriental Medicine (KIOM) to the Ministry of Science, ICT and Future
Planning (MSIP), Korea. Additionally, this study was also partially
supported by ICT Fusional Construction of Alternative Herbal
Medicine Resources (K14410), Characterization of Native Biological
Resources and Excavation of Alternative Herbal Medicine Resources
(K14411), KIOM to MSIP, Korea.
References
|
1
|
Heo J: Dong-eui-bo-gam. 14751610.(In
Korean).
|
|
2
|
Yang MY, Huang CN, Chan KC, Yang YS, Peng
CH and Wang CJ: Mulberry leaf polyphenols possess antiatherogenesis
effect via inhibiting LDL oxidation and foam cell formation. J
Agric Food Chem. 59:1985–1995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naowaboot J, Pannangpetch P,
Kukongviriyapan V, Kukongviriyapan U, Nakmareong S and Itharat A:
Mulberry leaf extract restores arterial pressure in
streptozotocin-induced chronic diabetic rats. Nutr Res. 29:602–608.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang NC, Jhou KY and Tseng CY:
Antihypertensive effect of mulberry leaf aqueous extract containing
γ-aminobutyric acid in spontaneously hypertensive rats. Food Chem.
132:1796–1801. 2012.
|
|
5
|
Oh KS, Ryu SY, Lee S, Seo HW, Oh BK, Kim
YS and Lee ΒΗ: Melanin-concentrating hormone-1 receptor antagonism
and anti-obesity effects of ethanolic extract from Morus
alba leaves in diet-induced obese mice. J Ethnopharmacol.
122:216–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JM, Bong HY, Jeong HI, Kim YK, Kim JY
and Kwon O: Postprandial hypoglycemic effect of mulberry leaf in
Goto-Kakizaki rats and counterpart control Wistar rats. Nutr Res
Pract. 3:272–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katsube T, Yamasaki M, Shiwaku K, Ishijima
T, Matsumoto I, Abe K and Yamasaki Υ: Effect of flavonol glycoside
in mulberry (Morus alba L.) leaf on glucose metabolism and
oxidative stress in liver in diet-induced obese mice. J Sci Food
Agric. 90:2386–2392. 2010.PubMed/NCBI
|
|
8
|
Hsu LS, Ho HH, Lin MC, Chyau CC, Peng JS
and Wang CJ: Mulberry water extracts (MWEs) ameliorated carbon
tetrachloride-induced liver damages in rat. Food Chem Toxicol.
50:3086–3093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SY, Gao JJ, Lee WC, Ryu KS, Lee KR and
Kim YC: Antioxidative flavonoids from the leaves of Morus
alba. Arch Pharm Res. 22:81–85. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katsube T, Imawaka N, Kawano Y, Yamazaki
Y, Shiwaku K and Yamane Y: Antioxidant flavonol glycosides in
mulberry (Morus alba L.) leaves isolated based on LDL
antioxidant activity. Food Chem. 97:25–31. 2006.
|
|
11
|
Mok JY, Jeong SI, Kim JH and Jang SI:
Synergic effect of quercetin and astragalin from mulberry leaves on
anti-inflammation. Kor J Ori Physiol Pathol. 25:830–836. 2011.
|
|
12
|
Bravo L: Polyphenols: chemistry, dietary
sources, metabolism, and nutritional significance. Nutr Rev.
56:317–333. 1998.PubMed/NCBI
|
|
13
|
Kähkönen MP, Hopia AI, Vuorela HJ, Rauha
JP, Pihlaja K, Kujala TS and Heinonen M: Antioxidant activity of
plant extracts containing phenolic compounds. J Agric Food Chem.
47:3954–3962. 1999.PubMed/NCBI
|
|
14
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
implications for inflammation, heart disease and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
15
|
Soobrattee MA, Neergheen VS, Luximon-Ramma
A, Aruoma OI and Bahorun T: Phenolics as potential antioxidant
therapeutic agents: mechanism and actions. Mutat Res. 579:200–213.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aruoma OI: Free radicals, oxidative
stress, and antioxidants in human health and disease. J Am Oil Chem
Soc. 75:199–212. 1998. View Article : Google Scholar
|
|
17
|
Fang J, Seki T and Maeda H: Therapeutic
strategies by modulating oxygen stress in cancer and inflammation.
Adv Drug Deliv Rev. 61:290–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doi K, Kojima T and Fujimoto Y: Mulberry
leaf extract inhibits the oxidative modification of rabbit and
human low density lipoprotein. Biol Pharm Bull. 23:1066–1071. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Folin O and Denis W: On
phosphotungstic-phosphomolybdic compounds as color reagents. J Biol
Chem. 12:239–243. 1912.
|
|
20
|
Blois MS: Antioxidant determinations by
the use of a stable free radical. Nature. 181:1199–1200. 1958.
View Article : Google Scholar
|
|
21
|
Li X, Wu X and Huang L: Correlation
between antioxidant activities and phenolic contents of radix
Angelicae sinensis(Danggui). Molecules. 14:5349–5361. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Navarro-Núñez L, Lozano ML, Palomo M,
Martínez C, Vicente V, Castillo J, Benavente-García O, Diaz-Ricart
M, Escolar G and Rivera J: Apigenin inhibits platelet adhesion and
thrombus formation and synergizes with aspirin in the suppression
of the arachidonic acid pathway. J Agric Food Chem. 56:2970–2976.
2008.PubMed/NCBI
|
|
23
|
Jung CH, Lee JY, Cho CH and Kim CJ:
Anti-asthmatic action of quercetin and rutin in conscious
guinea-pigs challenged with aerosolized ovalbumin. Arch Pharm Res.
30:1599–1607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reddy GB, Muthenna P, Akileshwari C,
Saraswat M and Petrash JM: Inhibition of aldose reductase and
sorbitol accumulation by dietary rutin. Curr Sci. 101:1191–1197.
2011.
|
|
25
|
Razavi SM, Zahri S, Zarrini G, Nazemiyeh H
and Mohammadi S: Biological activity of quercetin-3-O-glucoside, a
known plant flavonoid. Bioorg Khim. 35:414–416. 2009.PubMed/NCBI
|
|
26
|
Rogerio AP, Kanashiro A, Fontanari C, da
Silva EV, Lucisano-Valim YM, Soares EG and Faccioli LH:
Anti-inflammatory activity of quercetin and isoquercitrin in
experimental murine allergic asthma. Inflamm Res. 56:402–408. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi J, Kang HJ, Kim SZ, Kwon TO, Jeong SI
and Jang SI: Antioxidant effect of astragalin isolated from the
leaves of Morus alba L. against free radical-induced
oxidative hemolysis of human red blood cells. Arch Pharm Res.
36:912–917. 2013.PubMed/NCBI
|
|
28
|
Lee HB, Kim EK, Park SJ, Bang SG, Kim TG
and Chung DW: Isolation and anti-inflammatory effect of astragalin
synthesized by enzymatic hydrolysis of tea seed extract. J Sci Food
Agric. 91:2315–2321. 2011. View Article : Google Scholar : PubMed/NCBI
|