Introduction
Microwave technology has been widely used in
numerous applications, such as food sterilization, mobile
communication and heat therapy in medicine (1). Microwaves cause heating within a material
by exciting molecules to rotate. This rotation produces energy in
the form of heat. However, microwaves have a harmful influence on
the body.
Cataracts and testis disorders are well-known as
adverse effects of microwave (2–5);
additionally, microwaves can penetrate the cranium and reach the
deep brain. Certain studies have shown that microwaves in the
frequency range between 800 and 1,000 MHz can penetrate the cranium
and that <40% of these can reach the deep brain (6,7) where they
may penetrate <4–5 cm into the brain (8,9).
Numerous studies have investigated the pathological,
biochemical and behavioral changes of the central nervous system
resulting from microwave exposure from mobile phones (10–14). The
adverse effects of microwave exposure from the Global System for
Mobile Communication were studied in an animal model, using 900-MHz
radiation at an intensity (such as 0.9 W/kg) and time similar to
those of mobile phone emissions (15).
These studies focused on chronic changes.
Excessive microwave exposure has been reported to
cause trauma and fatal accidents in the early 1970s (16) and change blood-brain barrier
permeability (17,18). The higher the microwave output, the
larger the blood-brain barrier permeability (19). Although certain studies reported that
these changes were caused by high temperature, blood-brain barrier
permeability was reported to change in the absence of elevated
temperature (20,21). Pathological changes of neural cells
induced by microwave exposure have not been well-characterized.
The present study aimed to evaluate the change in
the number of neural cells and presence of apoptotic cells in rats
for one month following exposure to excessive microwave
radiation.
Materials and methods
Animal model of microwave
exposure
A microwave applicator was used to expose rat brains
to microwaves to inactivate all enzymes in the brain (Model MMW-05,
Muromachi microwave fixation system; Muromachi Kikai Co., Ltd.,
Tokyo, Japan) (Fig. 1). In animals
weighing 300 g, a microwave frequency of 2.45 GHz increased brain
temperature to 75–90°C at 5.0 kW for 1.40 sec. Microwave output was
set between 2 and 5 kW in 0.01-kW increments and exposure time was
set between 0.10 and 2.99 sec in 0.01-sec increments.
First, to determine the appropriate microwave
radiation intensity, 61 male Sprague-Dawley rats (350–400 g;
Charles River Laboratories Japan, Inc., Kanagawa, Japan) were used.
The microwave output resulting in 50% mortality was determined in
preliminary experiments and microwaves at 3.0 kW for 0.10 sec were
used. A total of 31 male Sprague-Dawley rats were used for the
following study. The head was positioned prone, fixed on a rat
holder and exposed to 3.0 kW of microwave radiation for 0.1 sec
under anesthesia (360 mg/kg of chloral hydrate, intraperitoneal
injection).
Animals were sacrificed after 24 h (n=3), or 3
(n=3), 7 (n=3), 14 (n=3) or 28 days (n=4) of exposure. Animals
without microwave exposure were used as controls (n=3). The rats
were perfused for fixation with 10% buffered formalin under
anesthesia (360 mg/kg of chloral hydrate, intraperitoneal
injection) and their brains were removed and fixed overnight.
All the protocols that involved the use of animals
were approved by the Animal Use Committee of Nippon Medical School
(approval no. 24–106).
Histological analysis
Fixed brains were cut into 2-mm coronal sections
with a brain slicer (Muromachi Kikai Co., Ltd.) and embedded in
paraffin using a routine procedure with a vacuum rotary. The
paraffin-embedded sections (3-µm) were subjected to hematoxylin and
eosin (H&E) staining and the terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick-end labeling
(TUNEL) assay. Although various methods to evaluate brain injury
have been reported, we chose to ascertain the extent of neural
injury by assessing apoptosis and number of surviving cerebral
neurons.
The neural cells were counted in the motor cortex
and hippocampus [cornu ammonis 1 (CA1) and CA2]. The percentage of
positive cells stained with TUNEL (ApopTag® Peroxidase In
Situ Apoptosis Detection kit; Chemicon, EMD Millipore,
Billerica, MA, USA) was measured, which detected apoptosis cell
death in the choroid plexus in the lateral ventricle, motor cortex
and hippocampus (CA1 and CA2). The number of cells differed between
sections in the choroid plexus of the lateral ventricle, so the
cells were not counted in the choroid plexus. Cells were counted by
two investigators and the average was used for analysis.
Statistical analysis
Unless otherwise stated, values are presented as
mean ± standard deviation. Data were analyzed by the Student's
t-test. Statistical analyses were performed with StatFlex version.
6.0 (Artech Co., Ltd., Osaka, Japan). P<0.05 was considered to
indicate a statistically significant difference.
Results
Survival rate
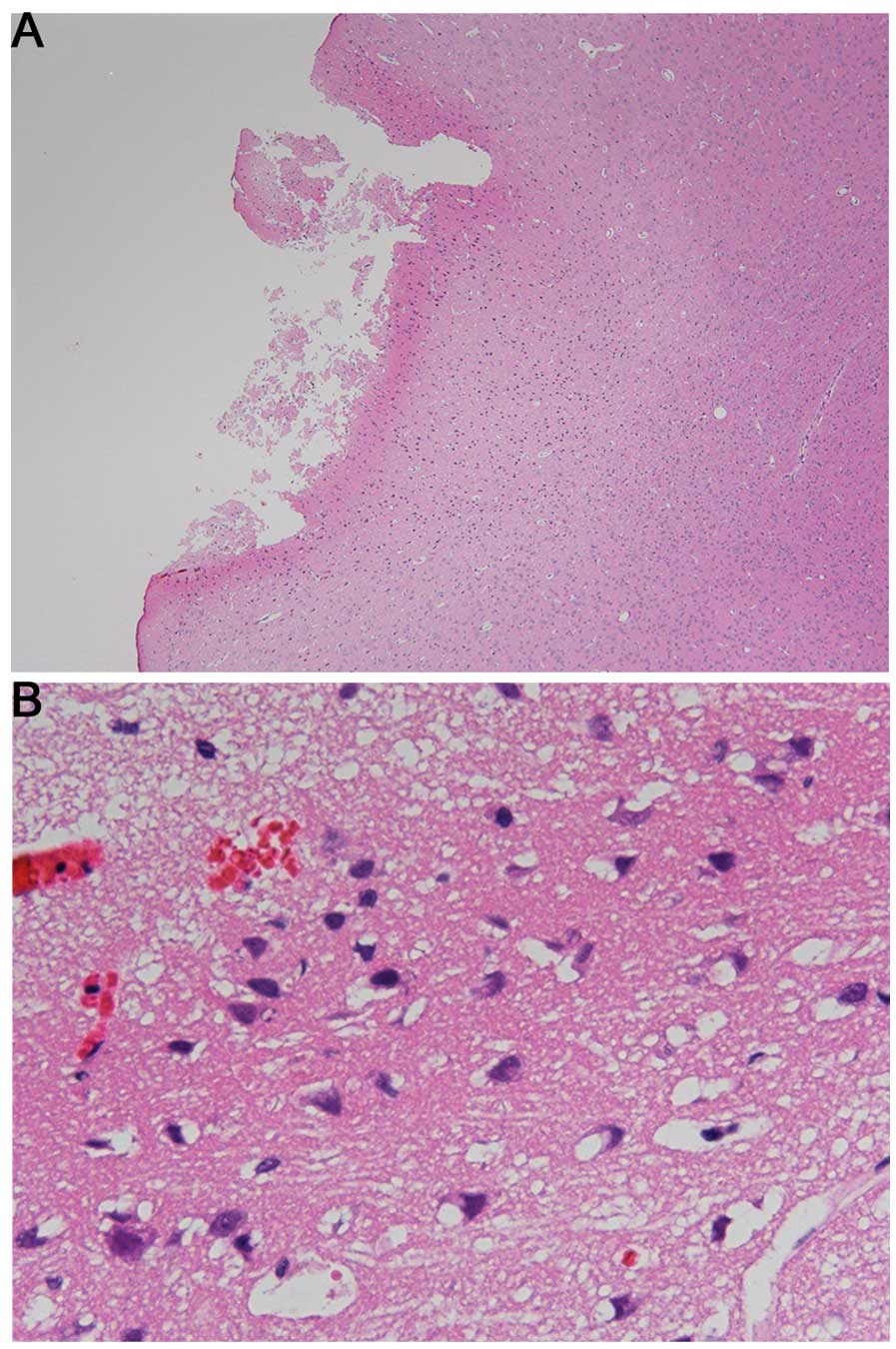
Preliminary experiments revealed that 3.2 kW of
microwave radiation for 0.1 sec caused cerebral injury of the
cortex after 24 h of exposure (Fig.
2).
When the radiation intensity was reduced to 3.0 kW,
6 of the 31 rats (19.4%) died within 24 h of microwave exposure and
in total, 15 rats (48.4%) died by day 28. Rats were exposed to
microwave radiation under these conditions and were examined
histologically.
Body weight changes
Body weight prior to microwave exposure was 384±11
g. The body weight significantly decreased to 363±14 g (P=0.007) on
day 1 after microwave exposure and 342±2 g (P<0.001) on day 3,
but significantly increased to 402±10 g (P=0.0151) by day 14. Four
rats survived on day 28, but the body weights of 3 had a deficit
and the other weighed 455 g. The body weight increased on day 28
after microwave exposure, but was not statistically significant
(Fig. 3).
Pathological changes
H&E staining showed no cerebral contusion or
cerebral edema after exposure to 3.0 kW.
In the CA1, the number of neural cells decreased
significantly by day 28 compared with the control (60.7 vs. 50.6,
P=0.0358), but did not decrease by day 28. In the CA2 and motor
cortex, the number of neural cells did not differ significantly
from that in the control (Fig. 4).
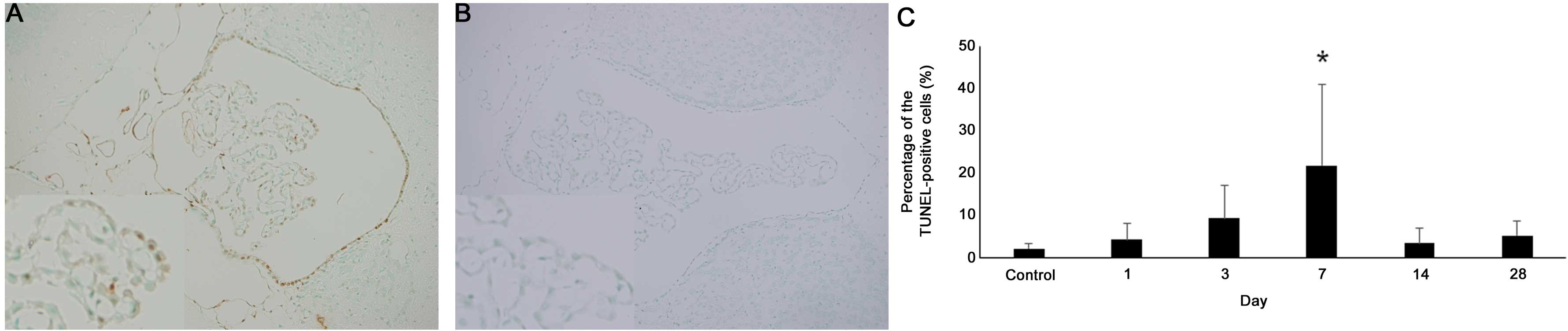
In the choroid plexus of the lateral ventricle, the
percentage of TUNEL-positive cells was 2.1±1.1% in the control
group, 4.2±3.7% (P=0.2191) on day 1, 9.2±7.9% (P=0.0547) on day 3
and 21.8±19.1% (P=0.0318) on day 7. There was a significant
increase on day 7 compared with the control. However, the
percentage of TUNEL-positive cells was 3.4±3.3% (P=0.3928) on day
14 and 5.1±3.4% (P=0.0596) on day 28. There were no significant
differences from controls in the percentage of TUNEL-positive cells
in the motor cortex and hippocampus (Fig.
5).
Discussion
The present study revealed that neural cells
decreased in the CA1 on day 28 and that the percentage of apoptotic
cells increased in the choroid plexus of the lateral ventricle on
day 7 after microwave exposure of 3.0 kW.
Animal models of traumatic brain injury have
included fluid percussion injury (22), controlled cortical impact injury
(23,24)
and weight-drop impact acceleration injury (25). Microwave exposure of 3.2 kW caused
cerebral contusion in the cortex. Although this level of microwave
exposure showed no effect on the body surface, cranium, brain
surface or cortex, it caused changes deep in the brain. The
microwave-induced neurotrauma model appears to be different from
other models of traumatic brain injury.
In CA1, the proportion of the apoptotic cells did
not increase on any day. Although the neural cells did not decrease
until day 14, they decreased significantly by day 28. The central
nervous system, particularly the hippocampus, is sensitive to
microwave radiation. Previous studies have shown that microwave
radiation damaged the hippocampal structure of rats, impaired
long-term potentization, reduced neurotransmitter concentration,
reduced synaptic vesicles and resulted in memory impairment
(26–30). The present study also showed that the
neural cells decreased in the hippocampus only, particularly in
CA1.
The proportion of apoptotic cells increased by day 7
in the choroid plexus of lateral ventricles for unknown reasons.
There was the potential to increase the temperature of the
ventricles by microwave excitation of water molecules.
Blast injury is known to cause injury confined to
the choroid plexus of lateral ventricles and surrounding tissue
(31).
Kaur et al (31)
reported that the choroid plexus in rats exhibited ultrastructural
changes following an open-field blast and that the intercellular
spaces between the choroid plexus epithelial cells were greatly
widened, coupled with the massive eruption and possible extrusion
of the apical cytoplasm into the ventricular lumen. Garman et
al (32) observed blood-brain
barrier permeability around the circumventricular organs following
the blast using the tube-blast injury model. Thus,
microwave-induced neurotrauma and blast injury caused injuries in
the choroid plexus of lateral ventricles; however, further studies
are required for evaluating damage to axons.
Although the present study showed high mortality, it
only showed small pathophysiological changes of the brain and the
cause of death was unclear. When comparing survival rate and body
weight, survival rate decreased parallel to body weight by day 3,
but survival rate did not continue to decrease after increasing by
day 14. There was no cardiac arrest or respiratory failure
immediately following microwave exposure of 3.0 kW.
Microwave-induced neurotraumas are simple and
reproducible in animal models of traumatic brain injury. The
intensity and time of microwave exposure can be precisely
varied.
In conclusion, the present study analyzed the
pathological changes in the rat brain following excessive microwave
exposure. Apoptotic cells increased in the choroid plexus of the
lateral ventricle on day 7 and neural cells decreased in the CA1 by
day 28. The effects of microwave exposure on the brain remain
unclear; however, microwave-induced neurotrauma shows the same
pathological changes as blast traumatic brain injury.
Acknowledgements
The present study was supported by Japan Society for
the Promotion of Science KAKENHI (grant no. 25462835). The authors
would like to thank Mr. Takayuki Asakura for performing the animal
experiments.
References
|
1
|
Kesari KK, Kumar S and Behari J:
Pathophysiology of microwave radiation: Effect on rat brain. Appl
Biochem Biotechnol. 166:379–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richardson AW, Duante TD and Hines HM:
Experimental cataract produced by 3 cm. Pulsed microwave
irradiations. AMA Arch Opthalmol. 45:382–386. 1951. View Article : Google Scholar
|
|
3
|
Hirsch FG and Parker JT: Bilateral
lenticular opacities occurring in a technician operating a
microwave generator. AMA Arch Ind Hyg Occup Med. 6:512–517.
1952.PubMed/NCBI
|
|
4
|
Kalant H: Physiological hazards of
microwave radiation: A survey of published literature. Can Med
Assoc J. 81:575–582. 1959.PubMed/NCBI
|
|
5
|
Ghanbari M, Mortazavi SB, Khavanin A and
Khazaei M: The Effects of Cell Phone Waves (900 MHz-GSM Band) on
Sperm Parameters and Total Antioxidant Capacity in Rats. Int J
Fertil Steril. 7:21–28. 2013.PubMed/NCBI
|
|
6
|
Kang XK, Li LW, Leong MS and Kooi PS: A
method of moments study of SAR inside spheroidal human head and
current distribution among handset wireless antennas. J Electromagn
Waves Appl. 15:61–75. 2001. View Article : Google Scholar
|
|
7
|
Barnett J, Timotijevic L, Shepherd R and
Senior V: Public responses to precautionary information from the
Department of Health (UK) about possible health risks from mobile
phones. Health Policy. 82:240–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dimbylow PJ and Mann SM: SAR calculations
in an anatomically realistic model of the head for mobile
communication transceivers at 900 MHz and 1.8 GHz. Phys Med Biol.
39:1537–1553. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rothman KJ, Chou CK, Morgan R, Balzano Q,
Guy AW, Funch DP, Preston-Martin S, Mandel J, Steffens R and Carlo
G: Assessment of cellular telephone and other radio frequency
exposure for epidemiologic research. Epidemiology. 7:291–298. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamblin DL, Wood AW, Croft RJ and Stough
C: Examining the effects of electromagnetic fields emitted by GSM
mobile phones on human event-related potentials and performance
during an auditory task. Clin Neurophysiol. 115:171–178. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sievert U, Eggert S and Pau HW: Can mobile
phone emissions affect auditory functions of cochlea or brain stem?
Otolaryngol Head Neck Surg. 132:451–455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferreri F, Curcio G, Pasqualetti P, De
Gennaro L, Fini R and Rossini PM: Mobile phone emissions and human
brain excitability. Ann Neurol. 60:188–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krause CM, Pesonen M, Haarala Björnberg C
and Hӓmӓlӓinen H: Effects of pulsed and continuous wave 902 MHz
mobile phone exposure on brain oscillatory activity during
cognitive processing. Bioelectromagnetics. 28:296–308. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumlin T, Iivonen H, Miettinen P, Juvonen
A, van Groen T, Puranen L, Pitkӓaho R, Juutilainen J and Tanila H:
Mobile phone radiation and the developing brain: Behavioral and
morphological effects in juvenile rats. Radiat Res. 168:471–479.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kesari KK, Kumar S and Behari J: 900-MHz
microwave radiation promotes oxidation in rat brain. Electromagn
Biol Med. 30:219–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McLaughlin JT: Tissue destruction and
death from microwave radiation (radar). Calif Med. 86:336–339.
1957.PubMed/NCBI
|
|
17
|
Cosquer B, Vasconcelos AP, Fröhlich J and
Cassel JC: Blood-brain barrier and electromagnetic fields: Effects
of scopolamine methylbromide on working memory after whole-body
exposure to 2.45 GHz microwaves in rats. Behav Brain Res.
161:229–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stam R: Electromagnetic fields and the
blood-brain barrier. Brain Res Brain Res Rev. 65:80–97. 2010.
View Article : Google Scholar
|
|
19
|
D'Andrea JA, Chou CK, Johnston SA and
Adair ER: Microwave effects on the nervous system.
Bioelectromagnetics. 24:(Suppl 6). S107–S147. 2003. View Article : Google Scholar
|
|
20
|
Salford LG, Brun A, Sturesson K, Eberhardt
JL and Persson BR: Permeability of the blood-brain barrier induced
by 915 MHz electromagnetic radiation, continuous wave and modulated
at 8, 16, 50 and 200 Hz. Microsc Res Tech. 27:535–542. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fritze K, Sommer C, Schmitz B, Mies G,
Hossmann KA, Kiessling M and Wiessner C: Effect of global system
for mobile communication (GSM) microwave exposure on blood-brain
barrier permeability in rat. Acta Neuropathol. 94:465–470. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dixon CE, Lyeth BG, Povlishock JT,
Findling RL, Hamm RJ, Marmarou A, Young HF and Hayes RL: A fluid
percussion model of experimental brain injury in the rat. J
Neurosurg. 67:110–119. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lighthall JW: Controlled cortical impact:
A new experimental brain injury model. J Neurotrauma. 5:1–15. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dixon CE, Clifton GL, Lighthall JW,
Yaghmai AA and Hayes RL: A controlled cortical impact model of
traumatic brain injury in the rat. J Neurosci Methods. 39:253–262.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marmarou A, Foda MA, van den Brink W,
Campbell J, Kita H and Demetriadou K: A new model of diffuse brain
injury in rats. Part I: Pathophysiology and biomechanics. J
Neurosurg. 80:291–300. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu S, Ning W, Xu Z, Zhou S, Chiang H and
Luo J: Chronic exposure to GSM 1800-MHz microwaves reduces
excitatory synaptic activity in cultured hippocampal neurons.
Neurosci Lett. 398:253–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Peng R, Hu X, Gao Y, Wang S, Zhao
L, Dong J, Su Z, Xu X, Gao R, et al: Abnormality of synaptic
vesicular associated proteins in cerebral cortex and hippocampus
after microwave exposure. Synapse. 63:1010–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao L, Peng RY, Wang SM, Wang LF, Gao YB,
Dong J, Li X and Su ZT: Relationship between cognition function and
hippocampus structure after long-term microwave exposure. Biomed
Environ Sci. 25:182–188. 2012.PubMed/NCBI
|
|
29
|
Wang H, Peng R, Zhou H, Wang S, Gao Y,
Wang L, Yong Z, Zuo H, Zhao L, Dong J, et al: Impairment of
long-term potentiation induction is essential for the disruption of
spatial memory after microwave exposure. Int J Radiat Biol.
89:1100–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Sun C, Xiong L, Yang Y, Gao Y,
Wang L, Zuo H, Xu X, Dong J, Zhou H, et al: MicroRNAs: Novel
Mechanism Involved in the Pathogenesis of Microwave Exposure on
Rats' Hippocampus. J Mol Neurosci. 53:222–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaur C, Singh J, Lim MK, Ng BL, Yap EP and
Ling EA: Studies of the choroid plexus and its associated epiplexus
cells in the lateral ventricles of rats following an exposure to a
single non-penetrative blast. Arch Histol Cytol. 59:239–248. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garman RH, Jenkins LW, Switzer RC III,
Bauman RA, Tong LC, Swauger PV, Parks SA, Ritzel DV, Dixon CE,
Clark RS, et al: Blast exposure in rats with body shielding is
characterized primarily by diffuse axonal injury. J Neurotrauma.
28:947–959. 2011. View Article : Google Scholar : PubMed/NCBI
|