Introduction
Prostate cancer is the most common type of cancer
diagnosis in males and the estimated new prostate cancer cases and
mortalities in 2013 were 238,590 and 29,720, respectively (1). Several studies have been performed and
their results suggested that the etiology of prostate cancer was
extremely complicated, and may be associated with several factors,
including smoking, environment, dietary habits, endocrine system,
age and ethnicity (2–7). However, the accurate etiology and
pathogenesis remain inconsistent. Recently, a number of studies
suggested that inflammation and genetic factors may play an
important role in the etiology of prostate cancer (8–14). The
prostaglandin-endoperoxide synthase 2 gene that encodes the
cyclooxygenase-2 enzyme has been verified to play an important role
in the development of prostate cancer in numerous studies (15–18).
The study by Woo et al (19)
observed that tumor infiltrating B-cells were increased in prostate
cancer tissue. The Lv et al (20) study suggested that hypoxia promoted
the invasiveness of prostate cancer PC3 cells via hypoxia-inducible
factor-1α- and tumor necrosis factor-α-induced stabilization of
Snail. McDonald et al (21)
investigated the associations between systemic inflammatory markers
and serum prostate-specific antigen (PSA) in 3,164 healthy males
and found that elevated serum PSA (194 males, 6.1% of the total)
was significantly associated with plasma fibrinogen, suggesting
that the markers of systemic inflammation were associated with
elevated PSA in males without a known prostate disease.
Among the cytokines involved in inflammation,
interleukin-6 (IL-6) plays a key role in the inflammation process.
IL-6 is one of the most potent proinflammatory cytokines during
acute inflammation, inducing and regulating the production of acute
phase proteins (22). Previously,
several studies have shown that the IL-6 polymorphism is
significantly associated with a number of diseases and plays an
important role in the etiology of diseases (23–29).
With regards to prostate cancer, the Mandić et al (30) study indicated that the IL-6 -174
single-nucleotide polymorphism (SNP) distribution may vary between
ethnicities and that a single cytokine-gene polymorphism probably
has only a minor influence on prostate cancer susceptibility. The
study by Pierce et al (31)
suggested that circulating IL-6 and its gene polymorphism did not
influence the prostate cancer risk, whereas Mandal et al
(32) had an opposing opinion. Due
to these conflicting results, the present meta-analysis was
conducted to provide a comprehensive assessment of the associations
of the IL-6 (-174 G/C) gene polymorphism with the risk of prostate
cancer.
Materials and methods
Search strategy
Databases, including PubMed, Embase, Web of Science,
the Cochrane Library, Chinese Biomedical Literature Database and
Wanfang database, were searched between January 1994 and March 2014
for all the possible studies using an analytical design (including
case-control and cohort studies) that mainly studied the
association between the IL-6 (-174 G/C) gene polymorphism and the
susceptibility of prostate cancer. The search terms used included:
‘Prostate cancer’ or ‘PCa;’ ‘interleukin 6’ or ‘IL-6;’ and
‘polymorphism,’ ‘single-nucleotide polymorphism,’ ‘SNP’ or
‘variation;’ and there was no language restriction in the
literature search. To find more eligible studies that may not have
been included in the initial search, the references of the
candidate studies were examined and searches of unpublished
literature were conducted.
Inclusion and exclusion criteria
The studies in the meta-analysis were included
according to the following criteria: i) Analytical design
(including case-control and cohort studies); ii) evaluation of the
prostate cancer risk and IL-6 (-174 G/C) gene polymorphism; iii)
sufficient data, including the number or frequency of alleles and
genotypes; and iv) genotype frequencies in control groups should be
abided by the Hardy-Weinberg equilibrium (HWE). The exclusion
criteria included: i) Case studies and reviews; ii) no sufficient
data reported; and iii) duplicated studies.
Data extraction
The quantitative data of all the eligible studies
were extracted according to the inclusion and exclusion criteria by
two investigators independently and a consensus was attempted if
the data for one investigator was inconsistent with the other. The
following characteristics of each study were collected: Authors,
year of publication, study design, ethnicity, group, sample size,
alleles and IL-6 genotypes. Certain studies included more than one
ethnicity, therefore the information data were extracted separately
according to the ethnicity.
Data synthesis and statistical
analysis
The pooled odds ratio (OR) with 95% confidence
interval (95% CI) was calculated to assess the associations between
the IL-6 (-174 G/C) gene polymorphism and prostate cancer according
to allele contrast (C vs. G), homozygote (CC vs. GG), heterozygote
(GC vs. GG), dominant (GC/CC vs. GG) and recessive (CC vs. GC/GG)
models, and P<0.05 was considered to indicate a statistically
significant difference. The subgroup analysis was also performed to
determine whether there was a significant association between the
IL-6 (-174 G/C) gene polymorphism and prostate cancer in different
ethnicities. The heterogeneity assumption was checked by a
χ2-based Q statistic test and quantified by the
I2 metric value. When I2>50% or P<0.10,
suggesting that a clear heterogeneity existed, the ORs were pooled
by the random-effect model, but for other cases the fixed-effect
model was used. In addition, the sensitivity analysis was performed
by individually removing the included studies to assess the impact
of each study on the combined effect of the present meta-analysis.
Stata 12.0 software (StataCorp, College Station, TX, USA) was used
to analyze the data in the study.
Results
Study characteristics
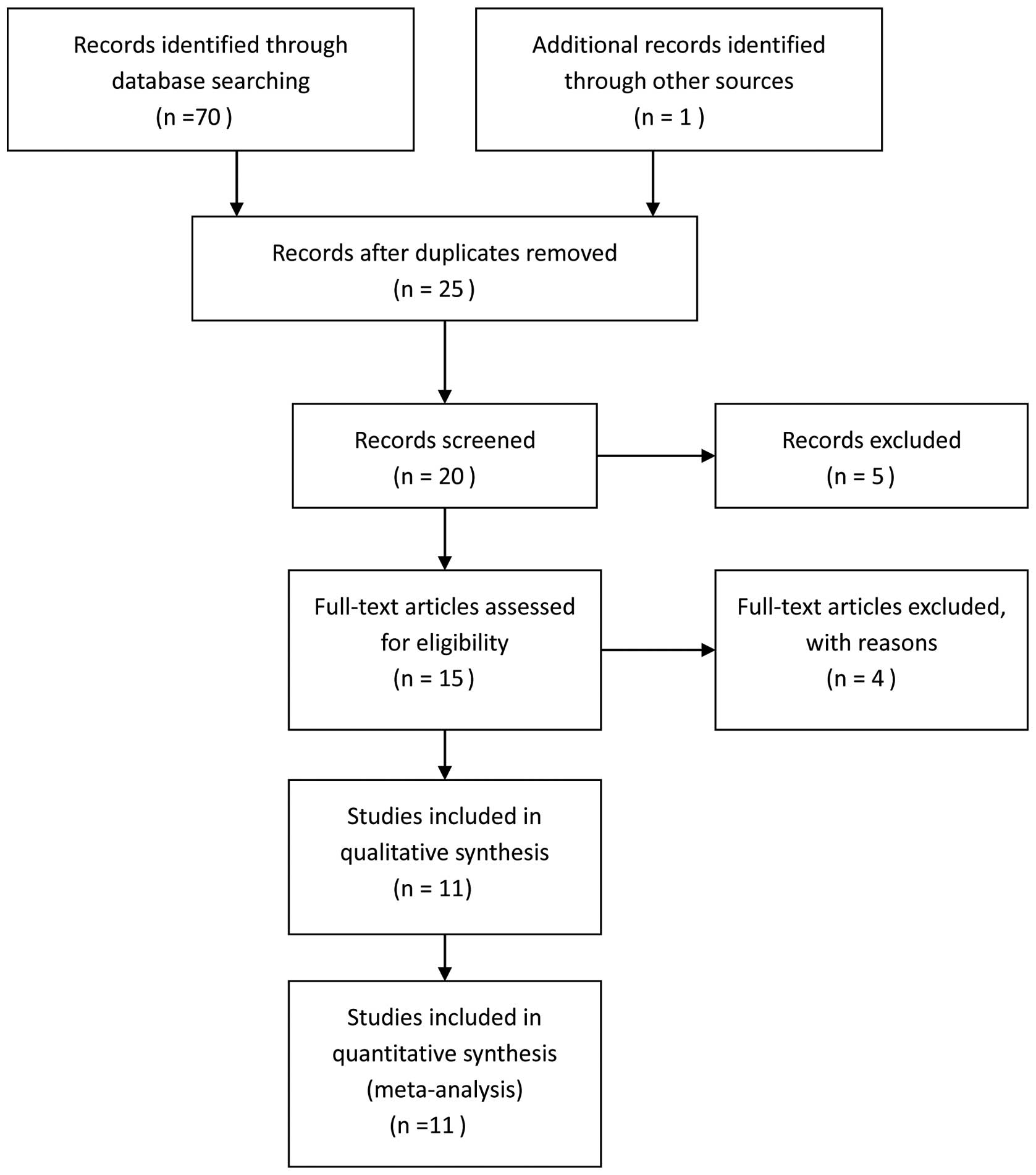
In the initial search, the total studies were
identified and 11 studies (30–40)
with 10,745 cases and 13,473 controls eventually satisfied the
eligibility criteria (Fig. 1).
Among these studies, 10 (30,32–40)
were case-control studies and only one (31) was a cohort study. Three studies
(31,32,40)
were conducted in Caucasian and African-American patients; five
(30,34–36,39)
reported the results in Caucasian patients only, two studied Asian
patients (37,38) and one (33) was conducted in a mixed population
(Caucasian and African-American). Additionally, 10 studies
(31–40) reported the alleles and genotypes of
IL-6 (-174 G/C) and one (30)
reported only the number of CC and GG+GC genotypes. The general
demographical characteristics of the studies included in the
meta-analysis are summarized in Table
I. The genotype distributions in the controls of all the
studies were consistent with HWE.
 | Table IGeneral characteristics of the studies
included in the meta-analysis. |
Table I
General characteristics of the studies
included in the meta-analysis.
| | | | | | IL-6 alleles | IL-6 genotypes | |
|---|
| | | | | |
|
| |
|---|
| Author | Year | Study design | Ethnicity | Group | Size | G | C | GG | GC | CC | (Refs.) |
|---|
| Mandal et
al | 2014 | Case-control | Caucasian | Case | 84 | 128 | 40 | 50 | 28 | 6 | (32) |
| Control | 78 | 82 | 74 | 26 | 30 | 22 | |
|
African-American | Case | 80 | 132 | 28 | 58 | 16 | 6 | |
| Control | 62 | 110 | 14 | 48 | 14 | 0 | |
| Mandic et
al | 2013 | Case-control | Caucasian | Case | 120 | - | - | 97a | - | 23 | (30) |
| Control | 120 | - | - | 104a | - | 16 | |
| Zhang et
al | 2010 | Case-control | Mixed
population | Case | 193 | 246 | 140 | 80 | 86 | 27 | (33) |
| Control | 197 | 275 | 119 | 100 | 75 | 22 | |
| Dossus et
al | 2010 | Case-control | Caucasian | Case | 7939 | 10406 | 5468 | 3594 | 3218 | 1125 | (34) |
| Control | 8508 | 11066 | 5950 | 3832 | 3402 | 1274 | |
| Zabaleta et
al | 2009 | Case-control | Caucasian | Case | 74 | 72 | 76 | 19 | 34 | 21 | (40) |
| Control | 401 | 415 | 387 | 126 | 163 | 112 | |
|
African-American | Case | 15 | 22 | 8 | 10 | 2 | 3 | |
| Control | 57 | 92 | 22 | 41 | 10 | 6 | |
| Wang et
al | 2009 | Case-control | Caucasian | Case | 250 | 298 | 202 | 91 | 116 | 43 | (35) |
| Control | 252 | 296 | 208 | 84 | 128 | 40 | |
| Moore et
al | 2009 | Case-control | Caucasian | Case | 957 | 867 | 1047 | 191 | 485 | 281 | (36) |
| Control | 847 | 793 | 901 | 196 | 401 | 250 | |
| Pierce et
al | 2009 | Cohort study | Caucasian | Case | 175 | 192 | 158 | 48 | 96 | 31 | (31) |
| Control | 1758 | 2101 | 1415 | 648 | 805 | 305 | |
|
African-American | Case | 40 | 73 | 7 | 34 | 5 | 1 | |
| Control | 260 | 475 | 45 | 216 | 43 | 1 | |
| Bao et
al | 2008 | Case-control | Asian | Case | 136 | 272 | 0 | 136 | 0 | 0 | (37) |
| Control | 120 | 240 | 0 | 120 | 0 | 0 | |
| Kesarwani et
al | 2008 | Case-control | Asian | Case | 200 | 288 | 112 | 102 | 84 | 14 | (38) |
| Control | 200 | 293 | 107 | 103 | 87 | 10 | |
| Michaud et
al | 2006 | Case-control | Caucasian | Case | 484 | 563 | 405 | 170 | 223 | 91 | (39) |
| Control | 613 | 753 | 473 | 230 | 293 | 90 | |
Meta-analysis results
In the meta-analysis, there were no associations
found between the IL-6 (-174 G/C) polymorphism and prostate cancer
susceptibility in the overall population in all the genetic models
indicated in Table II (allele
contrast: C vs. G; heterozygote model: GC vs. GG; and homozygote
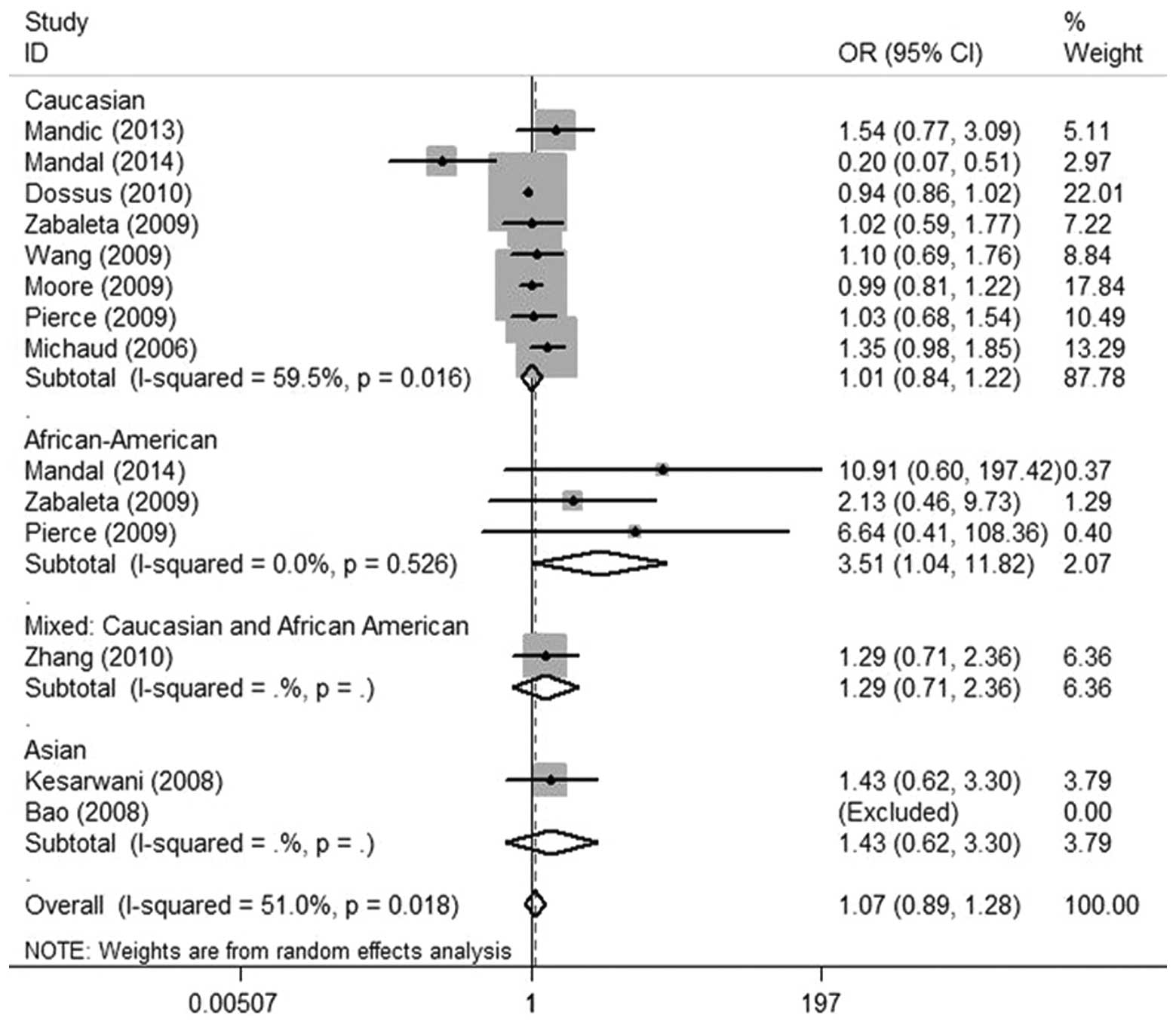
model: CC vs. GG), Fig. 2 (dominant
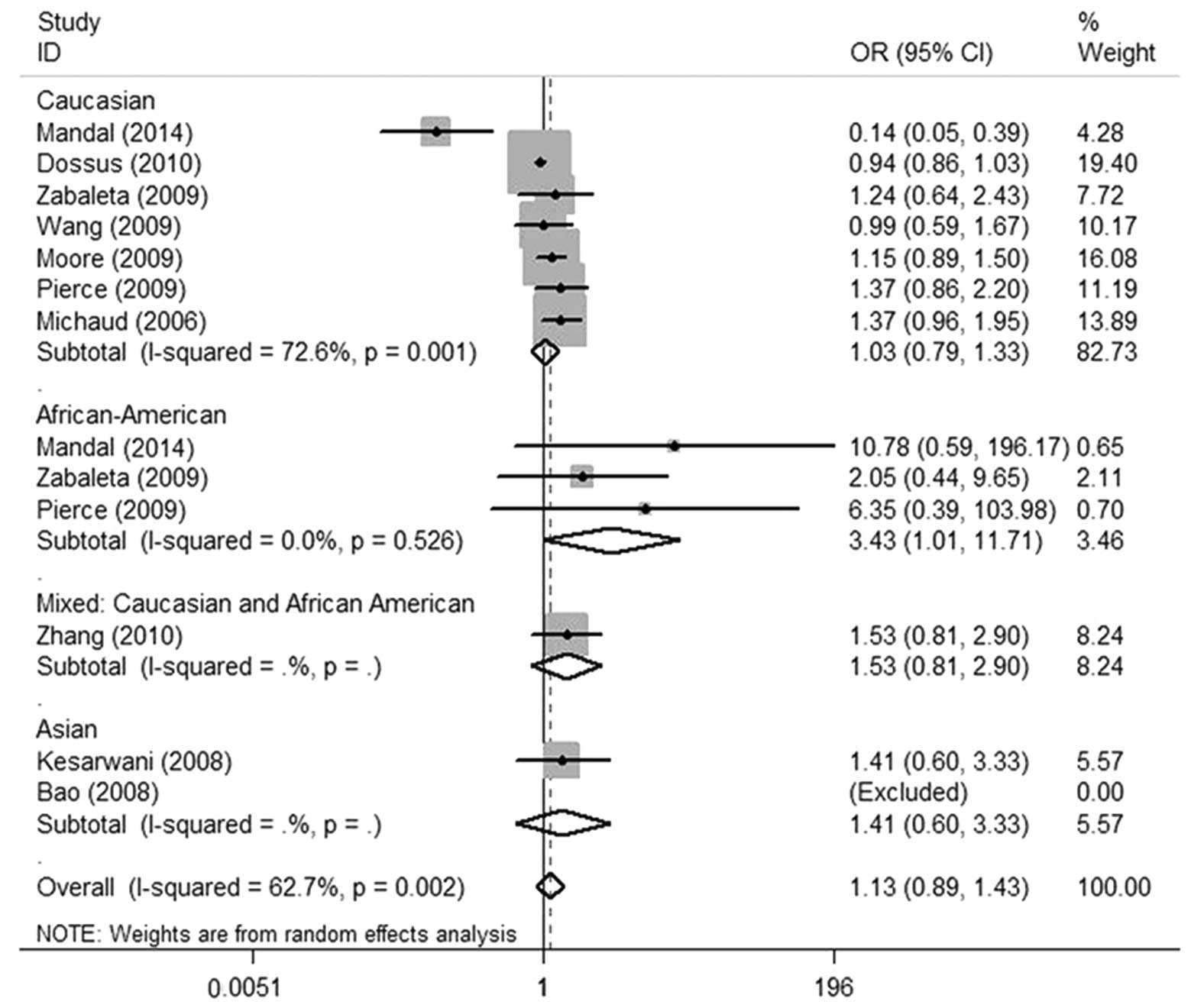
model: GC/CC vs. GG) and Fig. 3
(recessive model: CC vs. GG/GC). The subgroup analysis suggested
that the IL-6 (-174 G/C) polymorphism was not significantly
associated with prostate cancer in Asian, Caucasian and mixed
population patients under the allele contrast, homozygote,
heterozygote, dominant and recessive models. However, in
African-American patients, the subgroup analysis suggested that
there was a slightly significant association between the IL-6 (-174
G/C) polymorphism and prostate cancer risk in the homozygote and
recessive models (CC vs. GG: OR, 3.43; 95% CI, 1.01–11.71; P=0.049;
CC vs. GG/GC: OR, 3.51; 95% CI, 1.04–11.82; P=0.042, respectively)
(Figs. 3 and 4) and no significant association was found
in the allele contrast, heterozygote and dominant models (Table II and Fig. 2).
 | Table IIResults of the allele contrast,
heterozygote and homozygote models for the IL-6 (-174 G/C)
polymorphism and the risk of prostate cancer. |
Table II
Results of the allele contrast,
heterozygote and homozygote models for the IL-6 (-174 G/C)
polymorphism and the risk of prostate cancer.
| Association
test | Heterogeneity
test |
|---|
|
|
|
|---|
| Comparison | OR | 95% CI | P-value | P | I2
(%) |
|---|
| Overall |
| C vs. G | 1.05 | 0.93–1.28 | 0.43 | 0.00 | 67.00 |
| GC vs. GG | 1.03 | 0.97–1.10 | 0.28 | 0.08 | 38.70 |
| CC vs. GG | 1.13 | 0.89–1.43 | 0.32 | 0.002 | 62.70 |
| Ethnicity |
| Caucasian |
| C vs. G | 1.00 | 0.87–1.15 | 0.98 | 0.00 | 77.80 |
| GC vs. GG | 1.03 | 0.97–1.09 | 0.34 | 0.02 | 60.10 |
| CC vs. GG | 1.03 | 0.79–1.33 | 0.83 | 0.001 | 72.60 |
|
African-American |
| C vs. G | 1.40 | 0.88–2.22 | 0.16 | 0.65 | 0.00 |
| GC vs. GG | 0.85 | 0.47–1.52 | 0.58 | 0.93 | 0.00 |
| CC vs. GG | 3.43 | 1.01–11.71 | 0.049a | 0.53 | 0.00 |
| Asian |
| C vs. G | 1.06 | 0.78–1.45 | 0.69 | 1.00 | 0.00 |
| GC vs. GG | 0.97 | 0.65–1.46 | 0.90 | 1.00 | 0.00 |
| CC vs. GG | 1.41 | 0.60–3.33 | 0.43 | 1.00 | 0.00 |
| Mixed
population |
| C vs. G | 1.32 | 0.98–1.77 | 0.07 | 1.00 | 0.00 |
| GC vs. GG | 1.43 | 0.94–2.20 | 0.10 | 1.00 | 0.00 |
| CC vs. GG | 1.53 | 0.81–2.90 | 0.19 | 1.00 | 0.00 |
Sensitivity analysis
To evaluate the stability of the meta-analysis, a
leave-one-out sensitivity analysis was performed. The sensitivity
analysis suggested that the independent study by Mandal et
al (32) influenced the
interpretation of the results in the homozygote and recessive
models for African-American patients. When the Mandal et al
(32) study was removed from the
present meta-analysis, no significant association was found between
the IL-6 (-174 G/C) polymorphism and the risk of prostate cancer in
African-American patients under the homozygote (OR, 2.67; 95% CI,
0.69–10.36; P=0.17) and recessive models (OR, 2.64; 95% CI,
0.70–9.98; P=0.15). However, no single study influenced the results
in the overall population by the sensitivity analysis.
Publication bias
The Begg’s test was performed and the results did
not reveal any evidence of clear asymmetry (C vs. G, P=0.95; CC vs.
GG, P=0.73; GC vs. GG, P=0.54; GC/CC vs. GG, P=0.63; and CC vs.
GC/GG, P=0.06), suggesting the absence of publication bias in the
meta-analysis.
Discussion
Prostate cancer is a common cause of cancer
mortality in males and it is widely considered that age, diet,
ethnicity and environmental factors contribute to the prostate
cancer etiology (2,5–7), but
recently the genetic background and inflammation are considered as
sensitive factors for the differences in prostate cancer
susceptibility (37,40). Following the identification of the
IL-6 (-174 G/C) polymorphism, attention to determine whether the
IL-6 (-174 G/C) polymorphism is associated with prostate cancer,
not only in the overall population but also in different
ethnicities, has increased. Bao et al (37) used TaqMan polymerase chain reaction
to gene-type the IL-6 (-174 G/C) polymorphism for comparing the
prostate cases and controls in terms of allele frequency, genotype
frequency and risk of prostate cancer. The results suggested that
no significant association was found in the population of Han
people in the Hubei region, which was also identified in Caucasian
patients (36). Additionally, two
meta-analyses (41,42) based on studies published 4–10 years
ago also held the same conclusion. However, a recent study
published in January 2014 by Mandal et al (32) suggested that the GG genotype may be
associated with an increased risk of prostate cancer in Caucasian
subjects, whereas the CC genotype was associated with an increased
risk in the African-American subjects.
In order to determine whether the IL-6 (-174 G/C)
polymorphism is associated with the prostate cancer risk in the
overall population and different ethnic populations, the present
meta-analysis of 11 independent studies was performed, which
included 10,745 cases and 13,473 controls based on several recently
published studies, whose results were inconsistent with former
studies. In the meta-analysis, it was found that the IL-6 (-174
G/C) polymorphism is not a risk factor for prostate cancer in the
overall population. However, the present study suggested that there
was a slightly significant association between the IL-6 (-174 G/C)
polymorphism and prostate risk in African-American patients under
the homozygote and recessive models (CC vs. GG: OR, 3.43; 95% CI,
1.01–11.71; P=0.049; and CC vs. GG/GC: OR, 3.51; 95% CI,
1.04–11.82; P=0.042, respectively), which contradicts the results
of the Magalhaes et al (41)
meta-analysis. In addition, no significant associations were found
in Asians and Caucasians, which is consistent with the Magalhaes
et al (41) and Zhang et
al (42) studies, suggesting
that ancestral genetic factors in different populations may have an
impact on prostate cancer susceptibility. Additionally, the removal
of the Mandal et al (32)
study from the present meta-analysis showed that no significant
association was found between the IL-6 (-174 G/C) polymorphism and
the risk of prostate cancer in African-American patients under the
homozygote (OR, 2.67; 95% CI, 0.69–10.36; P=0.17) and recessive
models (OR, 2.64; 95% CI, 0.70–9.98; P=0.15). The potential
explanation for this may involve the different patients recruited
in each independent study, as well as their different lifestyles,
different experimental procedures and complex gene-gene and
gene-environment interaction, which may also have contributed to
these conflicting results.
Although the comprehensive analysis was conducted to
show the association between the IL-6 (-174 G/C) gene polymorphism
and prostate cancer risk, there are particular limitations that
should be identified. Firstly, for the African-American patients,
only three studies were conducted and the results of these studies
were contradictory. Therefore, it may be difficult to explore the
real association in African-American patients. Secondly, only two
studies that were conducted in Asian patients fulfilled the
inclusion criteria, which could not provide enough statistical
power to detect the possible effects of the IL-6 (-174 G/C) gene
polymorphism on prostate cancer in Asian patients. Thirdly, the
studies included in the meta-analysis were conducted in Caucasian,
Asian and African-American patients, which may not represent the
negative associations in all the worldwide ethnicities. In
addition, the possibility of gene-gene interactions or
environmental factors or the possibility of linkage disequilibrium
between the polymorphisms were also not considered in the study.
Therefore, larger-scale and well-designed studies are necessary to
estimate the association between the IL-6 (-174 G/C) polymorphism
and the risk of prostate cancer.
In conclusion, although there were certain
limitations in the meta-analysis, the study was based on a
substantial number of cases and controls and suggested that there
was no significant association between IL-6 (-174 G/C) polymorphism
and the prostate cancer risk in the overall population, as well as
in Caucasian and Asian patients, whereas the CC genotype may be
associated with an increased prostate cancer risk in the
African-American patients. Due to these limitations, more studies
that consider lifestyle, complex gene-gene and gene-environment
interactions or family history should be conducted to further
assess the associations of the IL-6 (-174 G/C) gene polymorphisms
with the risk of prostate cancer.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Discacciati A and Wolk A: Lifestyle and
dietary factors in prostate cancer prevention. Recent Results
Cancer Res. 202:27–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai GY, Giovannucci EL, Pollak MN, et al:
Association of C-peptide and leptin with prostate cancer incidence
in the Health Professionals Follow-up Study. Cancer Causes Control.
25:625–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu J and Yu E: Insulin-like growth factor
receptor-1 (IGF-IR) as a target for prostate cancer therapy. Cancer
Metastasis Rev. Jan 12–2014.(Epub ahead of print).
|
|
5
|
McGregor SE, Courneya KS, Kopciuk KA,
Tosevski C and Friedenreich CM: Case-control study of lifetime
alcohol intake and prostate cancer risk. Cancer Causes Control.
24:451–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rundle A, Jankowski M, Kryvenko ON, Tang D
and Rybicki BA: Obesity and future prostate cancer risk among men
after an initial benign biopsy of the prostate. Cancer Epidemiol
Biomarkers Prev. 22:898–904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nelson WG, Demarzo AM and Yegnasubramanian
S: The diet as a cause of human prostate cancer. Cancer Treat Res.
159:51–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sfanos KS, Isaacs WB and DeMarzo AM:
Infections and inflammation in prostate cancer. Am J Clin Exp Urol.
1:3–11. 2013.
|
|
9
|
Kwon OJ, Zhang L, Ittmann MM and Xin L:
Prostatic inflammation enhances basal-to-luminal differentiation
and accelerates initiation of prostate cancer with a basal cell
origin. Proc Natl Acad Sci USA. 111:E592–E600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boikos SA, Nirschl C, Martin A, Alme A,
Harris T and Drake CG: Prostate cancer cells up-regulate PD-L1 in
response to pro-inflammatory cytokines. Cancer Res. 73(Suppl 1):
Abstract 1437. 2013. View Article : Google Scholar
|
|
11
|
Ianni M, Porcellini E, Carbone I, et al:
Genetic factors regulating inflammation and DNA methylation
associated with prostate cancer. Prostate Cancer Prostatic Dis.
16:56–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyake M, Lawton A, Goodison S, Urquidi V
and Rosser CJ: Chemokine (C-X-C motif) ligand 1 (CXCL1) protein
expression is increased in high-grade prostate cancer. Pathol Res
Pract. 210:74–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dakhova O, Rowley D and Ittmann M: Genes
upregulated in prostate cancer reactive stroma promote prostate
cancer progression in vivo. Clin Cancer Res. 20:100–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heber D: Cancer and inflammation.
Immunonutrition: Interactions of Diet, Genetics, and Inflammation.
Aggarwal BB and Heber D: CRC Press; Boca Raton, FL: 101. 2014,
View Article : Google Scholar
|
|
15
|
Cheng I, Liu X, Plummer SJ, Krumroy LM,
Casey G and Witte JS: COX2 genetic variation, NSAIDs, and advanced
prostate cancer risk. Br J Cancer. 97:557–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Danforth KN, Hayes RB, Rodriguez C, et al:
Polymorphic variants in PTGS2 and prostate cancer risk: results
from two large nested case-control studies. Carcinogenesis.
29:568–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shahedi K, Lindström S, Zheng SL, et al:
Genetic variation in the COX-2 gene and the association with
prostate cancer risk. Int J Cancer. 119:668–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panguluri RC, Long LO, Chen W, et al:
COX-2 gene promoter haplotypes and prostate cancer risk.
Carcinogenesis. 25:961–966. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woo JR, Liss MA, Muldong MT, et al: Tumor
infiltrating B-cells are increased in prostate cancer tissue. J
Transl Med. 12:302014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv L, Yuan J, Huang T, et al:
Stabilization of Snail by HIF-1α and TNF-α is required for
hypoxia-induced invasion in prostate cancer PC3 cells. Mol Biol
Rep. Mar 8–2014.(Epub ahead of print).
|
|
21
|
McDonald AC, Vira MA, Vidal AC, Gan W,
Freedland SJ and Taioli E: Association between systemic
inflammatory markers and serum prostate-specific antigen in men
without prostatic disease - the 2001–2008. National Health and
Nutrition examination survey. Prostate. 74:561–567. 2014.PubMed/NCBI
|
|
22
|
Lehrer S, Diamond EJ, Mamkine B, Droller
MJ, Stone NN and Stock RG: C-reactive protein is significantly
associated with prostate-specific antigen and metastatic disease in
prostate cancer. BJU Int. 95:961–962. 2005. View Article : Google Scholar
|
|
23
|
Aulisa L, Papaleo P, Pola E, et al:
Association between IL-6 and MMP-3 gene polymorphisms and
adolescent idiopathic scoliosis: a case-control study. Spine (Phila
Pa 1976). 32:2700–2702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibata N, Ohnuma T, Takahashi T, et al:
Effect of IL-6 polymorphism on risk of Alzheimer disease:
genotype-phenotype association study in Japanese cases. Am J Med
Genet. 114:436–439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bader H: Clinical and systemic
implications of periodontal disease susceptibility: the importance
of IL-6 polymorphism. Dentistry. 4:1872014.
|
|
26
|
Yang Z, Liang Y, Qin B and Zhong R: A
meta-analysis of the association of IL-6 -174 G/C and -572 G/C
polymorphisms with systemic lupus erythematosus risk. Rheumatol
Int. 34:199–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HY, Feng L, Wu H and Xie XD: The
association of IL-6 and IL-6R gene polymorphisms with chronic
periodontitis in a Chinese population. Oral Dis. 20:69–75. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taniguchi K and Karin M: IL-6 and related
cytokines as the critical lynchpins between inflammation and
cancer. Semin Immunol. 26:54–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao X, Huang J, Zhong H, et al: Targeting
interleukin-6 in inflammatory autoimmune diseases and cancers.
Pharmacol Ther. 141:125–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mandić S, Sudarević B, Marczi S, et al:
Interleukin-6 polymorphism and prostate cancer risk in population
of Eastern Croatia. Coll Antropol. 37:907–911. 2013.PubMed/NCBI
|
|
31
|
Pierce BL, Biggs ML, DeCambre M, et al:
C-reactive protein, interleukin-6, and prostate cancer risk in men
aged 65 years and older. Cancer Causes Control. 20:1193–1203.
2009.PubMed/NCBI
|
|
32
|
Mandal S, Abebe F and Chaudhary J: -174G/C
polymorphism in the interleukin-6 promoter is differently
associated with prostate cancer incidence depending on race. Genet
Mol Res. 13:139–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Dhakal IB, Lang NP and Kadlubar
FF: Polymorphisms in inflammatory genes, plasma antioxidants, and
prostate cancer risk. Cancer Causes Control. 21:1437–1444. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dossus L, Kaaks R, Canzian F, et al: PTGS2
and IL6 genetic variation and risk of breast and prostate cancer:
results from the Breast and Prostate Cancer Cohort Consortium
(BPC3). Carcinogenesis. 31:455–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang MH, Helzlsouer KJ, Smith MW, et al:
Association of IL10 and other immune response- and obesity-related
genes with prostate cancer in CLUE II. Prostate. 69:874–885. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moore SC, Leitzmann MF, Albanes D, et al:
Adipokine genes and prostate cancer risk. Int J Cancer.
124:869–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bao S, Yang W, Zhou S and Ye Z:
Relationship between single nucleotide polymorphisms in -174G/C and
-634C/G promoter region of interleukin-6 and prostate cancer. J
Huazhong Univ Sci Technolog Med Sci. 28:693–696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kesarwani P, Ahirwar DK, Mandhani A and
Mittal RD: Association between -174 G/C promoter polymorphism of
the interleukin-6 gene and progression of prostate cancer in North
Indian population. DNA Cell Biol. 27:505–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Michaud DS, Daugherty SE, Berndt SI, et
al: Genetic polymorphisms of interleukin-1B (IL-1B), IL-6, IL-8,
and IL-10 and risk of prostate cancer. Cancer Res. 66:4525–4530.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zabaleta J, Su LJ, Lin HY, et al: Cytokine
genetic polymorphisms and prostate cancer aggressiveness.
Carcinogenesis. 30:1358–1362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Magalhães JF, Cortinhas AF, Albuquerque
CM, et al: Interleukin-6 gene -174G>C and -636G>C promoter
polymorphisms and prostate cancer risk. Mol Biol Rep. 40:449–455.
2013.
|
|
42
|
Zhang H, Xu Y, Li L, Liu R and Ma B: The
interleukin-6 -174G/C polymorphism and prostate cancer risk: a
systematic review and meta-analysis. Urol Int. 88:447–453. 2012.
View Article : Google Scholar : PubMed/NCBI
|