Introduction
Fetal bovine or calf serum (FBS or FCS,
respectively) includes unspecified amounts of growth factors, which
may modify the immune response and cell differentiation (1–4). In
addition, cell growth and differentiation may be affected in cell
culture, depending on the amount of FBS or FCS present.
A previous study demonstrated that FBS inhibited
cell growth and increased apoptosis (4). Compared to FBS-supplemented media,
serum-free media does not differ between individual batches, and
thus, it may be suitable for the cell culture performance. There
are several types of serum-free media, specific media is suitable
for stem cells and others are acceptable for primary cell culture
(5,6). These serum-free media do not affect
cell growth and differentiation in cell culture. STK2 is a
serum-free medium that has been developed for use with human
mesenchymal stem cells (hMSC) (7).
STK2 had a minimal effect on the gene expression and morphology of
hMSC after 50 days of culture, whereas Dulbecco’s modified Eagle’s
medium (DMEM) with 10% FBS significantly changed the expression of
~1,000 genes and the cell morphology (7). Although STK2 is suitable for hMSC
culture, it remains unknown whether STK2 can promote the growth of
normal and cancer cells.
CA9-22 and HSC-3 are human oral squamous cell
carcinoma cell lines that are often used for in vitro
assays. CA9-22 highly expresses epidermal growth factor receptor
(EGFR), whereas HSC-3 is prone to lymph node metastasis (8,9).
Compared to HSC-3, it appears likely that histone deacetylase 2 is
highly expressed in CA9-22 (10).
MSTO is a human malignant mesothelioma cell-line that highly
expresses EGFR (11). These cells
have been cultured previously using general medium with 10% FBS
(12–14). The present study aimed to examine
how STK1 or STK2 affected the cell growth of human gingival
fibroblasts (HGF-1), CA9-22, HSC-3 and MSTO by the
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-
methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. STK1
increased the proliferation of HGF-1 compared to DMEM, whereas STK1
and STK2 inhibited the proliferation of HSC-3 and MSTO. CA9-22 was
able to grow with STK, but the proliferation rate of CA9-22
cultured with STK was lower than with DMEM. These results indicate
that STK affects cell growth in the different cell lines, HGF-1,
CA9-22, HSC-3 and MSTO.
Materials and methods
Cell culture
HGF-1 and MSTO mesothelioma cells were obtained from
the American Type Culture Collection (Manassas, VA, USA). CA9-22
and HSC-3 human oral cancer cell lines were obtained from the
Japanese Cancer Research Resources Bank (Tokyo, Japan). These cells
were cultured with DMEM-high glucose (Sigma-Aldrich, St Louis, MO,
USA) supplemented with 10% FBS, STK1 or STK2 serum-free media (DS
Pharma Biomedical Co., Ltd., Osaka, Japan) at 37°C in a humidified
atmosphere of 95% air and 5% CO2. Images of the cells
were captured using a Zeiss microscope (Carl Zeiss, Oberkochen,
Germany).
Cell viability assay
The cell viability assay was performed using the MTS
assay. The cells were seeded in 96-well plates. After culturing for
24 h, the culture medium was changed to DMEM, STK1 or STK2, and the
culture was continued for 96 h. CellTiter 96®
AQueous One Solution reagent (Promega Corporation,
Madison, WI, USA) was added to each well and the cells were
incubated at 37°C for 1 h. The absorbance (OD490 nm) was
measured using a 96-well plate reader of iMark Microplate
Absorbance Reader (Bio-Rad, Tokyo, Japan).
Statistical analysis
The data are provided as mean ± standard deviation.
Student’s t-test was used for statistical analysis (P<0.05).
Results
STK inhibits the cell proliferation of
HSC-3 and MSTO
The affect of STK1 or STK2 was examined with regards
to the cell growth of normal and cancer cells by the MTS assay.
HGF-1, CA9-22, HSC-3 and MSTO cells were cultured with DMEM, STK1,
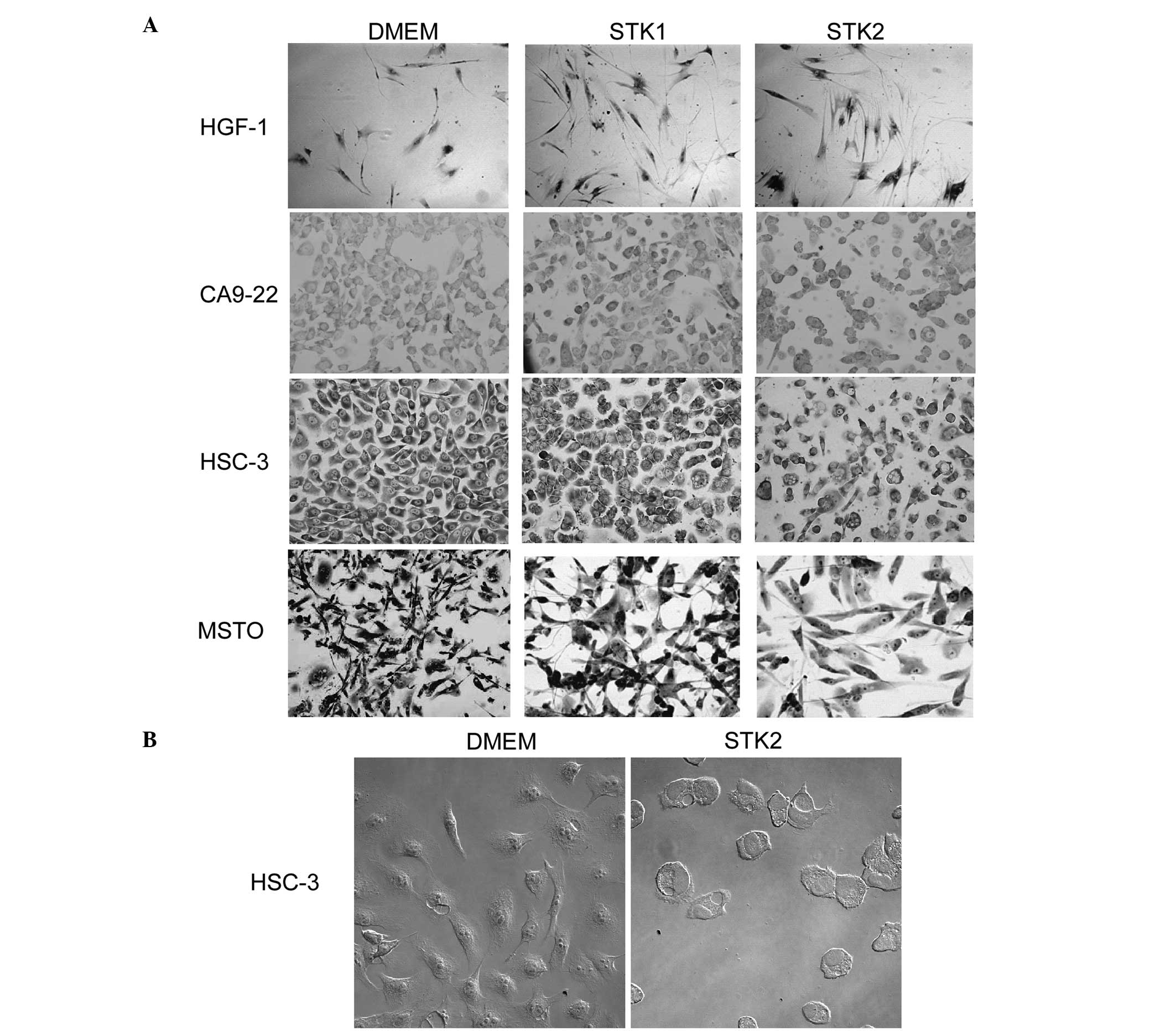
or STK2 for 24–96 h. The cell proliferation of HSC-3 at 96 h after
the medium was changed was markedly inhibited by STK1 and STK2. For
MSTO, some floating cells were observed when cultured with STK1
(Fig. 1A). STK2 also affected the
cell morphology of HSC-3, which changed to a round shape (Fig. 1B). The cell proliferation rates of
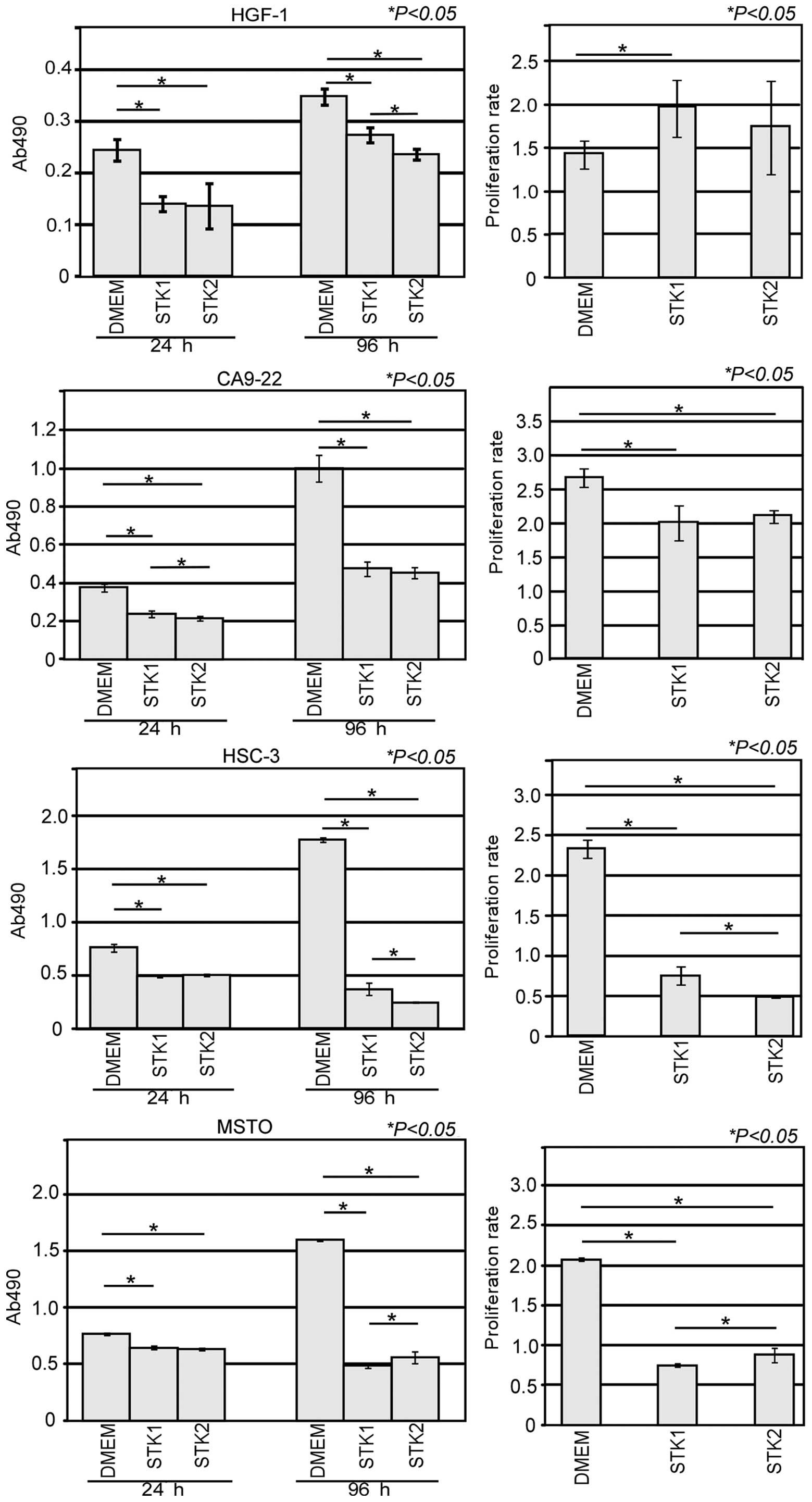
HGF-1 cultured with DMEM, STK1 and STK2 were 1.4, 2.0 and 1.7-fold,
respectively (Fig. 2). There was a
significant increase in the cell proliferation rate between DMEM
and STK1 (P<0.05), whereas there was no significant difference
between DMEM and STK2 or STK1 and STK2. The cell proliferation
rates of CA9-22 cultured with DMEM, STK1 and STK2 were 2.7, 2.0 and
2.1-fold, respectively. There was a significant decrease in the
cell proliferation rate between DMEM and STK1, or DMEM and STK2
(P<0.05), whereas there was no significant difference in the
cell proliferation rate between STK1 and STK2. The cell
proliferation rates of HSC-3 cultured with DMEM, STK1 and STK2 were
2.3, 0.8 and 0.5-fold, respectively. There were significant
decreases in the cell proliferation rates between DMEM and STK1,
between DMEM and STK2 (P<0.05), and between STK1 and STK2
(P<0.05). The cell proliferation rates of MSTO cultured with
DMEM, STK1 and STK2 were 2.1, 0.7 and 0.9-fold, respectively. There
were significant decreases in the cell proliferation rates between
DMEM and STK1, and DMEM and STK2 (P<0.05), and there was also a
significant increase in the cell proliferation rate between STK1
and STK2 (P<0.05).
Discussion
Previously, certain studies have performed cell
culture without FBS or FCS for pre-clinical data or embryonic stem
cells, as the serum affects the cell culture performance (15–17).
STK1 and STK2 are serum-free media with unclear roles in the
culture of normal and cancer cells. In the present study, the STK
media were demonstrated to inhibit the cell proliferation of HSC-3
and MSTO, whereas they increased the cell proliferation of HGF-1
and CA9-22. When cultured with DMEM, these cancer cells grew well
compared to STK1 and STK2. There are several possible explanations
as to why STK media inhibited HSC-3 and MSTO cell proliferation.
First, STK may have induced apoptosis or cell cycle arrest in HSC-3
and MSTO cells, as STK2 was found to induce a round shape in HSC-3.
In addition, MTSO cultured with STK1 gave rise to a number of
floating or detached cells. Whether this was due to apoptosis or
detachment, and the explanation for why STK1 did not induce as many
floating cells with HSC-3, could not be elucidated. STK1 may also
possibly affect MSTO cell adhesion molecules, including β-catenin,
claudins and E-cadherin (18,19).
CA9-22 and MSTO highly express EGFR, which may give
rise to different proliferation rates between these two cell types
when cultured with STK. Additionally, other factors may regulate
cell proliferation, including the clock genes, PER1 and
PER3, which are closely associated with cisplatin-induced
apoptosis in CA9-22 (8,20). PER1 is highly expressed in CA9-22,
whereas it is weakly expressed in HGF-1. Deleted in esophageal
cancer 1 (DEC1) regulates cell proliferation under a serum-free
condition (21). These molecules
are closely associated with the regulation of apoptosis, cell cycle
arrest and cell proliferation (20,22,23).
Therefore, it can be speculated that PER1, PER3 and DEC1 may be
involved in the regulation of cell proliferation by STK. These
studies indicate that HSC-3 and MSTO cells may require more
nutritional components than HGF-1 and CA9-22. Therefore, future
studies should clarify how STK affects apoptosis and the cell
cycle.
Acknowledgements
The present study was supported in part by
Grants-in-Aid for Science from the Ministry of Education, Culture,
Sports, Science and Technology of Japan (to Y.M. and Y.K.).
References
|
1
|
Kading VH, Blalock JE and Gifford GE:
Effect of serum on the antiviral and anticellular activities of
mouse interferon. Arch Virol. 56:237–242. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toldbod HE, Agger R, Bolund L and Hokland
M: Potent influence of bovine serum proteins in experimental
dendritic cell-based vaccination protocols. Scand J Immunol.
58:43–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qin ZL, Ju HP, Liu Y, Gao TT, Wang WB,
Aurelian L, Zhao P and Qi ZT: Fetal bovine serum inhibits hepatitis
C virus attachment to host cells. J Virol Methods. 193:261–269.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberg K, Ceder R, Farnebo L,
Norberg-Spaak L and Grafström RC: Multiple genotypic aberrances
associate to terminal differentiation-deficiency of an oral
squamous cell carcinoma in serum-free culture. Differentiation.
76:868–880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu ZZ, Chen P, Lu ZD, Cui SD and Dong ZM:
Enrichment of breast cancer stem cells using a keratinocyte
serum-free medium. Chin Med J (Engl). 124:2934–2936.
2011.PubMed/NCBI
|
|
6
|
Mark P, Kleinsorge M, Gaebel R, Lux CA,
Toelk A, Pittermann E, David R, Steinhoff G and Ma N: Human
mesenchymal stem cells display reduced expression of CD105 after
culture in serum-free medium. Stem Cells Int. 2013:6980762013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawada R, Yamada T, Tsuchiya T and
Matsuoka A: Microarray analysis of the effects of serum-free medium
on gene expression changes in human mesenchymal stem cells during
the in vitro culture. Yakugaku Zasshi. 130:1387–1393. 2010.(In
Japanese).
|
|
8
|
Hirai M, Gamou S, Minoshima S and Shimizu
N: Two independent mechanisms for escaping epidermal growth
factor-mediated growth inhibition in epidermal growth factor
receptor-hyperproducing human tumor cells. J Cell Biol.
107:791–799. 1988. View Article : Google Scholar
|
|
9
|
Matsui T, Ota T, Ueda Y, Tanino M and
Odashima S: Isolation of a highly metastatic cell line to lymph
node in human oral squamous cell carcinoma by orthotopic
implantation in nude mice. Oral Oncol. 34:253–256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang CC, Lin BR, Chen ST, Hsieh TH, Li YJ
and Kuo MY: HDAC2 promotes cell migration/invasion abilities
through HIF-1α stabilization in human oral squamous cell carcinoma.
J Oral Pathol Med. 40:567–575. 2011.PubMed/NCBI
|
|
11
|
Jänne PA, Taffaro ML, Salgia R and Johnson
BE: Inhibition of epidermal growth factor receptor signaling in
malignant pleural mesothelioma. Cancer Res. 62:5242–5247. 2002.
|
|
12
|
Bourguignon LY, Earle C, Wong G, Spevak CC
and Krueger K: Stem cell marker (Nanog) and Stat-3 signaling
promote MicroRNA-21 expression and chemoresistance in
hyaluronan/CD44-activated head and neck squamous cell carcinoma
cells. Oncogene. 31:149–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto K, Tominaga K, Sukedai M, Okinaga
T, Iwanaga K, Nishihara T and Fukuda J: Delivery of cytolethal
distending toxin B induces cell cycle arrest and apoptosis in
gingival squamous cell carcinoma in vitro. Eur J Oral Sci.
112:445–451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiang SL, Jiang SS, Wang YJ, Chiang HC,
Chen PH, Tu HP, Ho KY, Tsai YS, Chang IS and Ko YC:
Characterization of arecoline-induced effects on cytotoxicity in
normal human gingival fibroblasts by global gene expression
profiling. Toxicol Sci. 100:66–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SJ and Diamond B: Generation and
maturation of bone marrow-derived DCs under serum-free conditions.
J Immunol Methods. 323:101–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Yokohama-Tamaki T and Tanaka TS:
Short-term serum-free culture reveals that inhibition of Gsk3β
induces the tumor-like growth of mouse embryonic stem cells. PLoS
One. 6:e213552011.PubMed/NCBI
|
|
17
|
Millington M, Arndt A, Boyd M, Applegate T
and Shen S: Towards a clinically relevant lentiviral transduction
protocol for primary human CD34 hematopoietic stem/progenitor
cells. PLoS One. 4:e64612009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fassina A, Cappellesso R, Guzzardo V,
Dalla Via L, Piccolo S, Ventura L and Fassan M:
Epithelial-mesenchymal transition in malignant mesothelioma. Mod
Pathol. 25:86–99. 2012. View Article : Google Scholar
|
|
19
|
Ikari A, Atomi K, Takiguchi A, Yamazaki Y,
Hayashi H, Hirakawa J and Sugatani J: Enhancement of cell-cell
contact by claudin-4 in renal epithelial Madin-Darby canine kidney
cells. J Cell Biochem. 113:499–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato F, Wu Y, Bhawal UK, Liu Y, Imaizumi
T, Morohashi S, Kato Y and Kijima H: PERIOD1 (PER1) has
anti-apoptotic effects, and PER3 has pro-apoptotic effects during
cisplatin (CDDP) treatment in human gingival cancer CA9-22 cells.
Eur J Cancer. 47:1747–1758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Zhang H, Xie M, Hu M, Ge S, Yang D,
Wan Y and Yan B: Abundant expression of Dec1/stra13/sharp2 in colon
carcinoma: its antagonizing role in serum deprivation-induced
apoptosis and selective inhibition of procaspase activation.
Biochem J. 367:413–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhawal UK, Sato F, Arakawa Y, Fujimoto K,
Kawamoto T, Tanimoto K, Ito Y, Sasahira T, Sakurai T, Kobayashi M,
et al: Basic helix-loop-helix transcription factor DEC1 negatively
regulates cyclin D1. J Pathol. 224:420–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gery S, Komatsu N, Baldjyan L, Yu A, Koo D
and Koeffler HP: The circadian gene per1 plays an important role in
cell growth and DNA damage control in human cancer cells. Mol Cell.
22:375–382. 2006. View Article : Google Scholar : PubMed/NCBI
|