Introduction
Pulmonary fibrosis is a fatal disease characterized
by chronic inflammation and excessive collagen accumulation in the
lung, with an unknown pathogenesis (1). Although a number of scientific
advances have been made, no definitive and effective drug treatment
is available that could improve, or at least inhibit, the
progressive course of this disease (2). Therefore, identifying the molecular
mechanism and effective molecular targets are important for
preventive and therapeutic interventions.
Recent studies have demonstrated that
fibroblast-myofibroblast differentiation, induced by transforming
growth factor-β (TGF-β)/Smad, was involved in the etiology of
pulmonary fibrosis (1,3). Myofibroblasts are the primary
collagen-producing cells in fibrosis, which were commonly
identified by the expression of α-smooth muscle actin (α-SMA) and
intermediate features between the smooth muscle cells and the
fibroblasts. In addition to the TGF-β/Smad signaling pathway, the
Rho/Rho-associated coiled-coil-forming protein kinase (Rock) system
has been shown to play critical roles in organ fibrosis (4,5). The
Rho/Rock signaling pathway has been shown to regulate
myofibroblasts α-SMA expression and its inhibitors improved the
degree of organic fibrosis (6–8).
Whether the Rho/Rock system was involved in the
differentiation of lung fibroblasts and interacted with TGF-β/Smad
pathway remains unclear. In the present study, human embryonic lung
fibroblasts were stimulated by TGF-β1, Y-27632 and staurosporine.
Cell cycle progression, expression of the extracellular matrix
(ECM), RhoA, RhoC, Rock1 and Smad2 were evaluated to determine the
roles and molecular mechanisms of the TGF-β/Smad and the Rho/Rock
signaling pathway in the process of pulmonary fibrosis.
Materials and methods
Cell culture
WI-38 human embryonic lung fibroblasts were provided
by the Health Department of Dalian Medical University (Dalian,
China). The cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; Gibco-BRL, Carlsbad, CA, USA), supplemented with
penicillin/streptomycin (100 U/ml) and L-glutamine (2 mmol/l) with
10% fetal calf serum (FCS; Gibco-BRL) under conditions of
humidified 5% CO2 at 37°C. When cell confluence reached
70–80%, the medium was replaced with serum-free DMEM for 12 h. The
cells were divided into four groups, which were the normal, TGF-β1,
inhibitor of Rho/Rock (Y-27632) and inhibitor of TGF-β/Smad
(staurosporine) groups. The cells in each group were cultured in
DMEM with 10% FCS and stimulated for 48 h with empty vehicle, human
recombinant TGF-β1 (5 ng/ml; Sigma-Aldrich, St. Louis, MO, USA),
Y-27632 (1 μmol/l; Sigma-Aldrich) and TGF-β1 (5 ng/ml),
staurosporine (5 nmol/l; Sigma-Aldrich) and TGF-β1 (5 ng/ml),
respectively. All the experiments were repeated 3 times.
Assessment of α-SMA-positive cell
percentage by flow cytometry (FCM)
The cells were collected and the concentrations were
adjusted to 1×106 cells/ml. The cells were permeabilized
by adding 500 μl Permeabili Eirg Solution (BD Bioscience, Bedford,
MA, USA) to 100 μl of cell suspension for 10 min. Subsequent to
washing with phosphate-buffered saline (PBS), the supernatant was
discarded and the cell pellets were resuspended in 100 μl PBS with
or without 1 μl fluorescein isothiocyanate (FITC)-labeled α-SMA
monoclonal antibody (mAb; 1:100). Following incubation in the dark
for 30 min at room temperature, the cells were washed with PBS and
the supernatant was discarded. The cell pellets were resuspended in
500 μl PBS and analyzed using a flow cytometer (excitation light
488 nm, emission light 530 nm; BD Bioscience). All the data were
analyzed with CellQuest software (BD Bioscience).
Staining for α-SMA by
immunofluorescence
The cells were cultured on coverslips, washed twice
with cold PBS and fixed in cold acetone for 10 min. Subsequent to
thoroughly washing with PBS at room temperature 3 times, the cells
were blocked with non-immune animal serum for 30 min. The slides
were incubated with FITC-labeled α-SMA mAb (1:100) for 90 min at
37°C in a humidified chamber. The slides were visualized with a
fluorescence microscope following washing with PBS.
Cell cycle analysis by FCM
The cells were collected and the cell pellets were
fixed in 75% ethanol overnight at 4°C, and were washed twice with
ice-cold PBS. Each cell pellet (1×106) was resuspended
in 2 μl RNase A and incubated at room temperature for 10 min. For
each sample, 50 μl propidium iodide-staining solution was added,
followed by incubation in the dark for 10 min at 4°C. The cell
cycle profiles were determined for 2×105 cells using
CellQuest modfit software.
Type I collagen, laminin (LN),
fibronectin (FN) and tissue inhibitor of metalloproteinase-1
(TIMP-1) protein expressions by ELISA
The cells were incubated in 6 wells at
1×105 cells/ml in complete culture medium for 24 h,
followed by serum-free DMEM for 12 h. After incubation for 48 h,
the cell culture supernatant was collected for analysis of type I
collagen (Southern Biotech, Birmingham, AL, USA), LN
(Sigma-Aldrich), FN (Sigma-Aldrich) and TIMP-1 (Adlitteram
Diagnostic Laboratories, San Diego, CA, USA) using a sensitive
ELISA kit.
RhoA, RhoC, Rock1, Smad2 and TIMP-1 mRNA
expressions by quantitative polymerase chain reaction (qPCR)
The total cellular RNA was isolated with TRIzol and
mRNA was examined by qPCR according to the manufacturer’s
instructions for the Takara RNA PCR kit 3.0 (AMV; Takara Bio, Inc.,
Shiga, Japan). An equal amount of cDNA from each sample was
amplified using primers specific to each gene (Table I). DNA amplification was performed
using a thermocycler under the following conditions: For
RhoA, RhoC and Rock1, 30 cycles of
denaturation at 94°C for 30 sec, annealing at 51°C for 60 sec and
extension at 72°C for 60 sec; for Smad2, 40 cycles of
denaturation at 94°C for 45 sec, annealing at 51°C for 30 sec and
extension at 72°C for 30 sec; for TIMP-1, 30 cycles of
denaturation at 94°C for 30 sec, annealing at 58°C for 30 sec and
extension at 72°C for 60 sec; and for glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), 35 cycles of denaturation at 94°C for
60 sec, annealing at 60°C for 60 sec and extension at 72°C for 60
sec. The relative expression of the target genes was analyzed by
electrophoresis of the PCR products on 1.5% agarose gels by
comparing the band density to endogenous GAPDH.
 | Table IOligonucleotide primers of the target
genes. |
Table I
Oligonucleotide primers of the target
genes.
| mRNA species | mRNA | PCR product (bp) |
|---|
| RhoA | | 183 |
| Sense |
5′-CTGGTGATTGTTGGTGATGG-3′ | |
| Antisense |
5′-GCGATCATAATCTTCCTGCC-3′ | |
| RhoC | | 181 |
| Sense |
5′-TCCTCATCGTCTTCAGCAAG-3′ | |
| Antisense |
5′-GAGGATGACATCAGTGTCCG-3′ | |
| Rock1 | | 701 |
| Sense |
5′-AGTCTGTGGCAATGTGTGAG-3′ | |
| Antisense |
5′-CTTCAAGCCGACTAACAGTG-3′ | |
| Smad2 | | 248 |
| Sense |
5′-TCTTGATGGTCGTCTCCAGGTA-3′ | |
| Antisense |
5′-GAGGCGGAAGTTCTGTTAGGAT-3′ | |
| TIMP-1 | | 652 |
| Sense |
5′-TTGAATTCCCACCATGGCCCCCTTTGAGCC-3′ | |
| Antisense |
5′-CTGAATTCGCAGGATTCAGGCTATCTG-3′ | |
| GAPDH | | 448 |
| Sense |
5′-ACCACAGTCCATGCCATCAC-3′ | |
| Antisense |
5′-TCCACCACCCTGTTGCTGTA-3′ | |
RhoA, RhoC, Rock1, Smad2 and TIMP-1
protein expressions by western blotting
The total cell proteins were extracted in lysis
buffer containing protease inhibitor. The protein concentrations
were measured using a protein assay reagent kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). A 7.5% polyacrylamide gel
was used to resolve 20 μg of total protein by electrophoresis.
Following electrophoresis, the separated proteins were transferred
to polyvinylidene fluoride membranes (Pall Corporation, Port
Washington, NY, USA). Subsequent to blocking, the membranes were
incubated with primary antibodies (mouse monoclonal anti-TIMP-1 and
anti-RhoA 1:600; goat polyclonal anti-RhoC 1:800; mouse monoclonal
anti-Rock1 1:400; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA; and rabbit polyclonal anti-p-Smad2 1:800; New England Biolabs,
Ipswich, MA, USA) at 4°C overnight. The blots were washed and
incubated with a horseradish peroxidase-conjugated affinipure
rabbit anti-mouse immunoglobulin G (IgG) antibody (1:1,000), rabbit
anti-goat IgG (1:3,000) and goat anti-rabbit IgG (1:3,000;
Zhongshan Gold Bridge Biotech, Beijing, China) at 37°C for 1 h. The
bands were visualized by enhanced chemiluminescence (Santa Cruz
Biotechnology) according to the manufacturer’s instructions and
blots were quantified by a densitometrical analysis that involved
correcting for the background density of each gel. The membranes
were reprobed for GAPDH (Proteintech Group, Inc., Chicago, IL, USA)
to ensure equal loading.
Statistical analysis
Data analysis was performed using SPSS version 18.0
(SPSS, Chicago, IL, USA) statistical software. Data showed a normal
distribution and are expressed as mean ± standard deviation. The
results in different experimental groups were analyzed using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
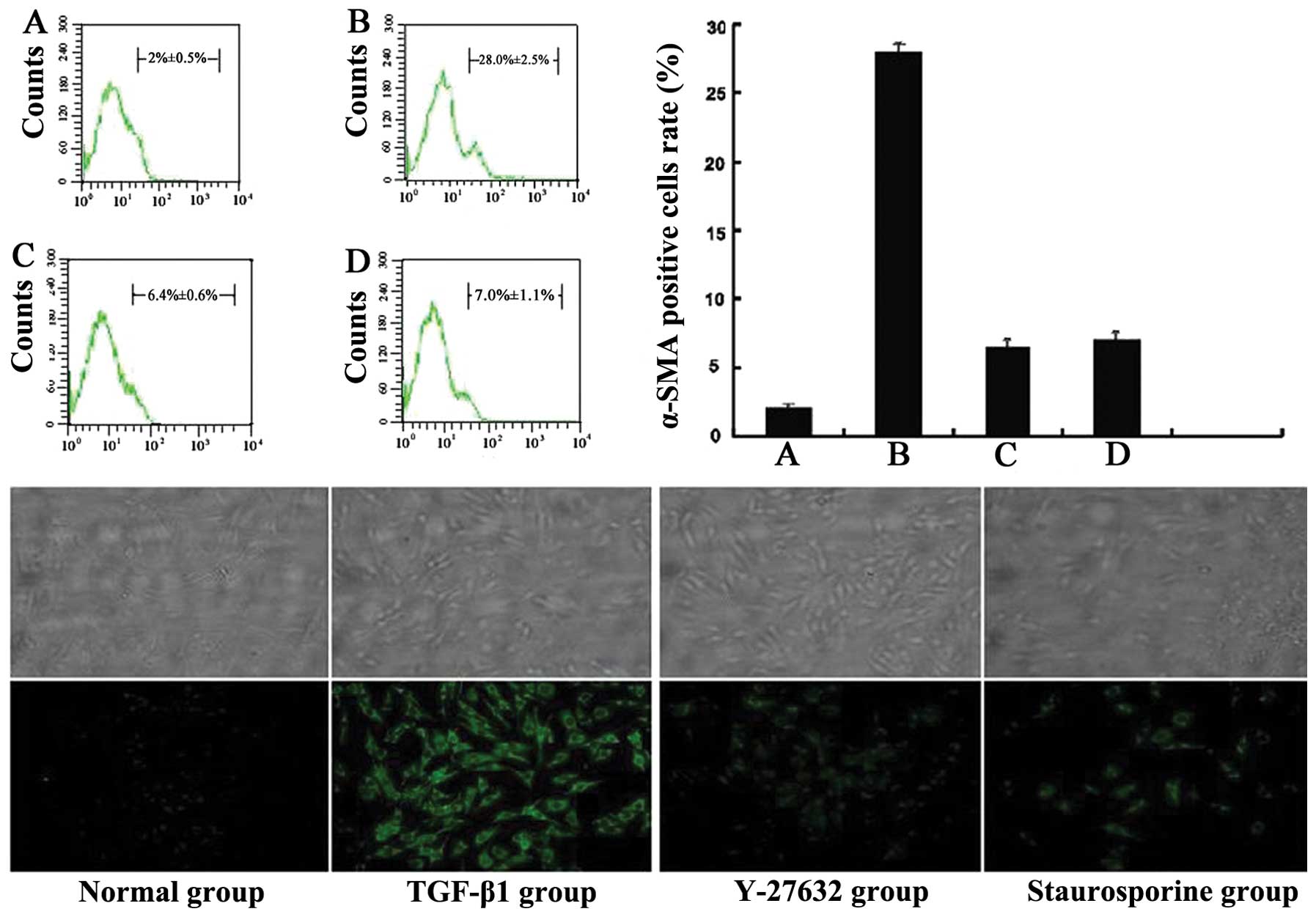
Results of α-SMA expression and
staining
In the TGF-β1 group, the percentage of
α-SMA-positive cells increased significantly compared to the normal
group (P<0.05). In the inhibitor of Rho/Rock and TGF-β/Smad
groups, the percentage decreased significantly compared to the
TGF-β1 group (P<0.05) (Fig.
1A–D).
Immunofluorescence staining revealed that α-SMA was
mainly expressed in cytoplasm. The expression was significantly
increased following induction by TGF-β1, but was suppressed by
Y-27632 and staurosporine (Fig.
1).
Results of cell cycle progression
In the TGF-β1 group, the cell population in the
G0/G1 phase decreased significantly compared
to the normal group (P<0.05), but increased significantly in the
S phase (P<0.05). Following treatment with TGF-β1 combined with
Y-27632 and staurosporine, the cell population in the
G0/G1 phase increased significantly compared
to the TGF-β1 group (P<0.05), but decreased significantly in the
S (P<0.05) and G2/M phase (P<0.05) (Table II).
 | Table IICell cycle progression in different
groups by flow cytometry. |
Table II
Cell cycle progression in different
groups by flow cytometry.
| Group |
G0/G1 | S | G2/M |
|---|
| Normal | 67.33±4.82 | 14.97±1.68 | 17.70±1.98 |
| TGF-β1 | 57.53±3.78a | 25.16±2.12a | 17.31±2.04 |
| Y-27632 | 72.48±6.31b | 18.87±1.38b | 8.65±1.07b |
| Staurosporine | 76.74±5.89b | 20.64±2.58b | 2.62±0.34b |
Results of ELISA
ELISA showed that the concentrations of type I
collagen, LN, FN and TIMP-1 in the cell culture supernatant in the
TGF-β1 group increased significantly compared to the normal group
(P<0.05). In the inhibitor of Rho/Rock and TGF-β/Smad groups,
the concentrations of these factors decreased significantly
compared to the TGF-β1 group (P<0.05) (Table III).
 | Table IIIExpression of type I collagen,
laminin (LN), fibronectin (FN) and tissue inhibitor of
metalloproteinase-1 (TIMP-1) in the cell culture supernatant (mean
± standard deviation). |
Table III
Expression of type I collagen,
laminin (LN), fibronectin (FN) and tissue inhibitor of
metalloproteinase-1 (TIMP-1) in the cell culture supernatant (mean
± standard deviation).
| Group | Type I collagen
(ng/ml) | LN (ng/ml) | FN (pg/ml) | TIMP-1 (μg/ml) |
|---|
| Normal | 193.47±19.37 | 9.28±0.64 | 23.93±7.16 | 4.75±0.86 |
| TGF-β1 |
309.29±13.10a | 16.68±1.97a | 50.60±8.25a | 9.32±1.56a |
| Y-27632 |
230.88±11.05b | 11.72±1.01b | 33.23±8.69b | 5.23±0.76b |
| Staurosporine |
221.43±15.24b | 12.46±1.84b | 29.54±8.02b | 5.08±0.92b |
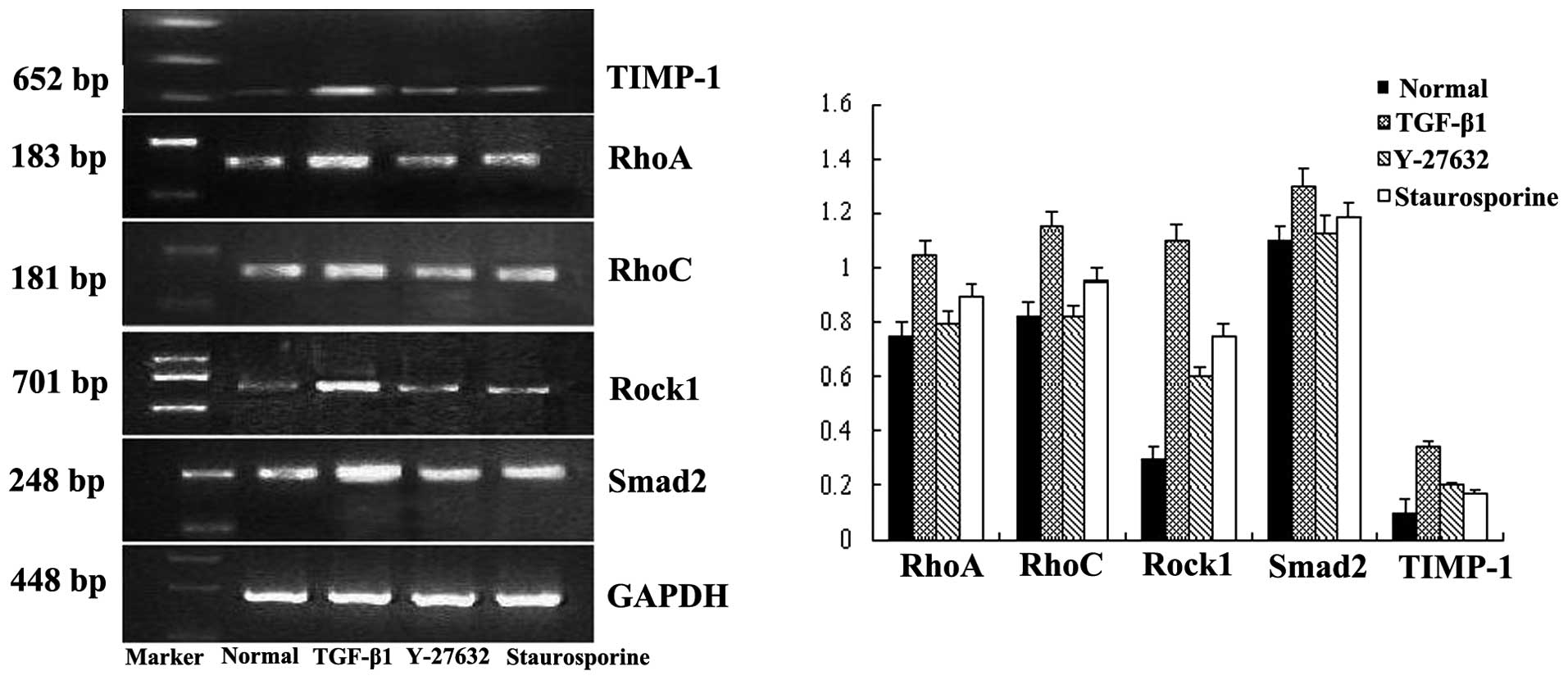
Results of qPCR
The results of qPCR showed that the mRNA expression
of RhoA, RhoC, Rock1, Smad2 and
TIMP-1 in the TGF-β1 group increased significantly compared
to the normal group (P<0.05). Compared to the expression in the
TGF-β1 group, RhoA, RhoC, Rock1, Smad2
and TIMP-1 decreased significantly in the inhibitor of
Rho/Rock group (P<0.05) and in the inhibitor of TGF-β/Smad
group, except for the RhoC expression (Fig. 2).
Results of western blotting
Furthermore, the protein expressions of RhoA, RhoC,
Rock1, Smad2 and TIMP-1 were detected using western blotting. The
results demonstrated that the protein expression of these factors
in the TGF-β1 group increased significantly compared to the normal
group (P<0.05). Compared to the TGF-β1 group, RhoA, RhoC, Rock1,
Smad2 and TIMP-1 decreased significantly in the inhibitor of
Rho/Rock group (P<0.05) and in the inhibitor of TGF-β/Smad
group, except for the RhoC expression (Fig. 3).
 | Figure 3Protein expressions of RhoA, RhoC,
Rho-associated coiled-coil-forming protein kinase (Rock) 1, Smad2,
tissue inhibitor of metalloproteinase-1 (TIMP-1) detected by
western blotting. The results showed that the protein expression of
these factors in the transforming growth factor-β1 (TGF-β1) group
increased significantly compared to the normal group (P<0.05).
Compared to the TGF-β1 group, RhoA, RhoC, Rock1, Smad2 and TIMP-1
decrased significantly in the inhibitor of Rho/Rock (Y-27632) group
(P<0.05) and the same in the inhibitor of TGF-β/Smad
(staurosporine) group, except for RhoC expression. GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Pulmonary fibrosis is a chronic, progressive,
fibrosing interstitial pneumonia of unknown aetiology. The
characteristics of the histopathology include fibroblast foci,
interstitial fibrosis and honeycomb changes, which are generated
due to fibroblast proliferation and excessive ECM deposition
(9). Fibroblast proliferation and
matrix deposition may be regulated by a number of mechanisms, such
as epithelial-mesenchymal transition, expansion of a local
fibroblast population and fibrocyte invasion (10).
In the presence of persisting injurious pathways,
TGF-β can initiate the transformation of fibroblast and fibrocyte
into myofibroblasts, which are resistant to apoptosis. The
resulting deposition of excessive ECM by these myofibroblasts
causes the development of pulmonary fibrosis (11). Once fibroblasts become activated,
they transform into α-SMA-expressing myofibroblasts that secrete
ECM components, factors promoting mesenchymal fibrosis and they
induce alveolar epithelial cells apotosis (12).
The FCM and immunofluorescence results showed that
the percentage of α-SMA-positive cells increased significantly
following stimulation by TGF-β1 compared to the normal group. In
the inhibitor of TGF-β/Smad group, the percentage decreased
significantly compared to the TGF-β1 group. These results indicate
that TGF-β1 was involved in regulating fibroblasts activation. The
cell cycle progression FCM results showed that the cell population
in the G0/G1 phase decreased, the S phase
increased by TGF-β1 stimulation, but the
G0/G1 phase increased and the S phase
decreased following inhibition by the TGF-β/Smad pathway. ELISA and
western blotting showed that the concentrations of type I collagen,
LN, FN and TIMP-1 in the cell culture supernatant of the TGF-β1
group increased significantly compared to the normal group. In the
inhibitor of TGF-β/Smad group, the concentrations of these factors
decreased significantly compared to the TGF-β1 group. The results
demonstrated that TGF-β1 plays important roles in regulating
fibroblasts differentiation and proliferation, ECM synthesis and
degradation through TGF-β1/Smad pathway.
In addition to TGF-β1/Smad, several signaling
pathways were involved in the pathogenesis of pulmonary fibrosis.
Previous studies have found that the fibrosis organ had abnormal
expressions of Rho and Rock, and inhibitors of Rock could improve
the models of organ fibrosis (13–15),
indicating that the Rho/Rock-mediated pathway may play a role in
pulmonary fibrosis. Rho, the small GTPase, and its target protein,
Rock, have been identified as major regulators of cell locomotion
mediated by reorganization of the actin cytoskeleton. Activated
Rock inhibits myosin phosphatase and this in turn induces
phosphorylation of the myosin light chain (MLC).
In the present study, Y-27632, an inhibitor of the
Rho/Rock signaling pathway, was used to observe whether the pathway
was involved in the development of fibroblast proliferation and
excessive ECM deposition.
Following stimulation by TGF-β1, the lung
fibroblasts expression of RhoA, RhoC, Rock1 increased, but was
decreased when fibroblasts were treated with TGF-β/Smad and the
Rho/Rock inhibitor. In the TGF-β1-stimulated fibroblasts, FCM and
immunofluorescence showed the percentage of α-SMA-positive cells
decreased following Y-27632 treatment. The cell cycle progression
results indicated that Y-27632 prevented the proliferation of
fibroblasts induced by TGF-β1. The concentrations of type I
collagen, LN, FN and TIMP-1 in the TGF-β1-stimulated fibroblasts
culture supernatant were reduced due to the inhibition of the
Rho/Rock pathway by Y-27632.
The aforementioned results indicated that the
Rho/Rock pathway was involved in lung fibroblast proliferation,
differentiation and excessive ECM deposition.
According to the study by Shimizu et al
(13), the Rho/Rock-mediated
pathway may contribute to the development of pulmonary fibrosis,
and furthermore, Y-27632 inhibited the Rock function at protein
levels, resulting in inhibition of muscle and non-muscle MLC 20
phosphorylation. Multiple smooth muscle cell (SMC)-specific
differentiation marker genes are regulated by RhoA-induced changes
in the actin cytoskeleton. RhoA activity is required for
SMC-specific promoter activity as C3 transferase, which ADP
ribosylates and irreversibly inactivates RhoA, completely inhibited
the activities of the SM22 and α-SMA promoters (16). Y-27632 can inhibit promoter
activity, which may be the mechanism for the inhibition of the
TGF-β1-stimulated fibroblast transformation to myofibroblasts.
Excessive ECM deposition was reduced due to Y-27632,
which is partly attributed to the decreased amounts of
myofibroblasts. In addition, the Rho/Rock pathway was involved in
ECM synthesis and decomposition by the mechanisms of the TGF-β/Smad
pathway, dependently or independently (17,18).
In the present study, staurosporine reduced the
expression of RhoA and Rock1, which were the key factors in
Rho/Rock pathway, and Y-27632 reduced the expression of Smad2,
which was a critical factor in the TGF-β/Smad pathway. The results
indicated that there may have been cross-talk between Rho/Rock and
the TGF-β/Smad pathway in the process of lung fibroblasts
transforming into myofibroblasts. A previous study has demonstrated
that the dominant-negative RhoA or the Rock inhibitor blocked the
nuclear translocation of Smad2 and Smad3 due to
Smad-phosphorylation inhibition and inhibition of the
Smad-dependent Smad binding elements (SBE) promoter activity,
whereas constitutively active RhoA significantly enhanced the SBE
promoter activity (19). The study
by Itoh et al (20)
indicated that the Rho/Rock pathway plays a role in relaying TGF-β
signal transduction to ECM synthesis in retinal pigment epithelial
cells in a Smad-dependent and Smad-independent way. Therefore, the
results showed that Smad signaling cross-talked with the Rho/Rock
pathway during the process of lung fibroblasts differentiation
induced by TGF-β1.
In conclusion, the Rho/Rock pathway and Smad
signaling were involved in lung fibroblasts transforming to
myofibroblasts induced by TGF-β1. The two pathways may undergo
cross-talk in the lung fibroblasts differentiation in vitro.
The specific cross points between the two pathways may be the
therapeutic targets for treating the disease and the process of
pulmonary fibrosis could be more effective.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30370620).
References
|
1
|
Warburton D, Shi W and Xu B: TGF-β-Smad3
signaling in emphysema and pulmonary fibrosis: an epigenetic
aberration of normal development? Am J Physiol Lung Cell Mol
Physiol. 304:L83–L85. 2012.
|
|
2
|
Antoniou KM, Margaritopoulos GA and
Siafakas NM: Pharmacological treatment of idiopathic pulmonary
fibrosis: from the past to the future. Eur Respir Rev. 22:281–291.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harris WT, Kelly DR, Zhou Y, et al:
Myfibroblast differentiation and enhanced TGF-β signaling in cystic
fibrosis lung disease. PLoS One. 8:e701962013.
|
|
4
|
Moriyama T and Nagatoya K: The Rho-ROCK
system as a new therapeutic target for preventing interstitial
fibrosis. Drug News Perspect. 17:29–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao HC, Zhao H, Zhang WQ, Li YQ and Ren
LQ: The role of the Rho/Rock signaling pathway in the pathogenesis
of acute ischemic myocardial fibrosis in rat models. Exp Ther Med.
5:1123–1128. 2013.PubMed/NCBI
|
|
6
|
Liu M, Gu M, Wu Y, et al: Therapeutic
effect of Y-27632 on chronic allograft nephropathy in rats. J Surg
Res. 157:e117–e127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu YB, Li X, Liang GN, Deng ZH, Jiang HY
and Zhou JH: Roles of Rho/Rock signaling pathway in silica-induced
epithelial-mesenchymal transition in human bronchial epithelial
cells. Biomed Environ Sci. 26:571–576. 2013.PubMed/NCBI
|
|
8
|
Tada S, Iwamoto H, Nakamuta M, et al: A
selective ROCK inhibitor, Y27632, prevents
dimethylnitrosamine-induced hepatic fibrosis in rats. J Hepatol.
34:529–536. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kosanovic D, Dahal BK, Wygrecka M, et al:
Mast cell chymase: an indispensable instrument in the pathological
symphony of idiopathic pulmonary fibrosis? Histol Histopathol.
28:691–699. 2013.PubMed/NCBI
|
|
10
|
Günther A, Korfei M, Mahavadi P, von der
Beck D, Ruppert C and Markart P: Unravelling the progressive
pathophysiology of idiopathic pulmonary fibrosis. Eur Respir Rev.
21:152–160. 2012.PubMed/NCBI
|
|
11
|
Coward WR, Saini G and Jenkins G: The
pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis.
4:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimizu Y, Dobashi K, Iizuka K, et al:
Contribution of small GTPase Rho and its target protein rock in a
murine model of lung fibrosis. Am J Respir Crit Care Med.
163:210–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagatoya K, Moriyama T, Kawada N, et al:
Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with
unilateral ureteral obstruction. Kidney Int. 61:1684–1695. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murata T, Arii S, Mori A and Imamura M:
Therapeutic significance of Y-27632, a Rho-kinase inhibitor, on the
established liver fibrosis. J Surg Res. 114:64–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mack CP, Somlyo AV, Hautmann M, Somlyo AP
and Owens GK: Smooth muscle differentiation marker gene expression
is regulated by RhoA-mediated actin polymerization. J Biol Chem.
276:341–347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu J, Nguyen D, Ouyang H, Zhang XH, Chen
XM and Zhang K: Inhibition of RhoA/Rho-kinase pathway suppresses
the expression of extracellular matrix induced by CTGF or TGF-β in
ARPE-19. Int J Ophthalmol. 6:8–14. 2013.
|
|
18
|
Zhou G, Li C and Cai L: Advanced glycation
end-products induce connective tissue growth factor-mediated renal
fibrosis predominantly through transforming growth factor
beta-independent pathway. Am J Pathol. 165:2033–2043. 2004.
View Article : Google Scholar
|
|
19
|
Chen S, Crawford M, Day RM, et al: RhoA
modulates Smad signaling during transforming growth
factor-beta-induced smooth muscle differentiation. J Biol Chem.
281:1765–1770. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Itoh Y, Kimoto K, Imaizumi M and Nakatsuka
K: Inhibition of RhoA/Rho-kinase pathway suppresses the expression
of type I collagen induced by TGF-beta2 in human retinal pigment
epithelial cells. Exp Eye Res. 84:464–472. 2007. View Article : Google Scholar : PubMed/NCBI
|