Introduction
Tumor invasion, metastasis and drug-resistance of
cancer cells are considered to play a vital role in cancer-related
mortality (1–5). In addition, cancer cell stemness is a
new challenge for cancer therapy as conventional chemotherapy is
inefficient in killing the stem/progenitor cells. Signaling
pathways, including the Hedgehog, Wnt/β-catenin, Notch, epidermal
growth factor receptor (EGFR), phosphorylated EGFR and KIT
pathways, are involved in this process.
The Wnt/β-catenin signal transduction pathway is a
major pathway regulating cancer cell fate. As indicated in certain
studies (6–8), the activity of this pathway is
necessary for the maintenance of stem cell self-renewal and
non-differentiation in normal tissues, and promotes the
amplification of stem cells in tumors. β-catenin, as the only
component in the third section of the pathway, may be an ideal
therapeutic target. However, the role of β-catenin signaling in
breast cancer is unclear.
The present study aimed to investigate whether
impairing β-catenin expression could decrease proliferation
and drug-resistance of tumor stem cells. β-catenin
expression in a highly metastatic breast cancer cell line
(MDA-MB-468) was repressed using small interfering RNA (siRNA). The
subsequent changes in stem/progenitor cells-related factors and in
the inhibition rates were monitored using siRNA and chemotherapy,
alone or in combination.
Materials and methods
Cell culture
The breast cancer cell line, MDA-MB-468, was
obtained from the Chinese-United Kingdom Medical Laboratory (Basic
Medical College, Zhengzhou University, Zhengzhou, China). The cells
were cultured at 37°C with 5% CO2 in Dulbecco’s modified
Eagle’s medium (HyClone, Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal calf serum (Hyclone, Thermo
Fisher Scientific, Inc.), 100 U/ml of penicillin G and 100 μg/ml of
streptomycin (Gibco-Invitrogen, Carlsbad, CA, USA).
RNA interference
Cell cultures were randomly assigned to the scramble
RNA or the siRNA groups. The scramble RNA group was transfected
with scramble siRNA and the siRNA group was transfected with
β-catenin siRNA. The siRNAs were obtained from Shanghai
GenePharma Co., Ltd., (Shanghai, China) and Lipofectamine™ 2000 was
obtained from Invitrogen. Interference was performed in 6- and
96-well culture plates, according to the manufacturer’s
instructions. Protein and total RNA 24, 48 and 72 h after
interference were extracted from 6 plates to perform western
blotting and semi-quantitative reverse transcription-polymerase
chain reaction (RT-qPCR).
Western blotting
Cells were lyzed in Western and immunoprecipitation
cell lysis solution (Beyotime Institute of Biotechnology, Haimen,
China). Protein concentrations were determined using the Lowry
method (9) in duplicate and the
results were averaged. Aliquots of the tissue samples corresponding
to 50 μg of total protein were heated at 100°C for 10 min with an
equivalent volume of 2X sample buffer (containing 4% SDS and 10%
mercaptoethanol) and loaded onto 10% polyacrylamide gels. The
proteins were electrotransferred to polyvinylidene difluoride
membranes (Bio-Rad, Hercules, CA, USA) in Tris-glycine-methanol
buffer. The membranes were blocked for 1 h at room temperature in a
blocking solution containing 5% skimmed milk. The membranes were
subsequently incubated overnight at 4°C with mouse monoclonal
anti-human β-catenin antibodies (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), followed by incubation with horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G. The
proteins of interest were visualized using the enhanced DAB western
blotting detection reagents and analyzed using the Quantity One
software (Bio-Rad).
Semi-RT-qPCR
Total RNA was extracted from cultured cells using
TRIzol (Qiagen, Venlo, Netherlands), according to the
manufacturer’s instructions. Total RNA (1–5 μg) was
reverse-transcribed using the TIANScript RT kit [Tiangen Biotech
(Beijing) Co., Ltd., Beijing, China] and the provided
oligo(dT)18 primers. The amount of cDNA was normalized
to the internal control, β-actin. All the primers and the annealing
temperature are listed in Table I.
PCR analysis was performed with the Golden Easy PCR system [Tiangen
Biotech (Beijing) Co., Ltd.].
 | Table IPolymerase chain reaction primers. |
Table I
Polymerase chain reaction primers.
| Primer | NCBI ID | Primers sequence
(5′-3′) | Product size, bp | Melting temperature,
°C |
|---|
| β-catenin | NM_001098209.1 | Forward:
CCCACTAATGTCCAGCGTTT
Reverse: AACCAAGCATTTTCACCAGG | 382 | 54.0 |
| Oct3/4 | NM_002701.4 | Forward:
TTCAGCCAAACGACCATC
Reverse: GGAAAGGGACCGAGGAGTA | 484 | 54.9 |
| BCRP | NM_004827.2 | Forward:
GCCATTCTCCCAGTCA
Reverse: GGGCGTCTATACACCAT | 494 | 49.7 |
| Survivin | NM_001012271.1 | Forward:
CTTGCCAGAGCCACGAA
Reverse: GGAACCTCACCCATAGCC | 632 | 55.4 |
| β-actin | NM_001101.3 | Forward:
GCCCTGAGGCACTCTTC
Reverse: GGCCGGACTCGTCATAC | 330 | 54.9 |
Chemotherapy
In the fluorouracil (5-FU) group, the cells were
treated with 5-FU alone (1 μg/ml final concentration). In the
5-FU/siRNA group, the cells were treated with β-catenin siRNA and
subsequently with 5-FU (1 μg/ml final concentration) 24 h after
siRNA.
Cell growth inhibition rate
The cells were inoculated in 96-well culture plates
at 5,000 cells/well and treated according to their specific group.
The Cell Counting kit-8 (CCK-8) solution (Beyotime Institute of
Biotechnology) was added at 24, 48 and 72 h in the siRNA group and
at 24 h in the 5-FU and 5-FU/siRNA groups, prior to incubation for
2 h. Following incubation, the absorbance (ABS) of each sample was
measured at 450 nm. Inhibition Rate (IR) = (ABS of experimental
group - ABS of scramble group)/ABS of scramble group × 100%.
Statistical analysis
All the experiments were repeated five times and the
mean values were used for statistical analyses. Statistical
analysis was performed using SPSS 13.0 (SPSS, Inc., Chicago, IL,
USA). Comparisons between the groups at each time interval were
performed using one-way analysis of variance. The associations
between the factors were determined by two-sided Pearson
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of β-catenin interference on mRNA
expression of β-catenin, Oct3/4, survivin and BCRP
β-catenin, Oct3/4, survivin and
BCRP mRNA expression was decreased at 24, 48 and 72 h after
interference in the siRNA group and the inhibition rate at 24 h was
the most evident (48%). The curves for each mRNA were consistent
(Fig. 1).
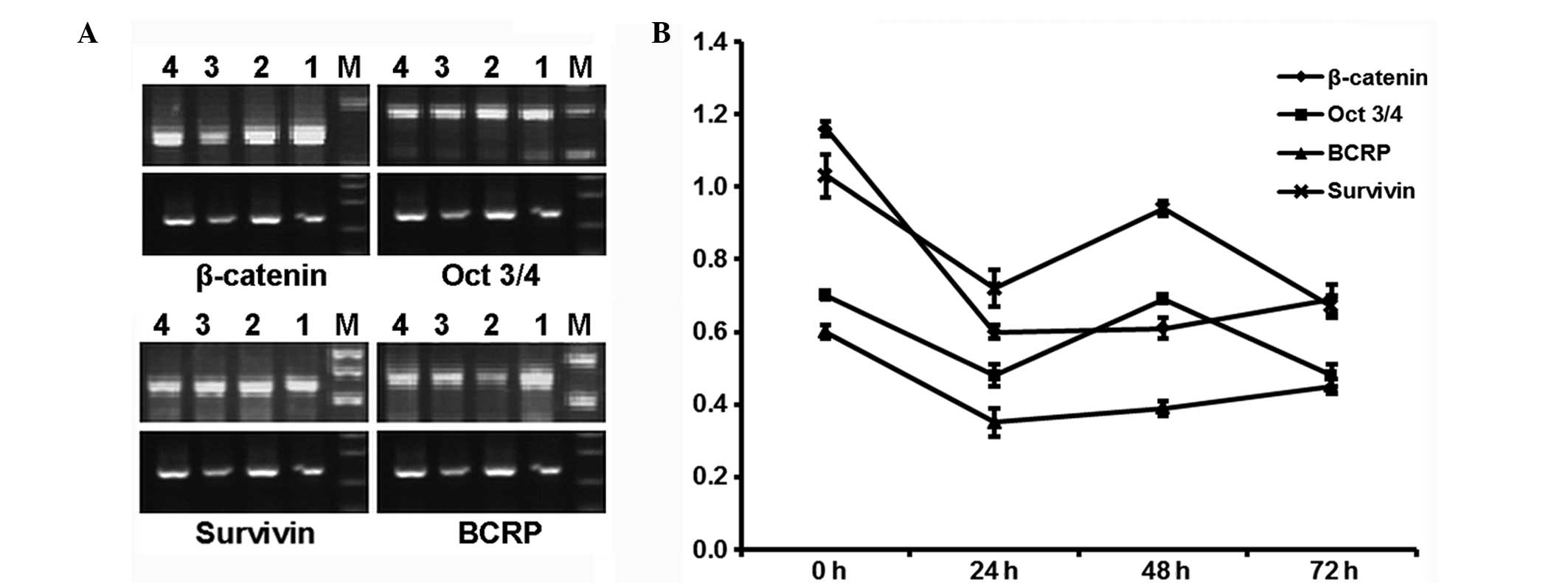 | Figure 1(A) mRNA expression of
β-catenin, Oct3/4, survivin and BCRP
following β-catenin small interfering RNA (siRNA) by
semi-quantitative reverse transcription-polymerase chain reaction.
In every image, the upper section is the mRNA of interest and the
lower section is the internal control, β-actin. Lanes 1, 2,
3 and 4 represent PCR products at 0, 24, 48 and 72 h after
transfection, respectively. M, marker (DL2000 for each mRNA, except
50-bp ladder for BCRP). (B) mRNA expression curve of
β-catenin, Oct3/4, survivin and BCRP
following β-catenin siRNA treatment. Each dot represents the
relative grayscale value (ratio of corresponding mRNA to
β-actin). All the values decreased following transfection
and the decline was most evident at 24 h. |
mRNA expression of β-catenin, Oct3/4,
survivin and BCRP under chemotherapy
The above data showed that β-catenin
interference was the most efficient at 24 h. Therefore, 24 h after
β-catenin siRNA transfection, 5-FU (1 μg/ml) or saline was
added to the MDA-MB-468 cells. RT-qPCR results showed that 5-FU
inhibited the mRNA expression of β-catenin, Oct3/4,
survivin and BCRP. Furthermore, β-catenin
siRNA transfection enhanced the inhibition rate of 5-FU on these
genes (Fig. 2). These results
indicated that β-catenin siRNA enhanced the inhibition of
Oct3/4, survivin and BCRP gene expression by
5-FU.
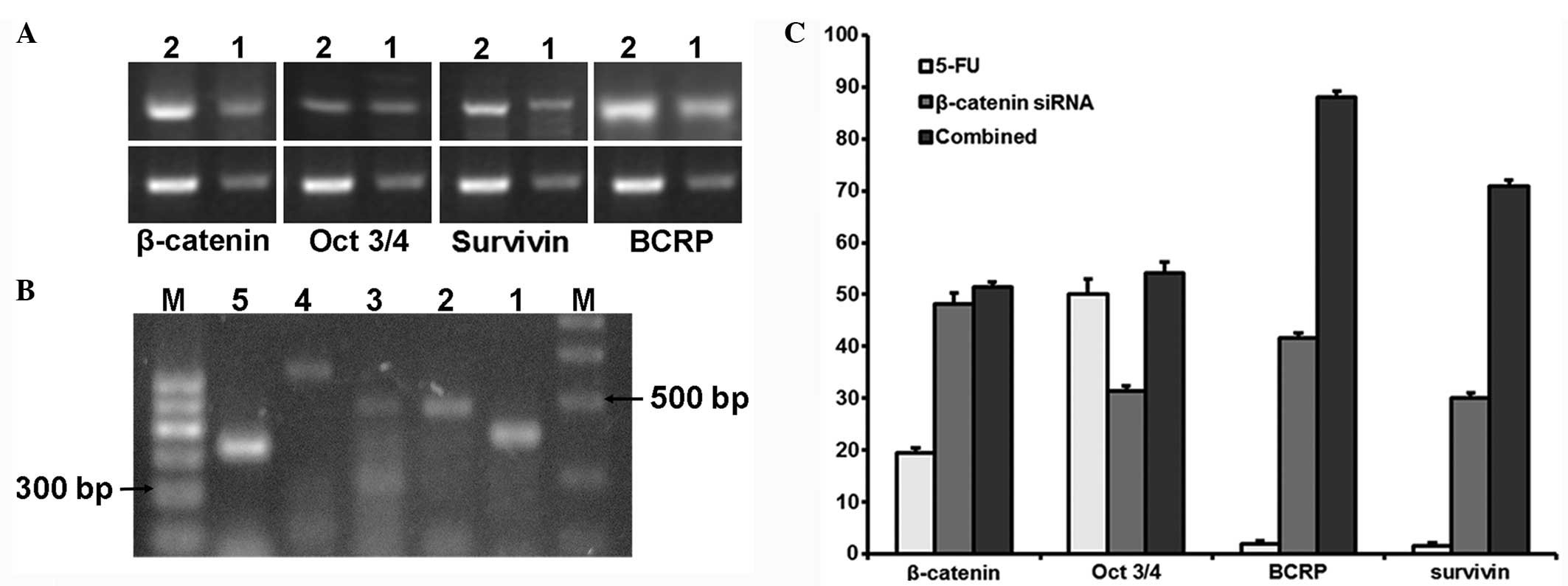 | Figure 2(A) mRNA expression of
β-catenin, Oct3/4, survivin and BCRP at
24 h after 5-FU treatment by reverse transcription-polymerase chain
reaction. In every image, the upper section is the mRNA of interest
and the lower section is the internal control, β-actin. Lane
2 is 24 h after chemotherapy in the 5-FU group, whereas lane 1 is
the blank control group. (B) mRNA expression of β-catenin,
Oct3/4, survivin and BCRP 24 h after
chemotherapy (48 h after transfection) in the 5-FU/mRNA group.
Lanes 1, 2, 3, 4 and 5 are β-catenin, Oct3/4,
BCRP, survivin and β-actin, respectively. M,
DNA marker (left, 100-bp ladder; right, DL2000). (C) Inhibition
rate of β-catenin, Oct3/4, survivin and
BCRP. The inhibition rates were obtained from the relative
grayscale value of each mRNA in the three experimental groups
divided by that of the control group. The inhibition rates of each
mRNA were the most efficient in the small interfering RNA
(siRNA)/5-FU group. |
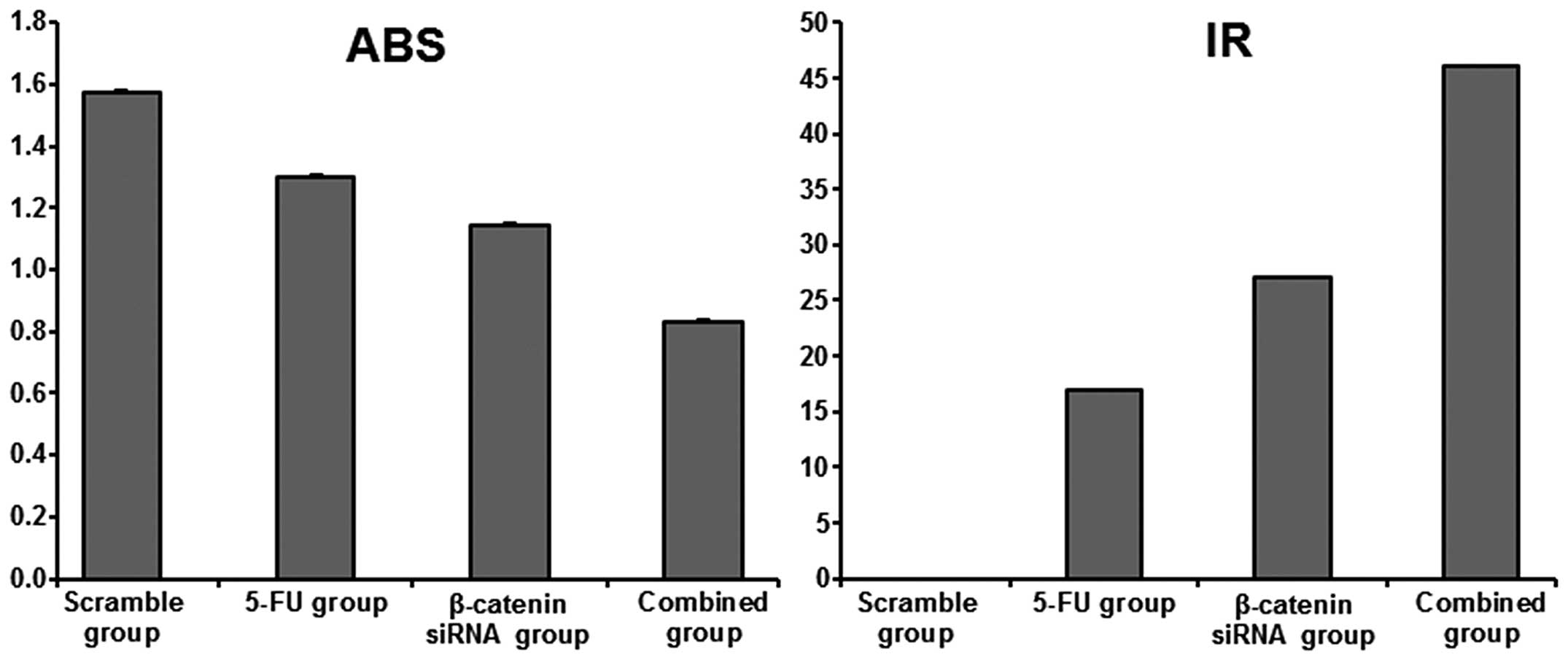
Cell growth inhibition rates
CCK-8 analyses were used to assess the cell growth
inhibition rate under different conditions. In the 5-FU or siRNA
groups, the cell growth inhibition rates were 17 and 27%,
respectively. In the 5-FU/siRNA group, the cell growth inhibition
rate was 46%, which was significantly different compared to the
other two groups (P<0.05). These results indicated that
β-catenin siRNA could enhance the inhibition rate of 5-FU
chemotherapy on cell growth (Fig.
3).
Discussion
Distant metastases and invasion are responsible for
>90% of cancer-related mortalities (10). The initial stage of metastatic
progression is essentially dependent upon important biological
events, including cell proliferation, epithelial-mesenchymal
transition, drug resistance and cancer cell stemness (11,12).
Wnt/β-catenin signaling has been demonstrated to
play an important role in metastasis development (13). In the study by DiMeo et al
(14), the downstream target genes
of the Wnt/β-catenin pathway were found to be significantly
upregulated in early breast cancer metastatic cells in the lungs of
a mouse model. Another study revealed that β-catenin is
upregulated in human cancers and correlates with poor prognosis
(15). The accumulation of nuclear
β-catenin in the invasive fronts of primary tumors further
emphasizes the critical role of β-catenin in the metastatic process
(16). Previous studies have
suggested that the β-catenin pathway may play a pivotal role in
cancer metastasis and invasion (14–16).
In the present study, it was shown that the β-catenin pathway
affects the expression of genes involved in cell proliferation,
drug resistance and cell stemness.
Following β-catenin siRNA transfection,
β-catenin mRNA expression showed a significant
downregulation compared to the scramble siRNA group, demonstrating
the validity of the transfection. In the present study, mRNA
expression of the stem/progenitor marker Oct3/4 decreased
when β-catenin was repressed, indicating that decreasing
β-catenin may be an effective way for reducing the
proportion of stem/progenitor cells. Simultaneously, the mRNA
expression of the anti-apoptosis factor survivin and the
drug-resistance factor BCRP decreased similarly, showing a
positive association between β-catenin, Oct3/4,
survivin and BCRP expression. These results showed
that stem/progenitor cells had inherent anti-apoptosis and
drug-resistant properties, consistent with previous studies
(5,17,18).
Oct3/4 encodes a transcription factor
involved in embryonic development, ensuring embryonic stem cell
pluripotency (19) and
participating in stem cell renewal (20). Oct3/4 is a stem/progenitor cell
general marker and represents the existence or quantity/functional
changes in stem/progenitor cells. BCRP is a member of the adenosine
triphosphate-binding cassette transporters superfamily involved in
multi-drug resistance of cancer stem cells (21–23).
Survivin encodes a negative regulatory protein that prevents
apoptotic cell death (17) and is
associated with drug-resistance of stem cells (5,18).
In the present study, Oct3/4, survivin
and BCRP expression were significantly inhibited in the
MDA-MB-468 cell line due to the knockdown of β-catenin.
Oct3/4, survivin and BCRP expression were
associated with breast cancer cell stemness, proliferation and drug
resistance. However, there was no detailed data that showed that
β-catenin knockdown could affect MDA-MB-468 cell stemness and drug
resistance. However, cell proliferation was inhibited significantly
following β-catenin knockdown. These results demonstrated that
β-catenin could be a potential candidate for breast cancer
therapy.
There are limited studies on the effects of
decreasing β-catenin expression to restrain cancer stem/progenitor
cells. Wang et al (24)
silenced the expression of β-catenin in the esophageal
cancer cell line, Eca-109, using little hairpin RNA and found that
the cell cycle was blocked in the G0/G1
stage, resulting from the decreased expression of cyclin D1. A
decrease in cell growth and colony formation rates were also
observed, which could be used as a surrogate for stem cells
quantity. These results are consistent with the aforementioned
previous studies.
The present study revealed that the β-catenin
inhibition rate (for mRNA and protein) was higher 24 h after
transfection. The inhibition rate in the siRNA/5-FU and 5-FU groups
was more evident compared to the siRNA group, possibly due to the
decreased capacity of anti-apoptosis and drug-resistance resulting
from decreased β-catenin protein expression. The data of the
present study indicated that combining 5-FU with gene silencing
could be an advantageous option for enhancing the curative effect
of chemotherapy in breast cancer and other malignancies.
In conclusion, these preliminary results indicate
that knockdown of β-catenin enhanced 5-FU-induced proliferation
inhibition of the breast cancer cell line MDA-MB-468.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81172179).
References
|
1
|
Eramo A, Ricci-Vitiani L, Zeuner A, et al:
Chemotherapy resistance of glioblastoma stem cells. Cell Death
Differ. 13:1238–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dean M: ABC transporters, drug resistance,
and cancer stem cells. J Mammary Gland Biol Neoplasia. 14:3–9.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
4
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst. 98:1777–1785. 2006.
|
|
5
|
Tanei T, Morimoto K, Shimazu K, et al:
Association of breast cancer stem cells identified by aldehyde
dehydrogenase 1 expression with resistance to sequential Paclitaxel
and epirubicin-based chemotherapy for breast cancers. Clin Cancer
Res. 15:4234–4241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Welm B, Podsypanina K, et al:
Evidence that transgenes encoding components of the Wnt signaling
pathway preferentially induce mammary cancers from progenitor
cells. Proc Natl Acad Sci USA. 100:15853–15858. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu BY, McDermott SP, Khwaja SS and
Alexander CM: The transforming activity of Wnt effectors correlates
with their ability to induce the accumulation of mammary progenitor
cells. Proc Natl Acad Sci USA. 101:4158–4163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
10
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steeg PS: Tumor metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y, Zheng S, An N, et al: β-catenin as a
potential key target for tumor suppression. Int J Cancer.
129:1541–1551. 2011.
|
|
14
|
DiMeo TA, Anderson K, Phadke P, et al: A
novel lung metastasis signature links Wnt signaling with cancer
cell self-renewal and epithelial-mesenchymal transition in
basal-like breast cancer. Cancer Res. 69:5364–5373. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin SY, Xia W, Wang JC, et al:
Beta-catenin, a novel prognostic marker for breast cancer: its
roles in cyclin D1 expression and cancer progression. Proc Natl
Acad Sci USA. 97:4262–4266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brabletz T, Jung A, Reu S, et al: Variable
beta-catenin expression in colorectal cancers indicates tumor
progression driven by the tumor environment. Proc Natl Acad Sci
USA. 98:10356–10361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan S, Aspe JR, Asumen MG, et al:
Extracellular, cell-permeable survivin inhibits apoptosis while
promoting proliferative and metastatic potential. Br J Cancer.
100:1073–1086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woodward WA, Chen MS, Behbod F, Alfaro MP,
Buchholz TA and Rosen JM: WNT/beta-catenin mediates radiation
resistance of mouse mammary progenitor cells. Proc Natl Acad Sci
USA. 104:618–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schöler HR, Dressler GR, Balling R,
Rohdewohld H and Gruss P: Oct-4: a germline-specific transcription
factor mapping to the mouse t-complex. EMBO J. 9:2185–2195.
1990.PubMed/NCBI
|
|
20
|
Schöler HR, Ruppert S, Suzuki N, Chowdhury
K and Gruss P: New type of POU domain in germ line-specific protein
Oct-4. Nature. 344:435–439. 1990.PubMed/NCBI
|
|
21
|
Nakanishi T, Chumsri S, Khakpour N, et al:
Side-population cells in luminal-type breast cancer have
tumour-initiating cell properties, and are regulated by HER2
expression and signalling. Br J Cancer. 102:815–826. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen NP, Almeida FS, Chi A, et al:
Molecular biology of breast cancer stem cells: potential clinical
applications. Cancer Treat Rev. 36:485–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abaan OD, Mutlu PK, Baran Y, Atalay C and
Gunduz U: Multidrug resistance mediated by MRP1 gene overexpression
in breast cancer patients. Cancer Invest. 27:201–205. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang JS, Zheng CL, Wang YJ, et al: Gene
silencing of beta-catenin by RNAi inhibits cell proliferation in
human esophageal cancer cells in vitro and in nude mice. Dis
Esophagus. 22:151–162. 2009. View Article : Google Scholar : PubMed/NCBI
|