Introduction
In skin, the sebaceous glands produce and secrete
sebum that consists of a complex mixture of lipids that include
functions, such as photoprotection, antimicrobial activity,
delivery of fat-soluble anti-oxidants to the skin surface and pro-
and anti-inflammatory activity exerted by specific lipids for
homeostasis (1,2). Excreted sebum also maintains the skin
surface moisture via the formation of a lipid film on the skin
(3). Sebum is excreted to the skin
surface through the skin pore, which is an opening of the
pilosebaceous unit (2). The skin pores
affect the topographical features at the skin surface. Under
specific conditions, enlarged funnel-shaped skin pores may be
present. Skin pore widening is induced by various exogenous and
endogenous factors, such as gender, genetic predisposition, aging,
chronic ultraviolet light exposure, comedogenic xenobiotics, acne
and seborrhea (4). Various factors
induce excessive sebum synthesis and accumulation in the
pilosebaceous unit, which also contributes to skin pore size
(5). The enlarged skin pores can be
visible as ‘orange peel skin’, which is a significant cosmetic
problem (6). In addition, lipid
oxidation solidifies accumulated sebum, which generates comedone
formation (7).
A cosmetic cleanser is required that reduces skin
pore size by decreasing excessive sebum production and
accumulation, and that eliminates comedones. The objective of the
present study was to evaluate the effect of a cosmetic cleanser on
the removal of sebum and on skin pore size. The cleanser contained
a mixture of Diospyros kaki folium, Polygonum
cuspidatum and Castanea crenata var. dulcis
(DPC).
Diospyros kaki folium (persimmon) is widely
cultured in eastern Asia (8,9). Diospyros kaki folium leaves are
used for herbal medicines as they contain abundant flavonoid
glycosides that are used for their microbial inhibition, radical
scavenging, neuroprotection, blood-pressure reduction and
thrombosis inhibitory effects (10,11).
Polygonum cuspidatum and Castanea crenata var.
dulcis are also used for herbal medicines in eastern Asia
(12). Polygonum cuspidatum has
been used in traditional medicine for the remedy of inflammatory
diseases, hepatitis, tumors, diarrhea, dermatitis and osteomyelitis
(13). Additionally, in recent
studies, the resveratrol present in high concentrations in
Polygonum cuspidatum has been found to be an
anti-inflammatory via reduction of cytokine levels in plasma and an
anti-oxidant (14,15). Castanea crenata var.
dulcis is representatively used as an antiwrinkle agent in
East Asia (16).
The overall purpose of the present study was to
determine the effects of DPC added to a cosmetic cleanser on
enlarged pores and sebum elimination. The associations between pore
size and sebum emission were assessed in the absence and presence
of DPC.
Materials and methods
Preparation of test materials
DPC was purchased from Ami Cosmetics (Seoul, Korea).
Each dried sample of Diospyros kaki folium and Castanea
crenata var. dulcis (1 kg) was incubated for 3 h in 5 l
water at 85°C. Polygonum cuspidatum was incubated for 3 h in
5 l water at 75°C. The extracts were filtered using a 0.45 µm
membrane and vacuum evaporated using an Evelyn-1100 evaporator
(EYELA, Tokyo Rikakikai Co., Ltd., Tokyo, Japan). The concentrated
extracts ware freeze-dried using a Bondiro FD8508 freeze dryer
(ilShinBioBase Co., Ltd., Dongducheon, Korea). All the extracts
were sterilized using 60-Co γ-radiation (10 kGy/h, 25 kGy) prior to
use.
The base cosmetic cleanser prepared for the study
contained 3.5% dipropylene glycol, 2.5% hydrolyzed algin, 0.1%
sodium hyaluronate, 2.5% panthenol, 1% disodium-EDTA, 0.01%
triethanolamine and 0.03% sodium lauroyl sarcosinate. The cosmetic
cleanser with the DPC extracts was prepared by adding 4% DPC
extracts to the base cosmetic cleanser.
Subjects and treatments
The study protocols were approved by the
Institutional Review Board of the Korea Institute for Skin and
Clinical Sciences (Seoul, Korea). Informed consent was obtained
from all the participants. In total, 23 patients (7 male and 16
female) were studied, aged 20–50 years, who had no skin disease or
hypersensitivity. Each side of the subject's nose was washed with
70% ethanol prior to application of the test materials. The control
cosmetic cleanser was applied to the left side and the DPC cosmetic
cleanser was applied to the right side of the nose. After a 20-min
application of the test materials, external solidified sebum was
harvested using a curette. Oil content and skin pore count and area
were measured. All the experiments were performed in a temperature-
and humidity-controlled room. The temperature was 22±1°C and the
humidity was 45±5%.
Evaluation of oil content in skin
The oil content in the skin was measured by first
applying sebum tape to each side of the nose, prior and subsequent
to application of the cleansers. The oil content of the sebum tape
was measured using a DermaLab USB sebum probe (Cortex Technology
ApS, Hadsund, Denmark).
Evaluation of skin pore count and
area
A PRIMOS Lite skin measurement device (field of view
45×30-simple, flexible 3D measuring; GFMesstechnik GmbH, Berlin,
Germany) was utilized for evaluation of skin pore count and area.
Each side of the nose for each subject was measured three
consecutive times. The measurements were taken prior and subsequent
to application of the test materials. The images were fitted into
the same position using 3-dimensional matching and were analyzed
using the PRIMOS Lite software (PRIMOS Lite version 5.6E).
Evaluation of external sebum
For evaluation of external sebum, emitted sebum was
harvested from a 1-cm2 skin surface area using a
comedone extractor. The sebum was diffused onto a glass slide and
photographed. The sebum was calculated and quantified using Image J
software (National Institutes of Health, Washington, DC, USA).
Statistical analysis
The statistical significance of the differences was
determined using the Student's paired t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of DPC on oil content of
skin
The effect of DPC on skin oil content was first
assessed. A DermaLab USB sebum probe was used on each side of the
nose where the control and DPC cosmetic cleanser was applied. As
indicated in Fig. 1, skin oil content
decreased by 12.70%, from 7.06 to 6.16, for the control cosmetic
cleanser. The skin oil content decreased by 77.3% (from 6.19 to
1.40) when the cleanser that contained DPC was used.
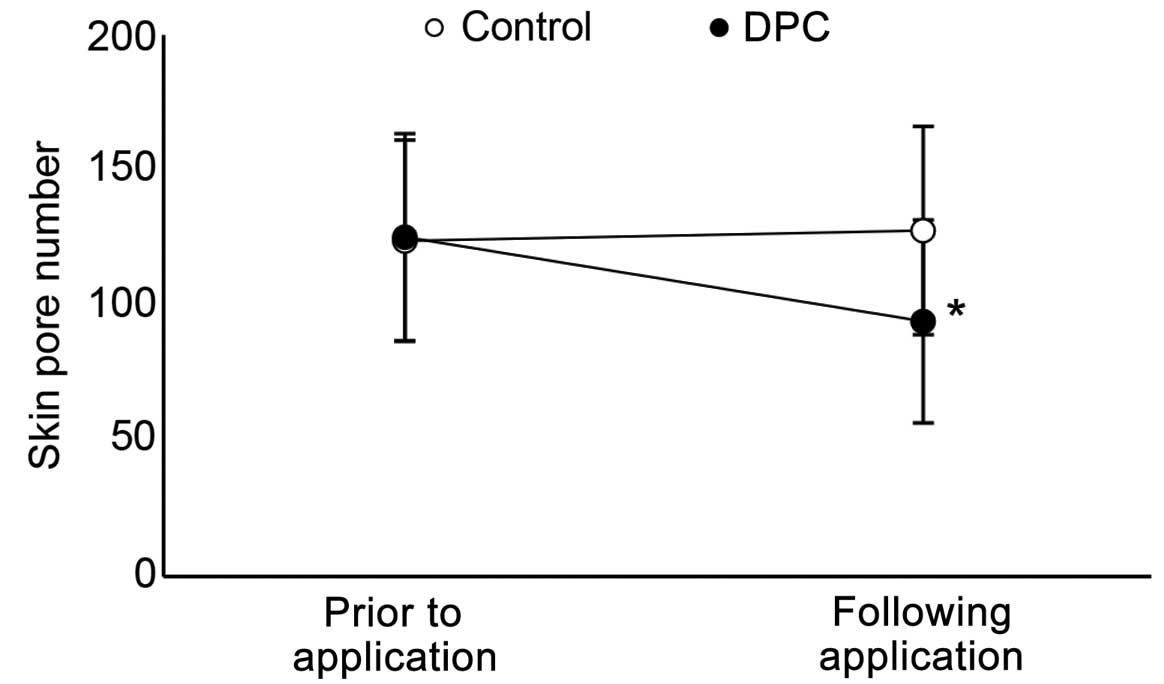
Effect of DPC on number and size of
skin pores
The numbers and sizes of skin pores were also
evaluated to reveal the effects of the DPC cosmetic cleanser on the
skin. Using the PRIMOS Lite, the numbers of skin pores were counted
prior and subsequent to application of the control cosmetic
cleanser. The number of skin pores increased by 3.05%, from 124.00
to 127.78 (Fig. 2). The number of skin
pores decreased by 24.83%, from 125.39 to 94.23, when the cleanser
containing DPC was used. PRIMOS Lite was also used to reveal the
skin pore size. The control cleanser increased skin pore size, from
0.05 to 0.06 µm3 (20% increase). The cleanser containing
DPC decreased skin pore size, from 0.07 to 0.02 µm3
(71.43% decrease) (Fig. 3).
Effect of DPC on amount of extracted
sebum
To quantify the amount of extracted sebum, the
straightened area was measured using Image J software. The
straightened area of sebum increased 335% when DPC cleanser was
used (Fig. 4A). In addition,
Demodex mites were detected in extracted sebum (Fig. 4B). Overall, this result suggested that
the cleanser containing DPC extracted a greater amount of sebum
compared to the control cleanser from human skin.
Discussion
To the best of our knowledge, the present study is
the first to reveal that a cosmetic cleanser containing DPC
decreases skin sebum and decreases the numbers and sizes of skin
pores. Oil content decreased by 12.60% when the control cleanser
was applied to the skin. However, pore size and number were
slightly increased, by 20 and 3.05%, respectively, when the control
cleanser was used. These results indicated that the cleanser
reduced skin oil content, but with the accompanying side effects of
increases in pore size and number. This result was consistent with
the results of an earlier study, which indicated that the detergent
contained in cleansers induces irritation, inflammation and
increases androgens (17–19). In general, it is well-known that
irritation, inflammation and androgen activation provokes
intercellular reactive oxygen species, which induce cell death,
inflammation, increase cytokine expression and can increase skin
pore size and number (18–20).
Additionally, in the DPC-containing cleanser applied
participants, extracted solidified sebum increased by 335%,
compared to the control cleanser. In the extracted solidified
sebum, Demodex mites, which is associated with the
development of rosacea and seborrheic dermatitis feed on sebum
(21,22), was detected by light microscopy. The
results suggested that the DPC cleanser facilitated extraction of
Demodex mites.
In conclusion, the skin oil content was
significantly decreased by the DPC cleanser, compared to the
control cleanser. The cleanser that contained DPC decreased pore
size and number, and the DPC cleanser easily removed solidified
sebum in skin.
Acknowledgements
The present study was supported by a grant from the
Korean Health Technology R&D Project (grant no. HN13C0075),
Ministry of Health & Welfare, Republic of Korea. Dr Hwa Jun Cha
was supported by the KU Research Professor Program of Konkuk
University.
References
|
1
|
Zouboulis CC, Baron JM, Böhm M,
Kippenberger S, Kurzen H, Reichrath J and Thielitz A: Frontiers in
sebaceous gland biology and pathology. Exp Dermatol. 17:542–551.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Picardo M, Ottaviani M, Camera E and
Mastrofrancesco A: Sebaceous gland lipids. Dermatoendocrinol.
1:68–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng Y, Dong Y, Wang J, et al:
Moisturizing and anti-sebum secretion effects of cosmetic
application on human facial skin. J Cosmet Sci. 60:7–14.
2009.PubMed/NCBI
|
|
4
|
Uhoda E, Piérard-Franchimont C, Petit L
and Piérard GE: The conundrum of skin pores in dermocosmetology.
Dermatology. 210:3–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roh M, Han M, Kim D and Chung K: Sebum
output as a factor contributing to the size of facial pores. Br J
Dermatol. 155:890–894. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roh M, Goo B, Jung J, Chung H and Chung K:
Treatment of enlarged pores with the quasi long-pulsed versus
Q-switched 1064 nm Nd:YAG lasers: A split-face, comparative,
controlled study. Laser Ther. 20:175–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cunliffe WJ, Holland DB and Jeremy A:
Comedone formation: Etiology, clinical presentation, and treatment.
Clin Dermatol. 22:367–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen G, Wei SH, Huang J and Sun J: A novel
C-glycosylflavone from the leaves of Diospyros kaki. J Asian Nat
Prod Res. 11:503–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamada Y, Yamamoto A, Yoneda N and
Nakatani N: Identification of kaempferol from the leaves of
Diospyros kaki and its antimicrobial activity against streptococcus
mutans. Biocontrol Sci. 4:97–100. 1999. View Article : Google Scholar
|
|
10
|
Bei W, Zang L, Guo J, et al:
Neuroprotective effects of a standardized flavonoid extract from
Diospyros kaki leaves. J Ethnopharmacol. 126:134–142. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakanaka S, Tachibana Y and Okada Y:
Preparation and antioxidant properties of extracts of Japanese
persimmon leaf tea (kakinoha cha). Food Chem. 89:569–575. 2005.
View Article : Google Scholar
|
|
12
|
Kirino A, Takasuka Y, Nishi A, Kawabe S,
Yamashita H, Kimoto M, Ito H and Tsuji H: Analysis and
functionality of major polyphenolic components of Polygonum
cuspidatum (itadori). J Nutr Sci Vitaminol (Tokyo). 58:278–286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leu YL, Hwang TL, Hu JW and Fang JY:
Anthraquinones from Polygonum cuspidatum as tyrosinase inhibitors
for dermal use. Phytother Res. 22:552–556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zahedi HS, Jazayeri S, Ghiasvand R,
Djalali M and Eshraghian MR: Effects of Polygonum cuspidatum
containing resveratrol on inflammation in male professional
basketball players. Int J Prev Med. 4 (Suppl 1):S1–S4.
2013.PubMed/NCBI
|
|
15
|
Ghanim H, Sia CL, Abuaysheh S,
Korzeniewski K, Patnaik P, Marumganti A, Chaudhuri A and Dandona P:
An antiinflammatory and reactive oxygen species suppressive effects
of an extract of Polygonum cuspidatum containing resveratrol. J
Clin Endocrinol Metab. 95:E1–E8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chi YS, Heo MY, Chung JH, Jo BK and Kim
HP: Effects of the chestnut inner shell extract on the expression
of adhesion molecules, fibronectin and vitronectin, of skin
fibroblasts in culture. Arch Pharm Res. 25:469–474. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slotosch CM, Kampf G and Löffler H:
Effects of disinfectants and detergents on skin irritation. Contact
Dermat. 57:235–241. 2007. View Article : Google Scholar
|
|
18
|
Nilius B, Prenen J and Owsianik G:
Irritating channels: The case of TRPA1. J Physiol. 589:1543–1549.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naha PC, Davoren M, Lyng FM and Byrne HJ:
Reactive oxygen species (ROS) induced cytokine production and
cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol Appl
Pharmacol. 246:91–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bikowski JB and Del Rosso JQ: Demodex
dermatitis: A retrospective analysis of clinical diagnosis and
successful treatment with topical crotamiton. J Clin Aesthet
Dermatol. 2:20–25. 2009.PubMed/NCBI
|
|
22
|
Jarmuda S, O'Reilly N, Zaba R, Jakubowicz
O, Szkaradkiewicz A and Kavanagh K: Potential role of Demodex mites
and bacteria in the induction of rosacea. J Med Microbiol.
61:1504–1510. 2012. View Article : Google Scholar : PubMed/NCBI
|