Introduction
The current understanding of human malignancy is
that it mainly arises due to the accumulation of multiple genetic
alterations, transforming normal cells into malignant cells
(1,2). Of these genetic alterations, a myriad
of genomic mutation data derived from a high-throughput DNA
sequencing technique provided a unique opportunity to profile the
mutation spectra underlying human cancers and a large number of
significant functional mutations in multiple genes were identified
in diverse types of cancer (1,3,4). These
genes can be defined as oncogenes or tumor suppressor genes and are
being used as molecular markers for diagnosis, staging and
prognosis of human cancers (5,6).
Ovarian carcinoma constitutes a heterogeneous group
of malignancies with significantly different clinical expression,
pathological characteristics and genetic etiology (7,8).
However, the majority of ovarian carcinomas shared certain common
genetic alterations, such as frequent tumor protein p53
(TP53) and PIK3CA, catalytic subunit α mutations (9,10), and
patients also exhibited subtype-specific mutations (11–13),
which are possibly essential for the differential clinical
expression and molecular-targeted therapy in ovarian carcinomas
(14,15). These observations emphasized the
requirement to identify novel subtype-specific molecular genetic
aberrations in ovarian carcinomas.
Recently, large-scale sequencing has identified
frequent mutations of the ribonuclease type III (DICER1)
gene in Sertoli-Leydig cell tumors of the ovary (3), CCCTC-binding factor (CTCF) gene
in transient abnormal myelopoiesis (16) and endometrial cancer (17), ribosomal protein L22 (RPL22)
gene in endometrial cancer (18),
DNA (cytosine-5-)-methyltransferase 3α (DNMT3A) gene in
hematological malignancies (4), the
transformation/transcription domain-associated protein
(TRRAP) gene in melanoma (19) and isocitrate dehydrogenase 1 and 2
(IDH1 and IDH2) genes in gliomas (1,20) and
acute myeloid leukemia (AML) (21),
respectively. Some of these mutations were closely associated with
cancer progression (22) and
prognosis (23,24).
Thus far, the mutation statuses of DICER1, CTCF,
RPL22, DNMT3A, TRRAP, IDH1 and IDH2 mutational hotspots
in ovarian carcinomas remain largely unknown. One critical concern
in cancer genetics is whether those cancer-associated mutations
identified in one type of cancer are also common in other types of
cancer. Therefore, a cohort of 251 Chinese patients with distinct
subtypes of ovarian carcinomas was recruited in the present study
to examine whether the hotspot mutations in these genes also
existed in these samples.
Materials and methods
Sample collection
The study included 251 archival formalin-fixed,
paraffin-embedded (FFPE) tissues with various subtypes of ovarian
carcinoma recruited from the Jiangxi Provincial Maternal and Child
Health Hospital (Nanchang, Jiangxi, China). Only those patients
with >70% of neoplastic cells were recruited in the study. The
sample cohort contained 76 ovarian serous carcinoma, 43 ovarian
clear cell carcinoma, 37 ovarian endometrioid carcinoma, 33 ovarian
germ cell tumor, 15 mucinous ovarian carcinoma, 18 ovarian sex
cord-stromal tumor, 12 other rare subtypes and 17 Krukenberg tumor,
and the available clinical data was as described previously
(25,26) and in Table I. Informed consent conforming to the
tenets of the Declaration of Helsinki was obtained from each
patient prior to the study. The Institutional Review Boards of the
Jiangxi Provincial Maternal and Child Health Hospital approved the
study.
 | Table IMutation frequencies of ribonuclease
type III (DICER1), CCCTC-binding factor (CTCF),
ribosomal protein L22 (RPL22), DNA
(cytosine-5-)-methyltransferase 3α (DNMT3A),
transformation/transcription domain-associated protein
(TRRAP), isocitrate dehydrogenase (IDH)1 and
IDH2 hotspot mutations in 251 Chinese patients with ovarian
carcinomas. |
Table I
Mutation frequencies of ribonuclease
type III (DICER1), CCCTC-binding factor (CTCF),
ribosomal protein L22 (RPL22), DNA
(cytosine-5-)-methyltransferase 3α (DNMT3A),
transformation/transcription domain-associated protein
(TRRAP), isocitrate dehydrogenase (IDH)1 and
IDH2 hotspot mutations in 251 Chinese patients with ovarian
carcinomas.
| Subtype/gene | No. | DICER1
p.E1705-D1709 | DICER1
p.D1810-E1813 | CTCF
p.T204fs* | RPL22
c.43delA | DNMT3A
p.R882 | TRRAP
p.S722 | IDH1
p.R132 | IDH2
p.R140 | IDH2
p.R172 | RNF43 p.I48V
and p.R40fs*11a | POLE1
p.S297Fb |
|---|
| Epithelial |
| Serous | 76 | 0/76 | 0/76 | 0/72 | 0/75 | 0/74 | 0/75 | 0/73 | 0/74 | 0/74 | 0/74 | 0/74 |
| Clear
cell | 43 | 0/43 | 0/43 | 0/42 | 0/43 | 0/43 | 0/43 | 0/42 | 0/41 | 0/41 | 0/41 | 0/41 |
|
Endometrioid | 37 | 0/37 | 0/37 | 0/35 | 0/35 | 0/36 | 0/37 | 0/35 | 0/37 | 0/37 | 0/37 | 3/37 |
| Mucinous | 15 | 0/15 | 0/15 | 0/14 | 0/15 | 0/15 | 0/15 | 0/14 | 0/15 | 0/15 | 2/15 | 0/15 |
|
Undifferentiated | 3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
|
Unclassified | 4 | 0/4 | 0/4 | 0/3 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| Transitional
cell | 3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Mixed | 2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Non-epithelial |
| Germ cell
tumor |
| Yolk
sac | 11 | 0/11 | 0/11 | 0/10 | 0/11 | 0/11 | 0/10 | 0/11 | 0/11 | 0/11 | 0/11 | 0/11 |
|
Dysgerminoma | 7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 |
|
Teratoma | 9 | 0/9 | 0/9 | 0/8 | 0/9 | 0/9 | 0/9 | 0/8 | 0/9 | 0/9 | 0/9 | 0/9 |
|
Mixed | 6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 |
| Sex cord-stromal
tumor |
|
Granulosa cell | 16 | 0/16 | 0/16 | 0/14 | 0/16 | 0/16 | 0/16 | 0/15 | 0/16 | 0/16 | 0/16 | 0/16 |
|
Sertoli-Leydig | 2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Krukenberg
tumors | 17 | 0/17 | 0/17 | 0/16 | 0/16 | 0/17 | 0/17 | 0/15 | 0/17 | 0/17 | 0/17 | 0/17 |
Mutation analysis of the DICER1, CTCF,
RPL22, DNMT3A, TRRAP, IDH1 and IDH2 genes
The Omega FFPE DNA kit (Omega Bio-tek Inc.,
Doraville, GA, USA) was used to isolate the DNA from the FFPE
tissues. The polymerase chain reaction (PCR) primers were as
summarized previously (25,27) and are shown in Table II. PCR reactions were performed in
a total volume of 25 µl, containing 50 ng genomic DNA, 2 units of
LA Taq DNA Polymerase (Takara Biotechnology Dalian Co. Ltd.,
Liaoning, China), 300 µM of each dNTP and 0.2 µM of each primer.
The amplification reaction was performed in a Thermal Cycler 2720
(Applied Biosystems, Foster City, CA, USA) and employed one
denaturation cycle of 94°C for 3 min, 35 amplification cycles of
94°C for 30 sec, 50–60°C (Table
II) (25,27) for 20 sec and 72°C for 30 sec, with
one final extension cycle of 72°C for 10 min. The PCR products were
purified and sequenced with an ABI 3730 DNA sequencer (Applied
Biosystems). DNA sequence analyses were performed with the DNASTAR
package software (DNASTAR Inc., Madison, WI, USA).
 | Table IIPrimers for the mutational analysis
of the ribonuclease type III (DICER1), CCCTC-binding factor
(CTCF), ribosomal protein L22 (RPL22), DNA
(cytosine-5-)-methyltransferase 3α (DNMT3A),
transformation/transcription domain-associated protein
(TRRAP), isocitrate dehydrogenase (IDH)1 and
IDH2 genes. |
Table II
Primers for the mutational analysis
of the ribonuclease type III (DICER1), CCCTC-binding factor
(CTCF), ribosomal protein L22 (RPL22), DNA
(cytosine-5-)-methyltransferase 3α (DNMT3A),
transformation/transcription domain-associated protein
(TRRAP), isocitrate dehydrogenase (IDH)1 and
IDH2 genes.
| Gene | Target regions | Amplicon, bp | Sample
detected | Annealing, ˚C | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| DICER1 | p.E1705.D1709 | 159 | 251/251 | 55 |
CGGATCCCCTCAGATTGTTA |
CGATGCAAAGATGGTGTTGT |
| DICER1 | p.D1810.E1813 | 171 | 251/251 | 55 |
TGGCCTTTTTGCTTACAAGTC |
TGCCAGACTGTCTCCAGTGA |
| CTCF | p.T204fs* | 212 | 237/251 | 56 |
GTTAAAGTGGGGGCCAATG |
AGCAGACCCTCCTGCTGTT |
| RPL22 | c.43delA | 190 | 247/251 | 60 |
TCTTGTTTTTCCGACTGACTGA |
CCGAGTGGCAATAAGGATGT |
| DNMT3A | p.R882 | 177 | 248/251 | 52 |
TGCCCTCTCTGCCTTTTCT |
CCATGTCCCTTACACACACG |
| TRRAP | p.S722 | 183 | 249/251 | 52 |
TCTGCTCTGTTTGCTACGAT |
GCACTACTTAGATTAAATGGAC |
| IDH1 | p.R132 | 269 | 239/251 | 50 |
TGCTGCAGAAGCTATAAAGAAG |
GCAAAATCACATTATTGCCAAC |
| IDH2 | p.R140 and
p.R172 | 209 | 246/251 | 50 |
GCTGCAGTGGGACCACTATT |
ACCCTGGCCTACCTGGTC |
Results and Discussion
The available clinical data of these patients are as
described previously (25,26). In the present study, a total of 251
Chinese samples with distinct subtypes of ovarian carcinoma were
screened for the presence of potential hotspot mutations in the
DICER1, CTCF, RPL22, DNMT3A, TRRAP, IDH1 and IDH2
genes. However, no mutations in these genes were detected in the
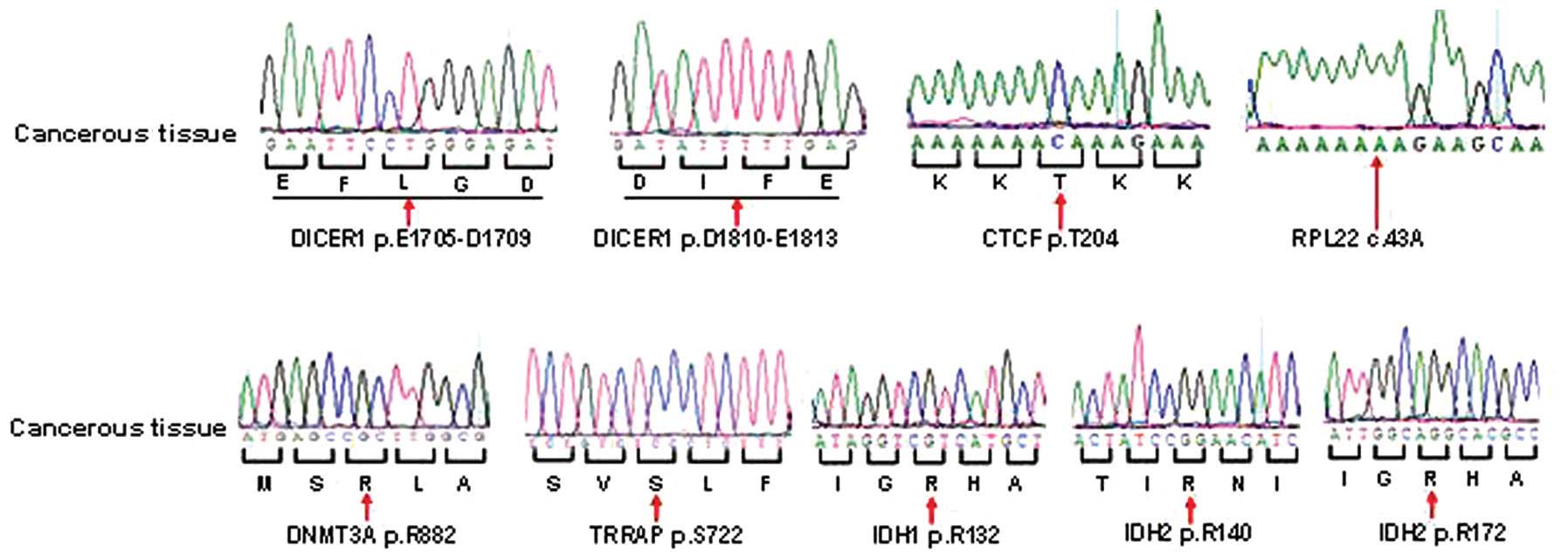
251 samples (Table I and Fig. 1).
Previous studies have found frequent DICER1
p.E1705-D1709 and p.D1810-E1813 mutations in Sertoli-Leydig cell
tumors (3,28). However, no DICER1 mutations
were detected in the two patients with Sertoli-Leydig cell tumors.
Therefore, it can be speculated that this discrepancy may be caused
mainly by the small sample size of the Sertoli-Leydig cell tumors
analyzed in the present study. In addition, DICER1 mutations
were not identified in other subtypes of ovarian carcinomas in the
samples, which is consistent with previous large-scale sequencing
results in which the DICER1 hotspot mutations were absent in
12 mucinous (29) or 316 serous
ovarian carcinomas (9).
Collectively, these results indicated that the DICER1
hotspot mutations may not be actively involved in the pathogenesis
of ovarian carcinoma, except for Sertoli-Leydig cell tumors.
CTCF p.T204fs* and RPL22 c.43delA
mutations have been observed frequently in endometrial carcinoma in
previously studies (17,18). Considering the fact that ovarian
carcinoma have certain overlapped genetic aberrations with
endometrial cancer, such as frequent TP53 (9,30) and
polymerase (DNA directed) ε, catalytic subunit (POLE1)
mutations (26,30), we hypothesized that ovarian
carcinomas may also harbor these mutations. However, neither
CTCF p.T204fs* nor RPL22 c.43delA mutations were
identified in the samples in the present study. The absence of the
CTCF and RPL22 mutations in ovarian cancer in a
previous study (29) and the
present study suggested that the CTCF and RPL22
hotspot mutations may play an extremely limited role in the
pathogenesis of ovarian cancer.
Prevalent TRRAP p.S722 mutation was initially
identified in melanomas in a whole-exome sequencing study (19). Subsequent extended studies failed to
identify these mutations in thyroid cancer (31) or splenic marginal zone lymphoma
(32). In the present study, no
TRRAP p.S722 mutations were detected in our ovarian cancer
patients with distinct subtypes. Also, TRRAP p.S722
mutations were not found in 12 mucinous (29) or 316 serous ovarian carcinomas
(9). These negative results led us
to speculate that TRRAP p.S722 mutations may not play a
crucial role in the malignant transformation of ovarian
carcinoma.
DNMT3A p.R882 mutations were identified
almost exclusively in hematological malignancies, including AML
(33), acute lymphoblastic leukemia
(34) and myelodysplastic syndromes
(35), and are generally infrequent
or absent in some solid tumors (9,29,36).
DNMT3A p.R882 mutations were not detected in the 251 samples
with distinct subtypes of ovarian carcinoma. Similarly, whole-exome
sequencing studies suggested that DNMT3A p.R882 mutations
were absent in 12 mucinous (29) or
316 serous ovarian carcinomas (9).
Taken together, the absence of DNMT3A p.R882 mutations in
ovarian carcinoma analyzed in the present study and in previous
studies (9,29) indicated that DNMT3A p.R882
mutations may be infrequent in ovarian carcinoma.
Frequent IDH1 p.R132, and IDH2 p.R140
and p.R172 mutations were identified in the central nervous system
tumors and AML (1,20,27).
However, no IDH1 or IDH2 mutations were detected in
the present samples. Similar results were observed in previous
studies in which IDH1 p.R132 mutations were not detected in
168 ovarian carcinomas or 8 ovarian cancer cell lines (20,37–39).
In addition, IDH1 and IDH2 hotspot mutations were
also not identified in 12 mucinous (29) or 316 serous ovarian carcinomas
(9). These combined results
suggested that IDH1 and IDH2 potential hotspot
mutations may not be common in patients with ovarian carcinoma.
Among these patients, the POLE1 mutation has
been previously found to be frequent in 37 ovarian endometrioid
carcinomas (26), whereas ring
finger protein 43 (RNF43) mutations were recurrent in 15
mucinous ovarian carcinomas (25)
(Table I). In the present study,
neither endometrioid nor mucinous ovarian carcinomas were detected
to harbor DICER1, CTCF, RPL22, DNMT3A, TRRAP, IDH1 and
IDH2 hotspot mutations. These results suggested that these
potential hotspot mutations observed in other (sub)types of cancer
may not play synergistic roles with POLE1 or RNF43
mutations in the carcinogenesis of endometrioid or mucinous ovarian
carcinomas, respectively.
The main limitation of the present study was that
only short DNA fragments spanning the potential hotspot mutations
were screened in the seven genes, and therefore, there is a
possibility that mutations in other residues of these genes may
exist in these samples. However, due to the shortage of DNA
amounts, this hypothesis was not tested.
In conclusion, DICER1, CTCF, RPL22, DNMT3A,
TRRAP, IDH1 and IDH2 hotspot mutations were not
identified in 251 Chinese patients with diverse subtypes of ovarian
carcinoma. These results were generally consistent with previous
studies and these combined results indicated that the hotspot
mutations in these genes may not be actively involved in the
carcinogenesis of Chinese patients with ovarian carcinoma, except
for DICER1 mutations in Sertoli-Leydig cell tumors.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81260384 and
81260097) and the Natural Science Foundation of Jiangxi Province
(no. 20114BAB215033).
References
|
1
|
Parsons DW, Jones S, Zhang X, et al: An
integrated genomic analysis of human glioblastoma multiforme.
Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu J, Jiao Y, Dal Molin M, et al:
Whole-exome sequencing of neoplastic cysts of the pancreas reveals
recurrent mutations in components of ubiquitin-dependent pathways.
Proc Natl Acad Sci USA. 108:21188–21193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heravi-Moussavi A, Anglesio MS, Cheng SW,
et al: Recurrent somatic DICER1 mutations in nonepithelial ovarian
cancers. N Engl J Med. 366:234–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ley TJ, Ding L, Walter MJ, et al: DNMT3A
mutations in acute myeloid leukemia. N Engl J Med. 363:2424–2433.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tefferi A, Lasho TL, Abdel-Wahab O, et al:
IDH1 and IDH2 mutation studies in 1473 patients with chronic-,
fibrotic- or blast-phase essential thrombocythemia, polycythemia
vera or myelofibrosis. Leukemia. 24:1302–1309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogino S, Liao X, Imamura Y, et al:
Predictive and prognostic analysis of PIK3CA mutation in stage III
colon cancer intergroup trial. J Natl Cancer Inst. 105:1789–1798.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: new opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar
|
|
9
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar
|
|
10
|
Wiegand KC, Shah SP, Al-Agha OM, et al:
ARID1A mutations in endometriosis-associated ovarian carcinomas. N
Engl J Med. 363:1532–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rafnar T, Gudbjartsson DF, Sulem P, et al:
Mutations in BRIP1 confer high risk of ovarian cancer. Nat Genet.
43:1104–1107. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loveday C, Turnbull C, Ramsay E, et al:
Germline mutations in RAD51D confer susceptibility to ovarian
cancer. Nat Genet. 43:879–882. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones S, Wang TL, Shih IeM, et al:
Frequent mutations of chromatin remodeling gene ARID1A in ovarian
clear cell carcinoma. Science. 330:228–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romero I, Sun CC, Wong KK, Bast RC Jr and
Gershenson DM: Low-grade serous carcinoma: new concepts and
emerging therapies. Gynecol Oncol. 130:660–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martini M, Vecchione L, Siena S, Tejpar S
and Bardelli A: Targeted therapies: how personal should we go? Nat
Rev Clin Oncol. 9:87–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida K, Toki T, Okuno Y, et al: The
landscape of somatic mutations in Down syndrome-related myeloid
disorders. Nat Genet. 45:1293–1299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zighelboim I, Mutch DG, Knapp A, et al:
High frequency strand slippage mutations in CTCF in MSI-positive
endometrial cancers. Hum Mutat. 35:63–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Novetsky AP, Zighelboim I, Thompson DM Jr,
Powell MA, Mutch DG and Goodfellow PJ: Frequent mutations in the
RPL22 gene and its clinical and functional implications. Gynecol
Oncol. 128:470–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei X, Walia V, Lin JC, et al: Exome
sequencing identifies GRIN2A as frequently mutated in melanoma. Nat
Genet. 43:442–446. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan H, Parsons DW, Jin G, et al: IDH1 and
IDH2 mutations in gliomas. N Engl J Med. 360:765–773. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abbas S, Lugthart S, Kavelaars FG, et al:
Acquired mutations in the genes encoding IDH1 and IDH2 both are
recurrent aberrations in acute myeloid leukemia: prevalence and
prognostic value. Blood. 116:2122–2126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe T, Nobusawa S, Kleihues P and
Ohgaki H: IDH1 mutations are early events in the development of
astrocytomas and oligodendrogliomas. Am J Pathol. 174:1149–1153.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Im AP, Sehgal AR, Carroll MP, et al:
DNMT3A and IDH mutations in acute myeloid leukemia and other
myeloid malignancies: associations with prognosis and potential
treatment strategies. Leukemia. 28:1774–1783. 2014. View Article : Google Scholar
|
|
24
|
Kihara R, Nagata Y, Kiyoi H, et al:
Comprehensive analysis of genetic alterations and their prognostic
impacts in adult acute myeloid leukemia patients. Leukemia.
28:1586–1595. 2014. View Article : Google Scholar
|
|
25
|
Zou Y, Wang F, Liu FY, et al: RNF43
mutations are recurrent in Chinese patients with mucinous ovarian
carcinoma but absent in other subtypes of ovarian cancer. Gene.
531:112–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou Y, Liu FY, Liu H, et al: Frequent
POLE1 p.S297F mutation in Chinese patients with ovarian
endometrioid carcinoma. Mutat Res Fundam Mol Mech Mutagen.
761:49–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou Y, Zeng Y, Zhang DF, Zou SH, Cheng YF
and Yao YG: IDH1 and IDH2 mutations are frequent in Chinese
patients with acute myeloid leukemia but rare in other types of
hematological disorders. Biochem Biophys Res Commun. 402:378–383.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Witkowski L, Mattina J, Schonberger S, et
al: DICER1 hotspot mutations in non-epithelial gonadal tumours. Br
J Cancer. 109:2744–2750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ryland GL, Hunter SM, Doyle MA, et al:
RNF43 is a tumour suppressor gene mutated in mucinous tumours of
the ovary. J Pathol. 229:469–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murugan AK, Yang C and Xing M: Mutational
analysis of the GNA11, MMP27, FGD1, TRRAP and GRM3 genes in thyroid
cancer. Oncol Lett. 6:437–441. 2013.PubMed/NCBI
|
|
32
|
Parry M, Rose-Zerilli MJ, Gibson J, et al:
Whole exome sequencing identifies novel recurrently mutated genes
in patients with splenic marginal zone lymphoma. PLoS One.
8:e832442013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan XJ, Xu J, Gu ZH, et al: Exome
sequencing identifies somatic mutations of DNA methyltransferase
gene DNMT3A in acute monocytic leukemia. Nat Genet. 43:309–315.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neumann M, Heesch S, Schlee C, et al:
Whole-exome sequencing in adult ETP-ALL reveals a high rate of
DNMT3A mutations. Blood. 121:4749–4752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walter MJ, Ding L, Shen D, et al:
Recurrent DNMT3A mutations in patients with myelodysplastic
syndromes. Leukemia. 25:1153–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim MS, Kim YR, Yoo NJ and Lee SH:
Mutational analysis of DNMT3A gene in acute leukemias and common
solid cancers. APMIS. 121:85–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bleeker FE, Lamba S, Leenstra S, et al:
IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in
high-grade gliomas but not in other solid tumors. Hum Mutat.
30:7–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang MR, Kim MS, Oh JE, et al: Mutational
analysis of IDH1 codon 132 in glioblastomas and other common
cancers. Int J Cancer. 125:353–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mauzo SH, Lee M, Petros J, et al:
Immunohistochemical demonstration of isocitrate dehydrogenase 1
(IDH1) mutation in a small subset of prostatic carcinomas. Appl
Immunohistochem Mol Morphol. 22:284–287. 2014. View Article : Google Scholar : PubMed/NCBI
|