Introduction
Lung cancer is a major cause of cancer-related
mortality in humans worldwide (1).
In Korea, 21.7% of cancer mortality in 2010 was due to lung cancer
and the number of new cases is predicted to continue to rise
(2,3). As the majority of lung cancer patients
have advanced disease at diagnosis, they are not candidates for
curative surgery. In addition, the methods for the early detection
of lung cancer have been proven to be elusive, and as a result,
prognostic improvement of this type of cancer has not been
successfully achieved (4).
Therefore, it is necessary to identify early diagnostic biomarkers
to improve the clinical outcome of lung cancer patients.
In the normal state, hemostasis and angiogenesis are
physiological processes that are strictly regulated to adjust to
tissue remodeling and wound healing requirements. However, this
ability is destroyed when cancer cells proliferate (5). For tumor growth, invasion and
metastasis, the activation of exogenous coagulation and
fibrinolysis is required. Therefore, the topical generation of
thrombin and fibrinolysis are extremely important factors for the
growth and spread of tumors (6,7). Tumor
cells release either coagulation factors, which directly activate
the coagulation pathway resulting in thrombin formation, or
plasminogen activators (PA), which directly activate the
fibrinolytic system. Thrombin acts as a growth factor for tumor
cells and facilitates tumor angiogenesis leading to fibrin
formation. The deposition of fibrin in cancer tissues acts as a
barrier against inflammatory cells that may destroy the tumor. PA
generates plasmin that promotes invasion and migration of tumor
cells into the circulation. As plasmin is also an active serine
protease in fibrinolysis (8), PA
affects the production of fibrin-fibrinogen degradation products
(FDP) in cancer cells. Numerous studies have demonstrated that
serum FDP is elevated in patients with various types of cancer
(9–11). Therefore, measuring serum FDP can be
useful for tumor detection.
The majority of FDP tests use latex agglutination,
turbidimetry or reflectometry, which mainly quantify the D and E
fragments of fibrin in plasma samples. The DR-70 immunoassay, a
commercially available polyclonal anti-FDP antibody-based
immunoassay, quantifies all the products of cancer-induced FDP,
including the D and E fragments and D-dimers in serum. Thus, the
DR-70 immunoassay is more sensitive than conventional FDP tests
(12). The cytokeratin 19
fragmentation antigen, CYFRA 21-1, is a polypeptide that recognizes
soluble cytokeratin 19 fragments and is a well-established
biomarker for lung cancer. Cytokeratin 19 is an acidic type I
cytokeratin that is expressed in all simple epithelia and in
carcinomas, including lung cancer, and it is a sensitive tumor
marker, particularly for non-small cell lung cancer (13–16).
Although the DR-70 immunoassay has been reported to
be clinically sensitive for the detection of several malignancies
(9,12,17–23),
extremely few studies have investigated the clinical efficiency of
DR-70 for the detection of lung cancer. A number of studies have
demonstrated that hemostatic alterations are frequently observed in
lung cancer patients and the degree of coagulation and fibrinolysis
activation has been correlated with the clinical progression of the
disease (5,24,25).
The aim of the present study was to investigate the diagnostic
value of the DR-70 immunoassay in lung cancer and to compare the
sensitivity and specificity of FDP with CYFRA 21-1 as biomarkers of
lung cancer.
Materials and methods
Patients
Serum samples were obtained from 193 patients
diagnosed with lung cancer between July 2007 and December 2009 at
Korea Cancer Center Hospital (Seoul, Korea) and from 84 healthy
controls from the blood specimen biobank of the hospital. An
additional 106 serum samples from patients with benign respiratory
diseases (86 asthma patients, 10 chronic obstructive pulmonary
disease, eight tuberculosis, one pneumonia and one aspergillosis
patient) were provided by Soonchunhyang University Bucheon Hospital
Biobank (Bucheon, Korea). All the samples of benign lung diseases
were obtained at the time of diagnosis, whereas the serum from
patients with cancer were collected prior to the surgery. Lung
cancer diagnosis and staging was determined using whole-body
computed tomography, percutaneous needle aspiration and
bronchoscopy with biopsy. Histopathological evaluation was
performed according to the revised World Health Organization
classification of lung tumors (26). The characteristics of the study
population are shown in Table I.
The median age of the patients with cancer was 62 years (range,
8–81 years) with a male-to-female ratio of 7.8:1. The
male-to-female ratio and the median age of the patients in the
benign lung disease group were 3.1:1 and 47 years (range, 17–80
years), respectively, whereas these values for the healthy control
group were 7.4:1 and 45 years (range, 28–75 years), respectively.
Smoking history was not available for 101 patients (12 lung cancer
patients, five benign lung disease group and all 84 healthy control
group patients). Among the remaining 282 patients, the smoker vs.
non-smoker ratios were 2.1:1 in the lung cancer patients and 0.5:1
in patients with benign lung diseases. The approval for the use of
human sera was obtained by the Institutional Review Board of Korea
Institute of Radiological and Medical Sciences
(K-1111-002-026).
 | Table ICharacteristics of the study
population. |
Table I
Characteristics of the study
population.
| Characteristics | Lung cancer patients
(n=193) | Benign lung disease
patients (n=106) | Healthy controls
(n=84) |
|---|
| Gender. n |
| Male | 171 | 80 | 74 |
| Female | 22 | 26 | 10 |
| Age, years
(range) | 62 (8–81) | 47 (17–80) | 45 (28–75) |
| Current smoking,
n | | | NE |
| Yes | 123 | 33 | |
| No | 58 | 68 | |
| Unknown | 12 | 5 | |
| Types, n (%) |
| SCLC | 7 (3.6) | | |
| ADC | 63 (32.6) | | |
| SCC | 72 (37.3) | | |
| Othersa | 51 (26.4) | | |
| Stage, n (%) |
| I | 58 (30.1) | | |
| II | 32 (16.6) | | |
| III | 38 (19.7) | | |
| IV | 65 (33.7) | | |
Detection of FDP and CYFRA 21-1
The concentrations of all the forms of FDP in the
serum were measured with the DR-70 immunoassay (AMDL Inc., Tustin,
CA, USA) in accordance with the manufacturer’s instructions. DR-70
is an enzyme-linked immunosorbent assay-based sandwich method.
Briefly, 100 μl of serum was diluted 1:200 and incubated in the
wells coated with affinity-purified rabbit anti-DR-70 antibodies
(AMDL Inc.,) for 30 min at room temperature. The wells were washed
and subsequently incubated with 100 μl horseradish
peroxidase-conjugated anti-DR-70 antibodies. Following additional
washes, 3,3′,5,5′-tetramethylbenzidine was added and the wells were
incubated in the dark at 25°C for 15 min. The reaction was
terminated by adding 100 μl stop solution and the intensity of the
color formed was read at 450 nm.
Serum CYFRA 21-1 was determined by an
electrochemiluminescence immunoassay system Roche Modular Analytics
E170 module (Roche Diagnostics GmbH, Mannheim, Germany).
Statistical analysis
The differences in serum concentration between the
groups were analyzed by the Student’s t-test and one-way analysis
of variance (ANOVA). Adjustment of age and gender was also
performed by two-way ANOVA. The optimal cut-off values for FDP and
CYFRA 21-1 were obtained by receiver operating characteristics
curve analysis. Diagnostic performance was described in terms of
sensitivity, specificity and area under the curve (AUC) by receiver
operating characteristics analysis. The association between FDP and
CYFRA 21-1 in lung cancer patients was assessed by Pearson’s
correlation test. P<0.05 was considered to indicate a
statistically significant difference. All the statistical data
analyses were performed using SALT Version 2.0 (Istech Inc., Korea)
software.
Results
Serum levels of tumor markers
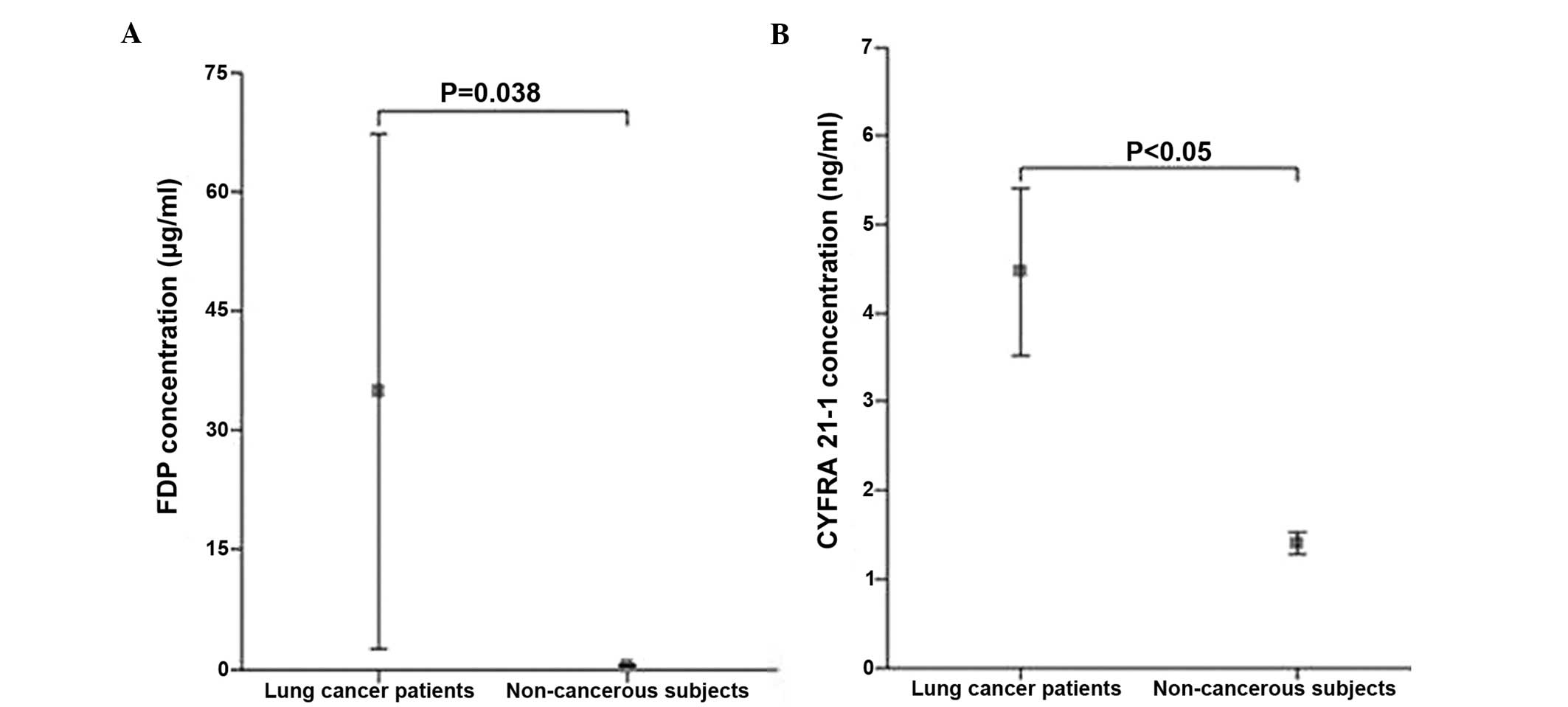
The mean serum FDP level in lung cancer patients was
35.01±229.02 μg/ml (range, 0.30–2200.00 μg/ml), which was
significantly higher compared to the non-cancerous subjects,
including patients with benign lung diseases and healthy controls
(0.60±0.75 μg/ml; range, 0.20–8.90 μg/ml; P=0.039). The mean serum
CYFRA 21-1 level was significantly higher in lung cancer patients
(4.50±6.70 ng/ml; range, 0.50–51.30 ng/ml) compared to the
non-cancerous subjects (1.40±0.83 ng/ml; range, 0.20–5.70 ng/ml;
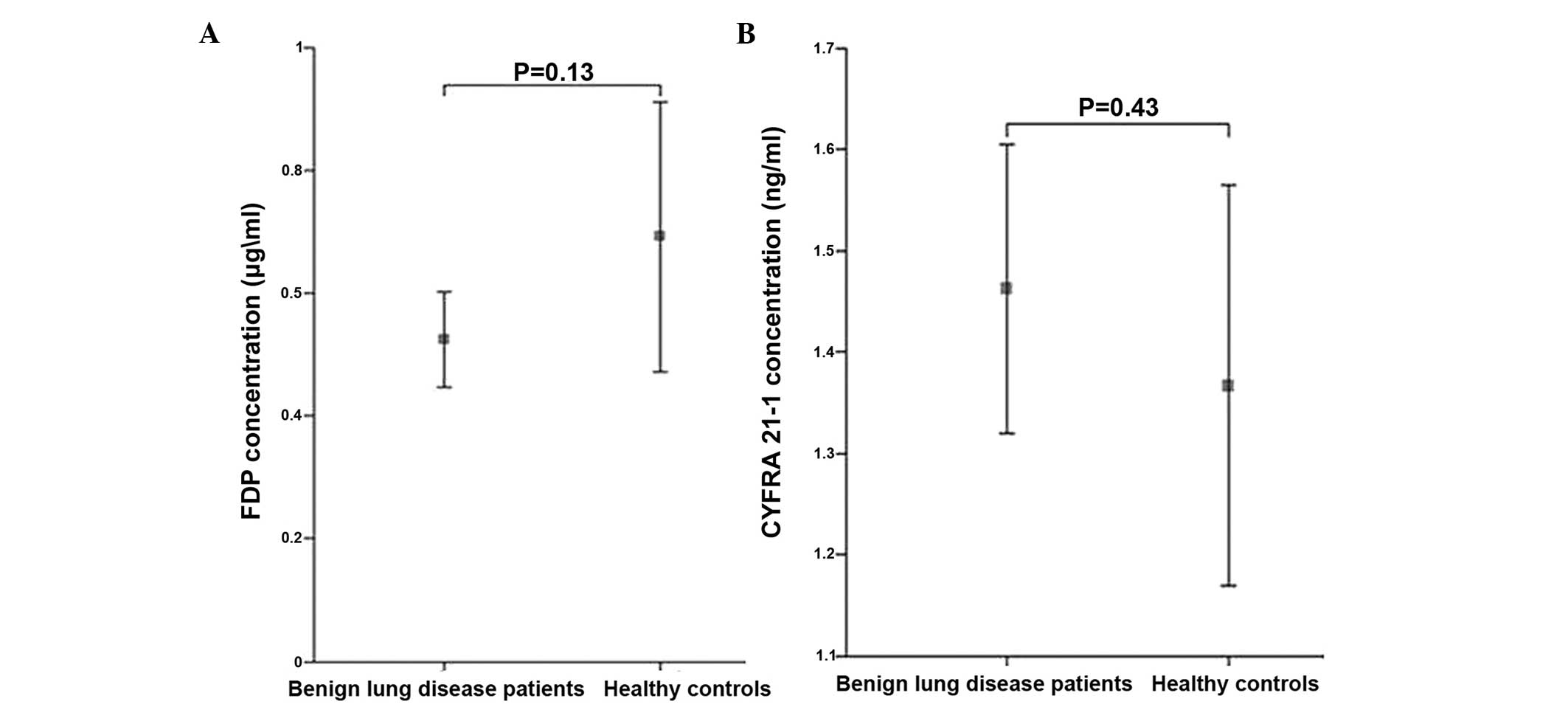
P<0.05) (Fig. 1). No significant
differences for the tumor markers were observed between the
patients with benign lung diseases and the healthy control group
(Fig. 2).
Association between the tumor markers and
pretreatment clinicopathological characteristics in lung
cancer
The mean levels of each tumor marker stratified by
clinicopathological characteristics of lung cancer patients are
listed in Table II. The levels of
FDP and CYFRA 21-1 were significantly higher in current smokers
compared to non-smokers (P=0.044 and P<0.001, respectively).
There were no significant differences in the serum levels of FDP or
CYFRA 21-1 based on age, gender and pathological cancer type or
tumor stage, with the exception of a significant increase in the
serum levels of CYFRA 21-1 in squamous cell carcinoma compared to
the other types of cancer.
 | Table IIFDP and CYFRA 21-1 concentrations in
patients with lung cancer. |
Table II
FDP and CYFRA 21-1 concentrations in
patients with lung cancer.
| Variables (n) | FDP, μg/ml mean
(range) | P-value | CYFRA 21-1, ng/ml
mean (range) | P-value |
|---|
| Age, years |
| ≤62 (105) | 10.3 (0.3–912.6) | 0.131 | 3.9 (0.5–51.3) | 0.263 |
| >62 (88) | 64.5
(0.3–2200.0) | | 5.1 (0.9–23.3) | |
| Gender |
| Male (171) | 39.4
(0.3–2200.0) | 0.464 | 5.4 (0.5–123.0) | 0.285 |
| Female (22) | 1.3 (0.3–2.9) | | 2.8 (1.0–14.6) | |
| Current
smokinga |
| Yes (123) | 54.0
(0.3–2200.0) | 0.044 | 5.6 (0.6–51.3) | <0.001 |
| No (58) | 1.7 (0.3–10.2) | | 2.5 (0.6–10.9) | |
| Type |
| SCLC (7) | 2.4 (0.7–9.4) | 0.629 | 4.3 (1.8–9.0) | 0.048 |
| ADC (63) | 46.2
(0.3–2200.0) | | 4.4 (0.9–51.3) | |
| SCC (72) | 51.8
(0.3–1892.8) | | 7.7 (0.6–123) | |
| Othersb (51) | 1.9 (0.3–10.2) | | 2.3 (0.6–20.1) | |
| Stage |
| I (58) | 96.8
(0.3–2200.0) | 0.095 | 4.9 (0.6–47.9) | 0.460 |
| II (32) | 1.4 (0.3–2.7) | | 6.1 (1.2–23.3) | |
| III (38) | 25.7
(0.3–912.6) | | 6.9
(1.3–123.0) | |
| IV (65) | 1.9 (0.3–9.4) | | 3.6 (0.5–51.3) | |
Diagnostic performance of tumor markers
for lung cancer
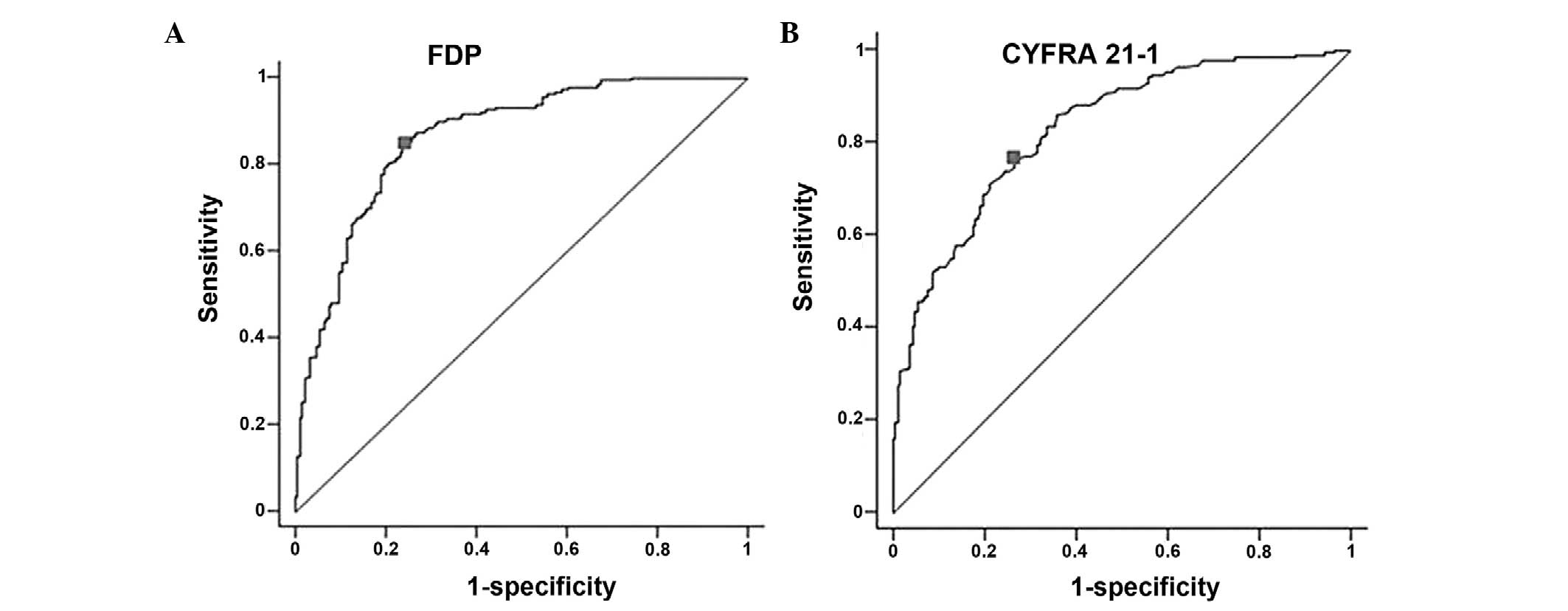
The accuracy, sensitivity and specificity of serum
FDP for the diagnosis of lung cancer at a cut-off value of 0.67
μg/ml were 80, 86 and 75%, respectively (Fig. 3A). The diagnostic accuracy,
sensitivity and specificity of CYFRA 21-1 in the same serum samples
at a cut-off value of 1.65 ng/ml were 75, 77 and 74%, respectively
(Fig. 3B). The AUC for FDP was 0.87
[95% confidence interval (CI), 0.83–0.90], which was higher than
that of the AUC for CYFRA 21-1 at 0.83 (95% CI, 0.79–0.87). The
sensitivity and specificity of serum FDP combined with serum CYFRA
21-1 were 95 and 57%, respectively.
Correlation of serum FDP with CYFRA
21-1
There was no correlation observed between the serum
FDP and CYFRA 21-1 levels in lung cancer patients (r=0.038;
P=0.599).
Discussion
The present study demonstrated that the mean serum
FDP level was higher in lung cancer patients compared to the
non-cancerous subjects. Consistent differences in the level of
components, such as FDP, are the pathophysiological basis for the
detection of lung cancer using tumor markers. Additionally, FDP had
a high lung cancer detection rate with a sensitivity of 85% and
specificity of 75% at a cut-off value of 0.67 μg/ml. These results
are comparable to those of CYFRA 21-1, which had a sensitivity and
specificity of 77 and 74%, respectively, at a cut-off value of 1.65
ng/ml. In comparison to CYFRA 21-1, FDP appeared to be improved
with regards to clinical performance expressed as AUC (0.87 vs.
0.83, respectively) for the detection of lung cancer.
The results of the present study are consistent with
previous studies. In 1995, Fields et al (27) first reported the results of a
clinical trial using the DR-70 immunoassay for the detection of
lung cancer. The overall sensitivity of the assay was 66% at a
specificity of 92%. The mean level of FDP in lung cancer patients
was ~4 times higher compared to the normal controls. Wu et
al (28) found that the FDP
levels increased in lung cancer patients, with an 86% diagnostic
sensitivity and a specificity of 96%. In contrast to the small
sample sizes used in earlier studies assessing the DR-70
immunoassay for lung cancer detection (9,27–29),
the sample size in the present study was considerably larger
(n=193), allowing the application of more accurate parametric
statistics.
The serum FDP levels were compared to CYFRA 21-1,
which is a relatively well established lung cancer marker. To the
best of our knowledge, this is the first study regarding the
comparison between these two biomarkers. When considering FDP as a
routine laboratory test for lung cancer, the present results could
provide certain practical information. No correlation was found
between FDP and CYFRA 21-1. The mechanisms by which these two
biomarkers are generated during carcinogenesis are different and
this may be the reason for the poor correlation. Although the
combination of FDP with CYFRA 21-1 increased the diagnostic
sensitivity <95%, this occurred at the expense of specificity.
Accordingly, it would not be recommended to assess the two markers
simultaneously for clinical purposes.
Kerber et al (18) demonstrated that the FDP levels
increased as the stage of gastrointestinal cancer advanced. In
addition, the level of FDP was positively correlated with the tumor
load and the number of metastatic sites. In the present study, the
serum FDP levels increased in lung cancer patients, but the
expression level was not correlated with the histological subtype
of the tumor or the tumor stage. The lack of correlation in the
present study may be due to the uneven number of patients among the
histological subtypes or stages of lung cancer.
The present study has certain limitations. The
significance of the serum FDP levels was not assessed as a marker
for monitoring lung cancer progression. Furthermore, the smoking
history of 101 subjects was unknown. The serum levels of FDP and
CYFRA 21-1 have been previously reported to be unaffected by
smoking (30). In the present
study, a difference in the mean serum FDP levels based on smoking
status was observed in lung cancer patients. However, this
difference was not observed in patients with benign lung disease.
The incomplete evaluation of the smoking history among the study
subjects makes it difficult to establish a link between the serum
FDP levels and smoking.
The present study focused on lung cancer patients in
Korea, and to the best of our knowledge, it is the first study to
compare lung cancer patients with healthy controls and patients
with benign lung diseases. The comparison of serum FDP with an
accepted lung cancer marker, CYFRA 21-1, also enhances the value of
the study. Further studies, including survival rate analysis and
long-term follow-up, are required to evaluate FDP as a monitoring
marker for lung cancer. Taken together, the results of the present
study indicate that the serum FDP levels measured by the DR-70
immunoassay can be used as a lung cancer marker in clinical
laboratories.
Acknowledgements
The biospecimens for the present study were provided
by the KIRAMS Radiation Biobank and the Soonchunhyang University
Bucheon Hospital Biobank, a member of the National Biobank of
Korea, which is supported by the Ministry of Health and
Welfare.
References
|
1
|
Kim HR, Oh IJ, Shin MG, et al: Plasma
proGRP concentration is sensitive and specific for discriminating
small cell lung cancer from nonmalignant conditions or non-small
cell lung cancer. J Korean Med Sci. 26:625–630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung KW, Won YJ, Kong HJ, et al: Cancer
statistics in Korea: incidence, mortality, survival and prevalence
in 2010. Cancer Res Treat. 45:1–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
van Zandwijk N: New methods for early
diagnosis of lung cancer. Lung Cancer. 38:S9–S11. 2002.PubMed/NCBI
|
|
5
|
Altiay G, Ciftci A, Demir M, et al: High
plasma D-dimer level is associated with decreased survival in
patients with lung cancer. Clin Oncol (R Coll Radiol). 19:494–498.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Falanga A and Rickles FR: Pathophysiology
of the thrombophilic state in the cancer patient. Semin Thromb
Hemost. 25:173–182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwaan HC and Keer HN: Fibrinolysis and
cancer. Semin Thromb Hemost. 16:230–235. 1990. View Article : Google Scholar
|
|
8
|
Unsal E, Atalay F, Atikcan S and Yilmaz A:
Prognostic significance of hemostatic parameters in patients with
lung cancer. Respir Med. 98:93–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu D, Zhou X, Yang G, et al: Clinical
performance of the AMDL DR-70 immunoassay kit for cancer detection.
J Immunoassay. 19:63–72. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oya M, Akiyama Y, Yanagida T, Akao S and
Ishikawa H: Plasma D-dimer level in patients with colorectal
cancer: its role as a tumor marker. Surg Today. 28:373–378. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerner C, Steinkellner W, Holzmann K, et
al: Elevated plasma levels of crosslinked fibrinogen gamma-chain
dimer indicate cancer-related fibrin deposition and fibrinolysis.
Thromb Haemost. 85:494–501. 2001.
|
|
12
|
Lin SZ, Chen CC, Lee KC, et al: DR-70
immunoassay for the surveillance of hepatocellular carcinoma. J
Gastroenterol Hepatol. 27:547–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stieber P, Dienemann H, Hasholzner U, et
al: Comparison of cytokeratin fragment 19 (CYFRA 21–1), tissue
polypeptide antigen (TPA) and tissue polypeptide specific antigen
(TPS) as tumour markers in lung cancer. Eur J Clin Chem Clin
Biochem. 31:689–694. 1993.
|
|
14
|
Pujol JL, Grenier J, Daurès JP, Daver A,
Pujol H and Michel FB: Serum fragment of cytokeratin subunit 19
measured by CYFRA 21–1 immunoradiometric assay as a marker of lung
cancer. Cancer Res. 53:61–66. 1993.PubMed/NCBI
|
|
15
|
Takada M, Masuda N, Matsuura E, et al:
Measurement of cytokeratin 19 fragments as a marker of lung cancer
by CYFRA 21-1 enzyme immunoassay. Br J Cancer. 71:160–165. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stieber P, Hasholzner U, Bodenmuller H, et
al: CYFRA 21-1. A new marker in lung cancer. Cancer. 72:707–713.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma J, Gong Q, Lin M, et al: Combined five
tumor markers in detecting primary hepatic carcinoma. Zhonghua Wai
Ke Za Zhi. 38:14–16. 2000.(In Chinese).
|
|
18
|
Kerber A, Trojan J, Herrlinger K, Zgouras
D, Caspary WF and Braden B: The new DR-70 immunoassay detects
cancer of the gastrointestinal tract: a validation study. Aliment
Pharmacol Ther. 20:983–987. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Qiao Z, Long X, Wei J and Cheng Y:
Serum concentration of AMDL DR-70 for the diagnosis and prognosis
of carcinoma of the tongue. Br J Oral Maxillofac Surg. 43:513–515.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee KH, Cho DH, Kim KM, Kim SM and Lee DJ:
Meaning of the DR-70(TM) immunoassay for patients with the
malignant tumor. Immune Netw. 6:43–51. 2006. View Article : Google Scholar
|
|
21
|
Shimwell NJ, Wei W, Wilson S, et al:
Assessment of novel combinations of biomarkers for the detection of
colorectal cancer. Cancer Biomark. 7:123–132. 2010.PubMed/NCBI
|
|
22
|
Ward DG, Wei W, Buckels J, et al:
Detection of pancreatic adenocarcinoma using circulating fragments
of fibrinogen. Eur J Gastroenterol Hepatol. 22:1358–1363. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Small-Howard AL and Harris H: Advantages
of the AMDL-ELISA DR-70 (FDP) assay over carcinoembryonic antigen
(CEA) for monitoring colorectal cancer patients. J Immunoassay
Immunochem. 31:131–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buccheri G, Torchio P and Ferrigno D:
Plasma levels of D-dimer in lung carcinoma: clinical and prognostic
significance. Cancer. 97:3044–3052. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pavey SJ, Hawson GA and Marsh NA: Impact
of the fibrinolytic enzyme system on prognosis and survival
associated with non-small cell lung carcinoma. Blood Coagul
Fibrinolysis. 12:51–58. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brambilla E, Travis WD, Colby TV, et al:
The new World Health Organization classification of lung tumours.
Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fields A, Poppema S and Jha Nl: Serum
levels of circulating extracellular matrix complex (CEMC) in lung
cancer patients: potential use as a tumor marker. In: 12th
International Conference on Human Tumor Markers; New York, USA.
June 11–14, 1995;
|
|
28
|
Wu D, Zhou X, Anderson G, et al:
Sensitivity and specificity of DR-70 lung cancer immunology. Anal
Lett. 32:1351–1362. 1999. View Article : Google Scholar
|
|
29
|
Ding L, Ping S and Jingmei Y: Application
of tumor marker of DR-70 in the diagnosis of malignant tumors.
Chongqing Med J. 28:1–3. 1999.
|
|
30
|
Kao CH, Hsieh JF, Ho YJ, Tsai SC and Lee
JK: Cytokeratin fragment 19 (CYFRA 21-1) in healthy smokers.
Anticancer Res. 19:4545–4546. 1999.PubMed/NCBI
|