Introduction
Hepatocellular carcinoma (HCC) is a common
malignancy of the liver with a poor prognosis. HCC is treated with
local ablation, surgical resection, transcatheter arterial
chemoembolization and systemic administration of chemotherapeutic
agents (1,2). The HCC tissue obtains nutrients and
oxygen from blood vessels by the process of angiogenesis (3,4).
Vascular endothelial cells proliferate by activating the
mitogen-activated protein (MAP) kinase pathway, which acts
downstream of the vascular endothelial cell growth factor (VEGF)
receptor (5). Sorafenib, a
multikinase inhibitor of VEGF, inhibits the MAP kinase pathway and
is used to treat HCC (6,7). Despite the sorafenib treatment,
>25% of patients with HCC succumb from disease progression
(8). Thus, it is necessary to
investigate alternative drugs/reagents alone or in combination with
sorafenib that can inhibit the signaling pathways involved in the
proliferation of HCC.
Mesenchymal-epithelial transition factor (c-Met) is
a receptor of the hepatocyte growth factor (HGF) (9). When HGF binds to c-Met, it stimulates
downstream signaling via the phosphatidylinositol-3 kinase pathway
and MAP kinase. Since c-Met is upregulated in HCC tissues compared
to the surrounding normal tissues (10,11),
inhibitors of c-Met, including
(3Z)-N-(3-chlorophenyl)-3-({3,5-dimethyl
-4-[(4-methylpiperazin-1-yl)carbonyl]-1H-pyrrol
-2-yl}methylene)-N-methyl-2-oxo-2,3-dihydro-1H-indole-5-sulf
onamide (SU11274), are considered to be promising candidates for
the treatment of HCC (12). SU11274
competes with adenosine triphosphate to bind to the activation loop
of c-Met (13). SU11274 is known to
suppress the proliferation of HCC cells (14), and if SU11274 can also suppress
angiogenesis, the anti-tumor effects are expected to be
stronger.
Thus, the aim of the present study was to establish
a co-culture of HCC and human umbilical vein endothelial cells
(HUVECs) as an in vitro model of the HCC tissue and to
investigate the anti-tumor effects of SU11274.
Materials and methods
Ethical statement
The study was approved by the Ethics Committee of
National Hospital Organization Shimoshizu Hospital (Yotsukaido,
Japan). Human fetal and adult liver RNA were collected with
informed consent from the donors and their relatives by Clontech
Laboratories, Inc., (Mountain View, CA, USA). All the human tissue
samples were collected with informed consent from the donors and
their relatives by BioChain (Hayward, CA, USA).
Cell culture
HCC cell lines (HLE, HLF, PLC/PRL/5, Hep3B and
HepG2) were purchased from RIKEN Cell Bank (Tsukuba, Japan). The
cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Sigma-Aldrich, St. Louis, MO, USA) and supplemented with 10% fetal
bovine serum (FBS; Life Technologies, Grand Island, NY, USA).
HUVECs and their culture medium [endothelial cell growth media
bullet kit (EGM)] were purchased from Lonza (Walkersville, NJ,
USA). The cell lines were cultured in 5% carbon dioxide at 37°C in
a humidified chamber. The cultured cells were observed under a
microscope (CKX41N-31PHP; Olympus, Tokyo, Japan).
Hematoxylin and eosin (H&E)
staining
The cells were spread onto 4-well chambers (Beckton
Dickinson, Franklin Lakes, NJ, USA), fixed with 100% methanol at
room temperature and stained with H&E staining (Muto Pure
Chemicals Co., Ltd., Tokyo, Japan). The specimens were observed and
images were captured using an AX80 microscope (Olympus).
Co-culture of HLF or PLC/PRL/5 cells with
HUVECs
HUVECs were spread at a density of
1.9×104 cells onto each well of a 24-well plate coated
with matrigel (Becton Dickinson) at the density of each well. After
1-day culture in EGM, the medium was discarded and
1.9×104 HLF cells or PLC/PRL/5 cells were spread onto
each well. The cells were cultured in DMEM supplemented with 10%
FBS.
Immunostaining
Serial sections of human healthy adult liver
(64-year-old male) and HCC tissue (60-year-old female) (BioChain)
were deparaffinized, autoclaved and incubated with hydrogen
peroxide, followed by 2% FBS in phosphate-buffered saline (PBS;
washing buffer) for 30 min. Following an overnight incubation with
rabbit monoclonal anti-c-Met antibody (1:300; Cell Signaling
Technology, Danvers, MA, USA), the specimens were rinsed with PBS
and incubated with horseradish peroxidase-labeled anti-rabbit
antibody (1:2,000; GE Healthcare, Pittsburgh, PA, USA) for 2 h.
Subsequently, diaminobenzidine (Dako, Glostrup, Denmark) was
applied to the tissue sections as a chromogen and the nuclei were
stained with hematoxylin for 15 sec. The specimens were observed
and the images were captured under an AX80 microscope (Olympus). A
specimen of HCC tissue incubated without the primary antibody was
used as a negative control.
Cell proliferation analysis
HLF cells, PLC/PRL/5 cells or HUVECs were
trypsinized, harvested, spread onto 96-well flat-bottom plates
(Asahi Techno Glass, Tokyo, Japan) at a density of 1,000 cells per
well and were incubated for 24 h in media supplemented with 10%
FBS. The cells were treated with the c-Met inhibitor, SU11274 (Wako
Pure Chemicals, Tokyo, Japan) at 0, 0.03, 0.1, 0.3, 1, 3, 10 and 30
μM for 72 h. These cells were subsequently used in the
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) assays, according to the manufacturer’s
instructions (Promega Corporation, Madison, WI, USA). MTS is
bioreduced by the cells into a colored formazan product into a
colored formazan product. The absorbance was analyzed at a
wavelength of 490 nm with an iMark Microplate Absorbance Reader
(Bio-Rad, Hercules, CA, USA). The absorbance was normalized against
that of 0 μM.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The cells were spread in 6-well plates (Asahi Techno
Glass) and cultured until they reached 80% confluency. SU11274 was
added to the media and 48 h later, total RNA was isolated using a
kit (Isogen; Nippon Gene, Tokyo, Japan). Complimentary DNA was
synthesized using SuperScript III and oligo(dT) primers (Life
Technologies), as per the manufacturer’s instructions. Total RNA
from adult human healthy liver was purchased from Clontech
Laboratories, Inc. The PCR primers and product sizes were as
follows: c-Met 77 [NM_000245; 5′-CATTGGGGAGCACTATGTC-3′ (forward),
5′-TGT 78 CCACCTCATCATCAGCG-3′ (reverse); 110 basepairs (bp)],
cyclin D1 (NM_053056; 5′-AGAGGCGGAGGA GAACAAACAG-3′,
5′-AGGCGGTAGTAGGACAGGAAG TTG-3′; 180 bp) and RPL19 (BC095445;
5′-CGAATGCCAG AGAAGGTCAC-3′, 5′-CCATGAGAATCCGCTTGTTT-3′; 157 bp).
RT-qPCR was performed at 40 cycles consisting of denaturing for 5
sec and annealing-extension for 5 sec. RPL19 primers were used as
the internal controls. RT-qPCR was performed using the Fast
SYBR-Green Master mix (Life Technologies) in the MiniOpticon system
(Bio-Rad). The expression level of the genes was analyzed
automatically with the system. The expression levels were
normalized against that of 0 μM.
Statistical analyses
One-way analysis of variance was performed with JMP
10.0.2 (SAS Institute, Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
c-Met expression
The expression of c-Met was analyzed
immunohistochemically in 99 surgical specimens of adult healthy
liver and HCC samples from 100 that were commercially available. In
the absence of the anti-c-Met antibody, HCC tissues did not show
any staining (Fig. 1A). While the
cell membrane of hepatocytes in the normal liver was weakly
positive for c-Met (Fig. 1B), the
cell membrane and cytoplasm of cells in the HCC tissue were
positive (Fig. 1C). These data
indicate that c-Met is upregulated in HCC cells as compared to the
normal hepatocytes.
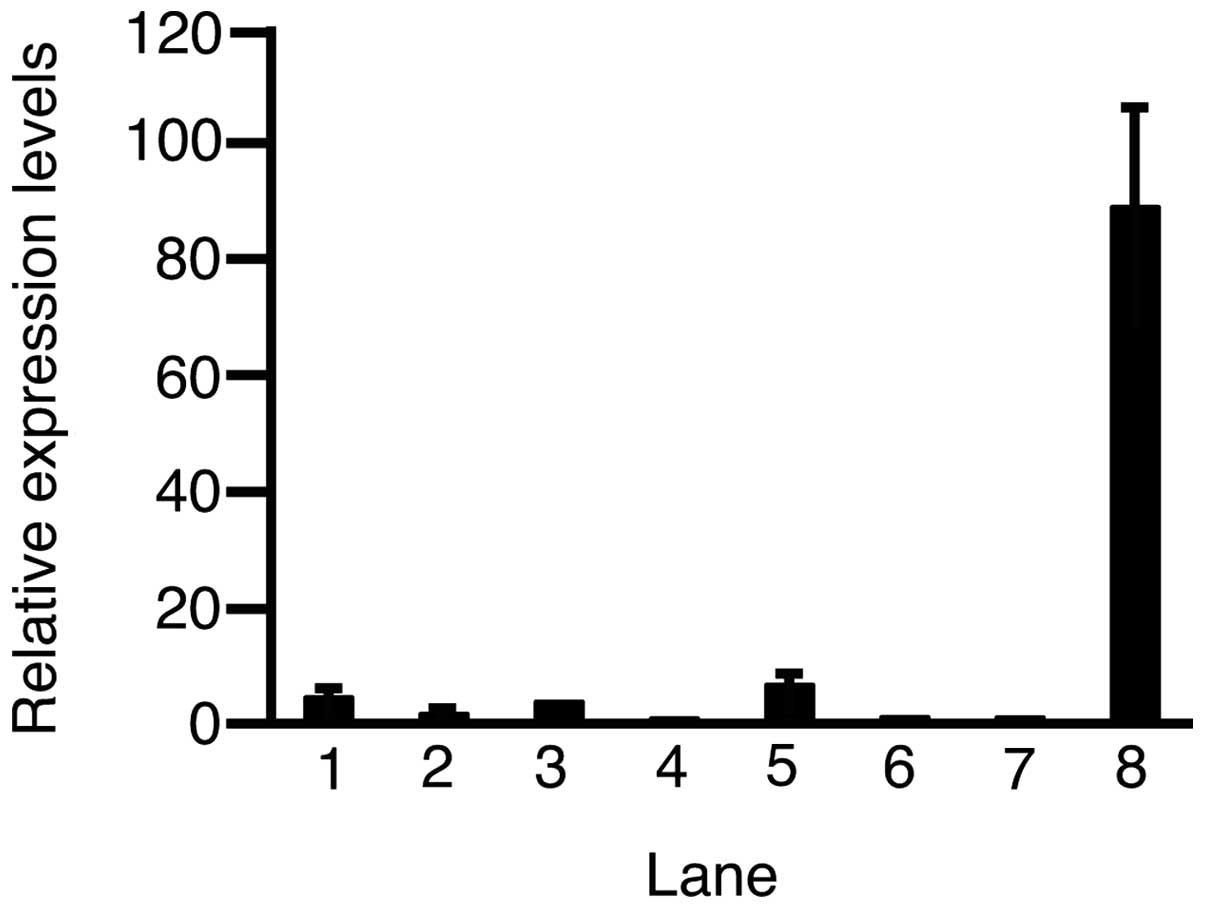
Subsequently, RT-qPCR was performed to assess the
expression level of c-Met in the HCC cell lines and HUVECs
(Fig. 2). The expression levels of
c-Met in HLE, HLF, PLC/PRL/5, Hep3B, Huh-6, HepG2, adult healthy
liver and HUVECs were 4.43±0.50, 1.61±0.18, 3.70±0.08, 0.81±0.18,
6.60±1.29, 1.06±0.35, 1.00±0.09 and 88.8±17.3 (mean ± standard
deviation), respectively. Except in Hep3B cells, the expression of
c-Met was higher in all the HCC cell lines and HUVECs as compared
to the adult healthy liver.
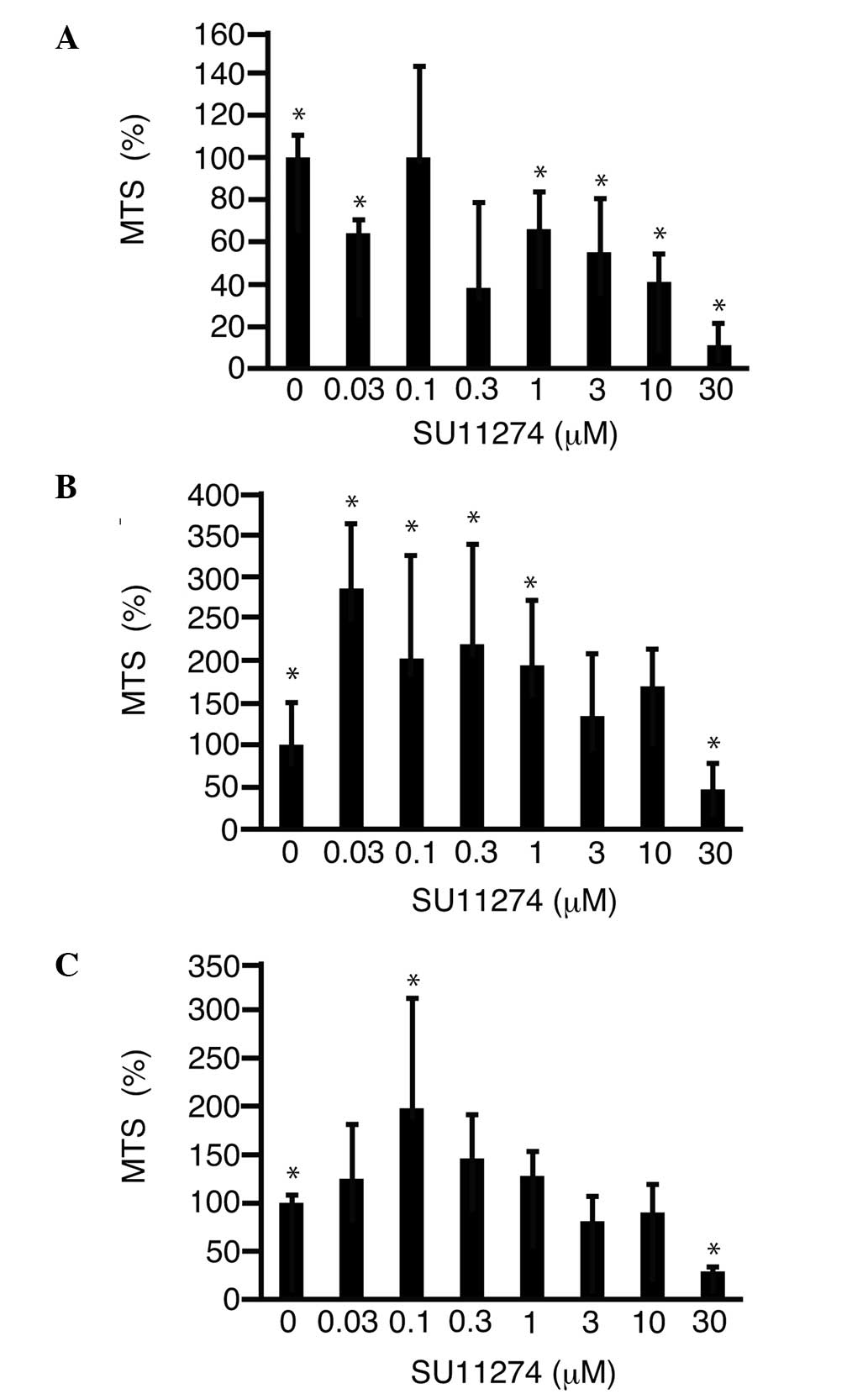
c-Met inhibition by SU11274
To address the possibility that inhibition of c-Met
suppresses cell proliferation, SU11274 was added to the media and
the MTS assay was performed. SU11274 was found to suppress the
proliferation of HLF cells in a dose-dependent manner and reached
11.0±9.4% (P<0.05) with 30 μM SU11274 as compared to 0 μM
(Fig. 3A). SU11274 (30 μM)
suppressed the proliferation of PLC/PRL/5 cells (Fig. 3B) and HUVECs (Fig. 3C) to 46.5±30.7 (P<0.05) and
29.4±5.0%, as compared to 0 μM (P<0.05), respectively.
To investigate the mechanism of cell proliferation
suppression by SU11274, the expression level of cyclin D1 was
analyzed by RT-qPCR and was found to decrease to 45.1±11.6%
(P<0.05) with 30 μM SU11274 in HLF cells, as compared to 0 μM
(Fig. 4A). The expression level of
cyclin D1 decreased to 30.1±10.3% (P<0.05) of SU11274 in
PLC/PRL/5 cells, as compared to 0 μM (Fig. 4B).
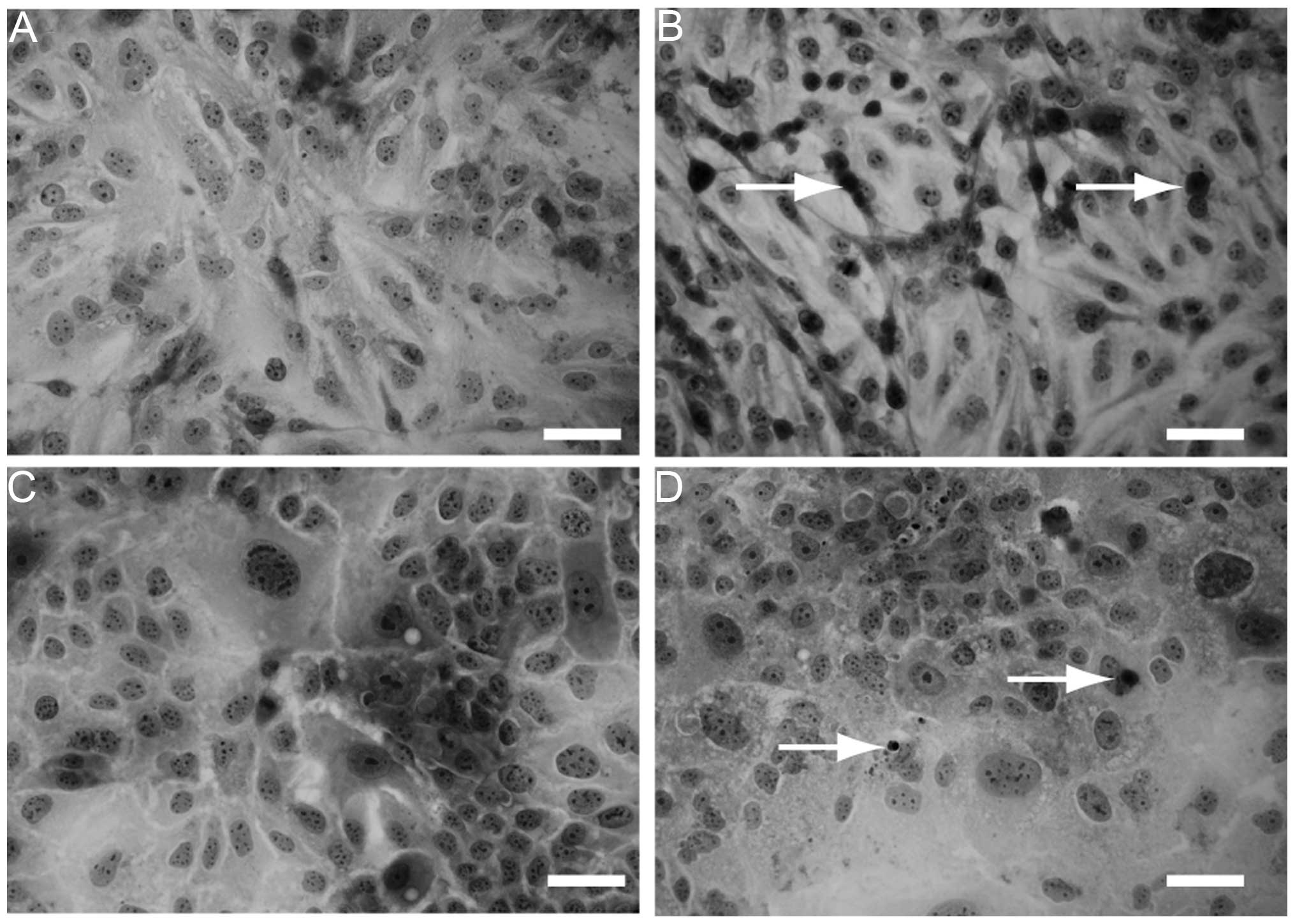
In addition, H&E staining was performed to
observe the morphological changes of the cells cultured with or
without SU11274. Compared to HLF (Fig.
5A) and PLC/PRL/5 cells (Fig.
5C) without SU11274 treatment, apoptotic cells were observed
with 30 μM SU11274 treatment (Fig. 5B
and D).
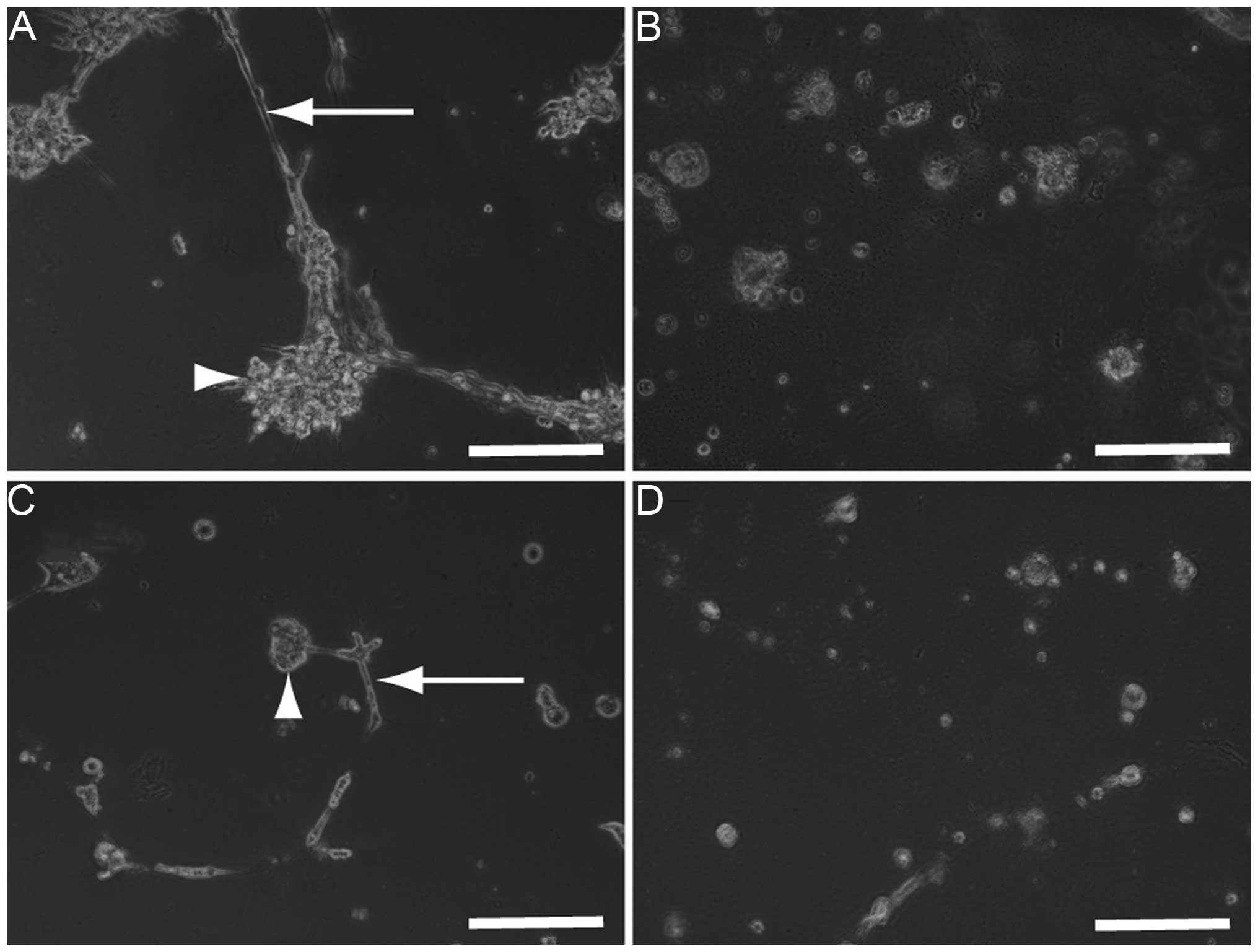
Co-cultures of HLF (Fig.
6A) or PLC/PRL/5 cells (Fig.
6C) with HUVECs were established to analyze the possibility
that SU11274 damages the co-culture used as an in vitro
model of hepatoma tissues. Three days after the addition of 30 μM
SU11274, the morphology of the co-cultures of HLF (Fig. 6B) or PLC/PRL/5 cells (Fig. 6D) with HUVECs were found to be
damaged and as a result, all the cells died.
Discussion
SU11274 suppresses proliferation and induces
apoptosis of HCC cells (15). In
the present study, proliferation of HCC cells was suppressed via
the downregulation of cyclin D1, a protein involved in cell cycle
progression (16,17). These results indicate that SU11274
can suppress cell cycle progression. In addition, H&E staining
showed apoptosis in cells cultured with SU11274. SU11274 is known
to activate caspase-3 (15). Taken
together, these data indicate that SU11274 suppresses the
proliferation of HLF and PLC cells by downregulating cyclin D1 and
inducing apoptosis.
c-Met is expressed in vascular endothelial cells and
is upregulated along with HGF in response to environmental stress
(18,19). The results of the present study show
that c-Met is expressed in HUVECs and the proliferation of HUVECs
is suppressed by SU11274. This indicates that SU11274 may suppress
the proliferation of HCC cells and HUVECs. Co-cultures of HLF or
PLC/PRL/5 cells and HUVECs were established to assess the
anti-proliferative effects of SU11274. The data clearly show that
these co-cultures were damaged by SU11274 treatment, indicating
that SU11274 may be useful for the suppression of proliferation of
HCC cells, as well as angiogenesis.
In conclusion, except for Hep3B, c-Met is highly
expressed in hepatoma cells and HUVECs. SU11274 (30 μM) suppresses
the proliferation of HLF, PLC/PRL/5 and HUVECs and it was found
that 30 μM SU11274 damaged the co-culture of HLF or PLC/PRL/5 cells
with HUVECs.
References
|
1
|
Lencioni R, Petruzzi P and Crocetti L:
Chemoembolization of hepatocellular carcinoma. Semin Intervent
Radiol. 30:3–11. 2013. View Article : Google Scholar
|
|
2
|
Kim HY and Park JW: Clinical trials of
combined molecular targeted therapy and locoregional therapy in
hepatocellular carcinoma: past, present, and future. Liver Cancer.
3:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomizawa M, Kondo F and Kondo Y: Growth
patterns and interstitial invasion of small hepatocellular
carcinoma. Pathol Int. 45:352–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miyahara K, Nouso K, Morimoto Y, et al:
Okayama Liver Cancer Group: Pro-angiogenic cytokines for prediction
of outcomes in patients with advanced hepatocellular carcinoma. Br
J Cancer. 109:2072–2078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie B, Wang DH and Spechler SJ: Sorafenib
for treatment of hepatocellular carcinoma: a systematic review. Dig
Dis Sci. 57:1122–1129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Furuse J, Ishii H, Nakachi K, Suzuki E,
Shimizu S and Nakajima K: Phase I study of sorafenib in Japanese
patients with hepatocellular carcinoma. Cancer Sci. 99:159–165.
2008.PubMed/NCBI
|
|
7
|
Hu H, Duan Z, Long X, et al: Sorafenib
combined with transarterial chemoembolization versus transarterial
chemoembolization alone for advanced-stage hepatocellular
carcinoma: a propensity score matching study. PLoS One.
9:e966202014. View Article : Google Scholar
|
|
8
|
Nishikawa H, Takeda H, Tsuchiya K, et al:
Japanese Red Cross Liver Study Group: Sorafenib therapy for BCLC
stage B/C hepatocellular carcinoma; clinical outcome and safety in
aged patients: a multicenter study in Japan. J Cancer. 5:499–509.
2014. View
Article : Google Scholar
|
|
9
|
Organ SL and Tsao MS: An overview of the
c-MET signaling pathway. Ther Adv Med Oncol. 3(Suppl 1): S7–S19.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grigioni WF, Fiorentino M, D’Errico A, et
al: Overexpression of c-met protooncogene product and raised Ki67
index in hepatocellular carcinomas with respect to benign liver
conditions. Hepatology. 21:1543–1546. 1995.PubMed/NCBI
|
|
11
|
Kang GH, Lee BS, Lee ES, Kim SH, Lee HY
and Kang DY: Prognostic significance of p53, mTOR, c-Met, IGF-1R,
and HSP70 overexpression after the resection of hepatocellular
carcinoma. Gut Liver. 8:79–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scagliotti GV, Novello S and von Pawel J:
The emerging role of MET/HGF inhibitors in oncology. Cancer Treat
Rev. 39:793–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mughal A, Aslam HM, Sheikh A, Khan AM and
Saleem S: c-Met inhibitors. Infect Agent Cancer. 8:132013.
View Article : Google Scholar
|
|
14
|
Inagaki Y, Qi F, Gao J, et al: Effect of
c-Met inhibitor SU11274 on hepatocellular carcinoma cell growth.
Biosci Trends. 5:52–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dang Y, Luo D, Rong M and Chen G:
Underexpression of miR-34a in hepatocellular carcinoma and its
contribution towards enhancement of proliferating inhibitory
effects of agents targeting c-MET. PLoS One. 8:e610542013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Insulin-like growth factor I
receptor involvement in proliferation of NOR-P1 cells in serum-free
media. J Cell Biochem. 113:2714–2720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tomizawa M, Shinozaki F, Motoyoshi Y, et
al: Niclosamide suppresses hepatoma cell proliferation via the Wnt
pathway. Onco Targets Ther. 6:1685–1693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harrington LS, Sainson RC, Williams CK, et
al: Regulation of multiple angiogenic pathways by DII4 and Notch in
human umbilical vein endothelial cells. Microvasc Res. 75:144–154.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu SY, Duan HF, Li QF, et al: Hepatocyte
growth factor protects endothelial cells against gamma ray
irradiation-induced damage. Acta Pharmacol Sin. 30:1415–1420. 2009.
View Article : Google Scholar : PubMed/NCBI
|