Introduction
Atherosclerosis is a chronic vascular disease that
is now recognized as an inflammation of the arterial wall (1). Clinical and experimental studies show the
consistent association between various markers of inflammation and
cardiovascular diseases (2,3). Previous studies indicated the important
roles of pro-inflammatory cytokines in the pathogenesis of
atherosclerosis, such as C-reactive protein (CRP) (4,5),
interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α (TNF-α)
(6,7).
Peroxisome proliferator-activated receptors (PPARs),
as members of the nuclear receptor family of transcription factors,
participate in the regulation of lipid metabolism, blood pressure,
cell growth and migration, oxidative stress and inflammation
(8–10).
As the important anti-inflammatory cytokines, PPARα and PPARγ have
been gaining increasing attention with regards to the study of the
mechanisms involved in etiology and pathogenesis of
atherosclerosis. PPARα and PPARγ may regulate the expression of a
number of inflammatory response genes through interference with
pro-inflammatory transcription factor pathways, such as activator
protein-1 and nuclear factor-κB (11).
Significantly low levels of PPARγ have been found in
atherosclerotic lesions and its activation reduces monocyte
recruitment by the plaque (12). This
PPAR-dependent inhibition may prevent the rupture of
atherosclerotic plaques and the formation of subsequent thrombosis
(13).
Matrix metalloproteinases (MMPs) are specialized
enzymes for the degradation of extracellular matrix. In the vessel
wall, dysregulated functions of MMPs often lead to impaired
endothelial barrier function, infiltration of inflammatory cells,
migration and proliferation of vascular smooth muscle cells (VSMCs)
and finally to the development of atherosclerosis (14). MMP-9 belongs to the gelatinase
subfamily of MMPs and remodels the extracellular matrix as a part
of inflammatory response, which leads to plaque destablization and
triggers atherosclerotic diseases. The plasma MMP-9 level is
associated with atherosclerosis in the femoral artery (15). Therefore, MMP-9 is regarded as a
promising biomarker for plaque vulnerability and cardiovascular
events.
The existing evidence has confirmed that fibrinogen
is a key regulator of inflammation, except for its vital roles in
blood clotting. A number of studies report that a high plasma
fibrinogen level is an independent and major risk marker of
atherosclerosis (16,17). Additionally, a high fibrinogen level is
associated with the prevalence and extent of coronary artery
disease (CAD), and appears to be indicated in the pathophysiology
and prognosis of CAD. This association was independent of any other
atherothrombotic risk factor (18).
Although the pleiotropic roles of fibrinogen in
cardiovascular diseases have been suggested, there have been few
studies demonstrating its direct pro-inflammatory effect on the
vascular cells. Recently, we reported that fibrinogen, fibrin and
fibrinogen degradation products (FDP) induce the CRP generation in
VSMCs (19) and fibrinogen, and FDP
also upregulates the expression of IL-6, TNF-α and inducible nitric
oxide synthase in VSMCs (20). On the
basis of our previous study, the present experiment further
examined whether fibrinogen regulated the expression of PPARα, PPARγ
and MMP-9 in VSMCs to provide more evidence for its
pro-inflammatory and pro-atherosclerotic effects.
Materials and methods
Reagents
Plasminogen-depleted fibrinogen and plasmin were from
Calbiochem-Merck Co. (Darmstadt, Germany). Fetal bovine serum (FBS)
was purchased from HyClone (Logan, UT, USA). Dulbecco's modified
Eagle's medium (DMEM) was produced by Gibco BRL (Carlsbad, CA,
USA). Penicillin and streptomycin were purchased from Sigma-Aldrich
(St. Louis, MO, USA). The rat MMP-9 enzyme-linked immunosorbent
assay (ELISA) kit was obtained from R&D Systems (Minneapolis,
MN, USA). Anti-α-actin antibody was provided by ZSGB-BIO (Beijing,
China). Anti-PPARα and anti-PPARγ antibodies were from Abcam
(Cambridge, UK). PrimeScript® reverse transcription (RT) master mix
was purchased from Takara Bio, Inc. (Shiga, Japan). Agarose gel was
from Spanish Biochemicals Corp. (Pronadisa, Madrid, Spain).
Culture of rat VSMCs
Male Sprague-Dawley rats were provided by the
Laboratory Animal Center of Xi'an Jiaotong University School of
Medicine (Xi'an, Shaanxi, China). VSMCs were isolated from the
thoracic aorta of rats and cultured using the explant method as
previously described (21). In brief,
rats were anesthetized with intraperitoneal injection of sodium
pentobarbital (30 mg/kg). The thoracic aorta was removed and freed
of connective tissue and adherent fat. The endothelial cell layer
of intima was removed mechanically and the aortic artery was cut
into cubes of ~3 mm. These were subsequently placed in DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere of 5% CO2 and 95%
air at 37°C until the VSMCs exhibited a typical ‘hill and valley’
growth pattern. Finally, VSMCs were identified by morphological
examination and showed 99% purity as estimated with the
immunocytochemical staining for α-actin. The cells were passaged by
brief trypsinization, and the cells between passages 3 and 8 were
used for the experiments. When the cells were grown to confluence,
the cells were starved for 24 h in the serum-free medium before the
experiments. All the experimental procedures were performed in
accordance with the international, national and institutional
rules, and approved by the Institutional Animal Care Committee of
Xi'an Jiaotong University.
Reverse transcription-polymerase chain
reaction (RT-PCR) assay
Total RNA was isolated from VSMCs and reverse
transcribed into complementary DNA with PrimeScript® RT master mix
(Takara Bio, Inc.) following the manufacturer's instructions.
Reaction conditions of PCR amplification were 94°C for 3 min, 35
cycles at 94°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec, and
the final extension of PCR products was performed for 5 min at 72°C.
Primers for rat PPARα, PPARγ, MMP-9 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were
synthesized by Shanghai Sangon Biotechnology Co., Ltd. (Shanghai,
China) (Table I shows the sequences).
GAPDH was used as an internal control. The samples were run
in triplicate. PCR products were run on a 2% agarose gel containing
0.5 µg/ml ethidium bromide and resolved by electrophoresis. Images
were digitally captured and the band intensity was analyzed using
Gel Pro Analyzer software, version 4.0 (Media Cybernetics, Inc.,
Rockville, MD, USA). The relative mRNA expression of PPARα,
PPARγ and MMP-9 was normalized to that of
GAPDH.
 | Table I.Primers used for reverse transcription
polymerase chain reaction analysis. |
Table I.
Primers used for reverse transcription
polymerase chain reaction analysis.
| Gene | Primer sequence | Accession number |
|---|
| PPARα |
5′-CGGGTCATACTCGCAGGAAAG-3′ | NM_013196 |
|
|
5′-TGGCAGCAGTGGAAGAATCG-3′ |
|
| PPARγ |
5′-GGAAGCCCTTTGGTGACTTTATGG-3′ | NM_013124 |
|
|
5′-GCAGCAGGTTGTCTTGGATGTC-3′ |
|
| MMP-9 |
5′-GGCACCATCATAACATCACCTA-3′ | NM_031055 |
|
|
5′-GACACCAAACTGGATGACAATG-3′ |
|
| GAPDH |
5′-GCCTTCTCCATGGTGGTGAA-3′ | NM_017008 |
|
|
5′-GGTCGGTGTGAACGGATTTG-3′ |
|
Western blot analysis
VSMCs were washed, lysed and homogenized in 10
mmol/l Tris-HCl (pH 7.4) containing 0.1% sodium dodecylsulfate and
a protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim,
Germany). Subsequently, the protein extract was boiled in
electrophoresis buffer. Total cell extract protein (25 µg) was
resolved on 10% SDS-PAGE gels and transferred onto nitrocellulose
membranes. The membranes were blocked with 5% skimmed dry milk in
Tris-buffered saline containing 0.1% Tween-20 and incubated with
anti-PPARα (Cat. no. ab24509) or anti-PPARγ antibodies (Cat. no.
ab27649; both from Abcam), followed by the incubation with a
secondary peroxidase-conjugated antibody (Cat. no. ZB-2301;
ZSGB-BIO). GAPDH was used as loading control. Signals were
visualized by an enhanced chemiluminescence detection reagent.
Reagents (Pierce Biotechnology, Inc., Rockford, USA) for
strengthening chemiluminescence were applied to the blots and the
light signals were detected by X-ray film. Optical density of the
bands was scanned and quantified with Gel Doc 2000 (Bio-Rad,
Hercules, CA, USA). Data were normalized against those of the
corresponding GAPDH. Results are expressed relative to the
control.
ELISA
Following stimulation of the cells for the indicated
time, MMP-9 concentration in the culture supernatant was measured
by ELISA using the quantitative sandwich enzyme immunoassay
technique according to the manufacturer's instructions.
Statistical analysis
The experiments were repeated three times and all
the data are expressed as means ± standard error of the mean. All
analyses were performed by one-way analysis of variance using the
SPSS 12.0 software package (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference between the groups and treatments.
Results
Fibrinogen downregulates mRNA and
protein expression of PPARα in VSMCs
Fig. 1 shows that
fibrinogen significantly downregulated mRNA and protein expression
of PPARα in VSMCs in concentration- and time-dependent manners
compared to control. The maximal inhibition of PPARα was reached at
10 µmol/l fibrinogen and the inhibitory rate was 48.9% for mRNA
expression and 71.8% for protein expression.
Fibrinogen reduces mRNA and protein
expression of PPARγ in VSMCs
The results in Fig. 2
exhibit that fibrinogen also reduced mRNA and protein expression of
PPARγ in VSMCs in a concentration-dependent manner. mRNA and
protein expression of PPARγ was significantly reduced after
stimulation of the cells with 5 µmol/l fibrinogen for 12 h and
reached a minimum at 10 µmol/l fibrinogen. The maximal inhibition
of mRNA and protein expression of PPARγ was 63.7 and 79.9%,
respectively.
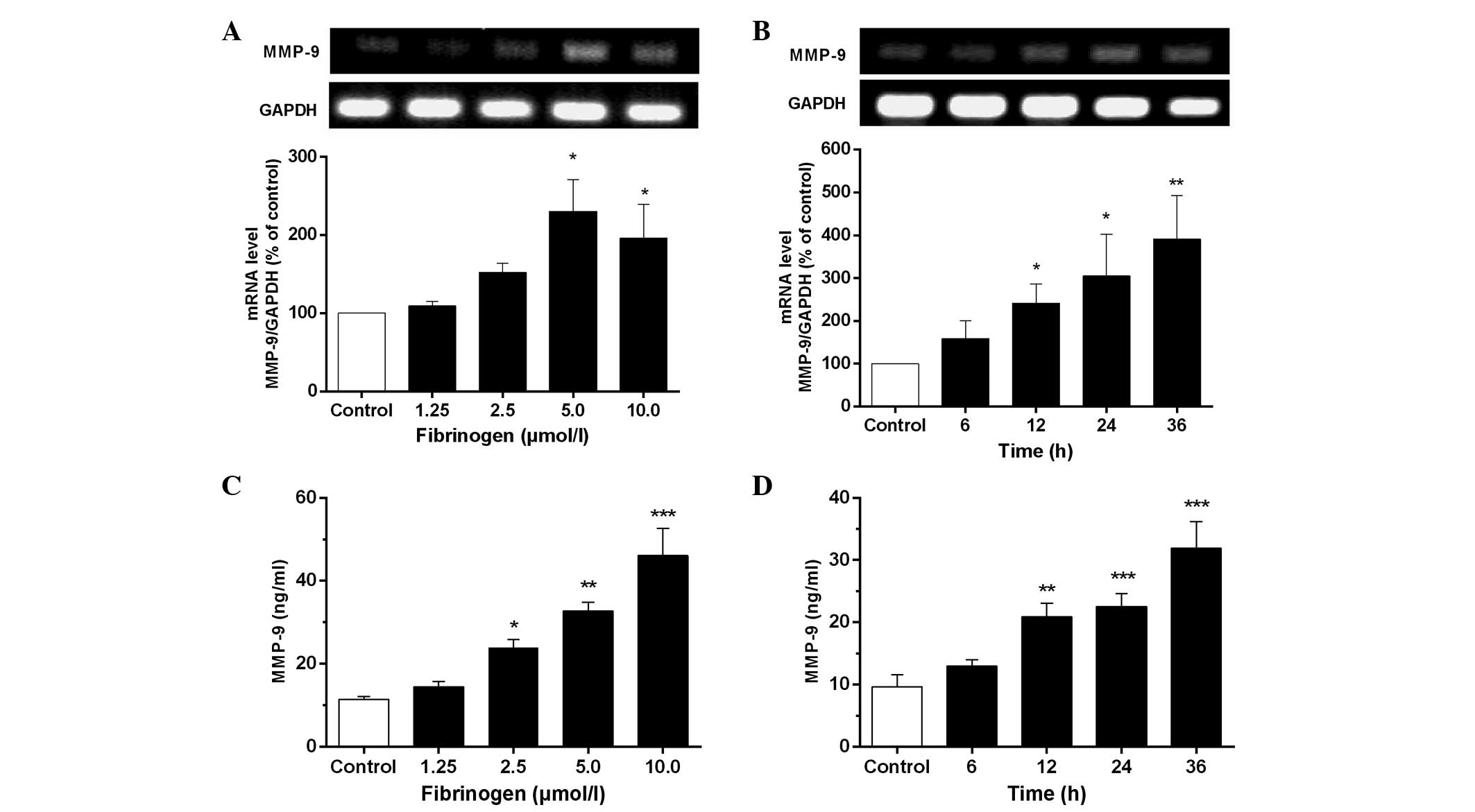
Fibrinogen increases mRNA and protein
expression of MMP-9 in VSMCs
As observed in Fig. 3A and
B, fibrinogen at 1.25–10 µmol/l markedly increased MMP-9
mRNA expression in VSMCs compared to the control. The results from
ELISA (Fig. 3C and D) indicated that
fibrinogen caused an apparent time- and concentration-dependent
increase of the MMP-9 level in the culture supernatants of VSMCs.
The maximal generation of MMP-9 was detected after treatment of the
cells with 10 µmol/l fibrinogen for 12 h, which was 4 times over the
control.
Discussion
An increasing body of evidence supports that
fibrinogen is not only a blood coagulation factor but also an
inflammatory marker (22). Fibrinogen,
fibrin and FDP are components of stable and unstable
atherosclerotic plaques (23).
Additionally, fibrinogen has been identified as an independent risk
indicator for ischemic heart disease and the severity of
atherosclerosis (24). Although
certain possible mechanisms regarding how fibrinogen participates in
atherosclerosis have been postulated, the underlying mechanism has
not yet been completely elucidated.
A number of studies report that an elevated
fibrinogen level participates in the formation of atherosclerosis
through activating platelet aggregation, increasing plasma
viscosity, injuring endothelial cells, stimulating migration and
proliferation of VSMCs (19,25). Certain studies also indicate that
fibrinogen has the ability to stimulate the production of the
pro-inflammatory cytokines such as monocyte chemoattractant
protein-1, IL-8 and endothelin-1 in endothelial cells (26,27), and the
synthesis of IL-6 and TNF-α in the peripheral blood mononuclear
cells (28). Extravascular fibrinogen
induces macrophage chemokine expression through Toll-like receptor
4, thereby promoting immune surveillance at sites of inflammation
(29). The present study showed that
fibrinogen downregulated expression of PPARα and PPARγ, and
upregulated MMP-9 production in VSMCs at the mRNA and protein
levels.
It is well-known that PPARα and PPARγ are involved
in all the stages of atherosclerosis (30). In addition to other beneficial
characteristics (31), PPARα- and
PPARγ-mediated inhibition of atherosclerosis is also associated
with their anti-inflammatory effect. In vitro studies
demonstrate that PPARα and PPARγ agonists reduce the gene
expression and secretion of pro-inflammatory cytokines, including
TNF-α, IL-1β and IL-6, consequently inhibiting macrophage and
endothelial activation (32,33).
MMP-9 is able to efficiently degrade major
components of the plaque extracellular matrix to lead to the
expansion and eventual rupture of atherosclerotic plaques (34). Previous studies showed that serum MMP-9
is increased in subjects at risk of various forms of chronic
inflammation and cardiovascular diseases (35). Certain studies confirm that activation
of monocytes by fibrinogen increases MMP-9 secretion and MMP-9
itself enhances monocyte recruitment by the plaque (36).
In conclusion, the present results demonstrate that
fibrinogen exerts a pro-inflammatory effect on VSMCs through
inhibiting the expression of anti-inflammatory cytokines PPARα and
PPARγ, and stimulating production of pro-inflammatory cytokine
MMP-9, which contributes to its atherogenic effect. The findings
provide new evidence for the pro-inflammatory and
pro-atherosclerotic effects of fibrinogen. However, the exact
molecular mechanisms for the effects remain unknown and further
studies are required to characterize the mechanisms responsible for
the pro-inflammatory effect of fibrinogen.
References
|
1
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gram J: Inflammation in atherosclerosis
and acute coronary syndromes. Int Congr Ser. 1229:95–102. 2002.
View Article : Google Scholar
|
|
3
|
Kaperonis EA, Liapis CD, Kakisis JD,
Dimitroulis D and Papavassiliou VG: Inflammation and
atherosclerosis. Eur J Vasc Endovasc Surg. 31:386–393. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yasojima K, Schwab C, McGeer EG and McGeer
PL: Generation of C-reactive protein and complement components in
atherosclerotic plaques. Am J Pathol. 158:1039–1051. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ridker PM: Clinical application of
C-reactive protein for cardiovascular disease detection and
prevention. Circulation. 107:363–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bermudez EA, Rifai N, Buring J, Manson JE
and Ridker PM: Interrelationships among circulating interleukin-6,
C-reactive protein and traditional cardiovascular risk factors in
women. Arterioscler Thromb Vasc Biol. 22:1668–1673. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lind L: Circulating markers of
inflammation and atherosclerosis. Atherosclerosis. 169:203–214.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han SH, Quon MJ and Koh KK: Beneficial
vascular and metabolic effects of peroxisome proliferator-activated
receptor-α activators. Hypertension. 46:1086–1092. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchesi C and Schiffrin EL: Peroxisome
proliferator-activated receptors and the vascular system: Beyond
their metabolic effects. J Am Soc Hypertens. 2:227–238. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moraes LA, Piqueras L and Bishop-Bailey D:
Peroxisome proliferator-activated receptors and inflammation.
Pharmacol Ther. 110:371–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bouhlel MA, Staels B and Chinetti-Gbaguidi
G: Peroxisome proliferator-activated receptors - from active
regulators of macrophage biology to pharmacological targets in the
treatment of cardiovascular disease. J Intern Med. 263:28–42.
2008.PubMed/NCBI
|
|
12
|
Marx N, Sukhova G, Murphy C, Libby P and
Plutzky J: Macrophages in human atheroma contain PPARgamma:
Differentiation-dependent peroxisomal proliferator-activated
receptor gamma (PPARgamma) expression and reduction of MMP-9
activity through PPARgamma activation in mononuclear phagocytes in
vitro. Am J Pathol. 153:17–23. 1998.PubMed/NCBI
|
|
13
|
Shu H, Wong B, Zhou G, Li Y, Berger J,
Woods JW, Wright SD and Cai TQ: Activation of PPARalpha or gamma
reduces secretion of matrix metalloproteinase 9 but not interleukin
8 from human monocytic THP-1 cells. Biochem Biophys Res Commun.
267:345–349. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galis ZS and Khatri JJ: Matrix
metalloproteinases in vascular remodeling and atherogenesis: The
good, the bad and the ugly. Circ Res. 90:251–262. 2002.PubMed/NCBI
|
|
15
|
Olson FJ, Schmidt C, Gummesson A,
Sigurdardottir V, Hulthe J, Wiklund O and Fagerberg B: Circulating
matrix metalloproteinase 9 levels in relation to sampling methods,
femoral and carotid atherosclerosis. J Intern Med. 263:626–635.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eidelman RS and Hennekens CH: Fibrinogen:
A predictor of stroke and marker of atherosclerosis. Eur Heart J.
24:499–500. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tribouilloy C, Peltier M, Colas L, Senni
M, Ganry O, Rey JL and Lesbre JP: Fibrinogen is an independent
marker for thoracic aortic atherosclerosis. Am J Cardiol.
81:321–326. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Luca G, Verdoia M, Cassetti E, Schaffer
A, Cavallino C, Bolzani V and Marino P: Novara Atherosclerosis
Study Group (NAS): High fibrinogen level is an independent
predictor of presence and extent of coronary artery disease among
Italian population. J Thromb Thrombolysis. 31:458–463. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo F, Liu J, Wang C, Liu N and Lu P:
Fibrinogen, fibrin, and FDP induce C-reactive protein generation in
rat vascular smooth muscle cells: Pro-inflammatory effect on
atherosclerosis. Biochem Biophys Res Commun. 390:942–946. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu PP, Liu JT, Liu N, Guo F, Ji YY and
Pang X: Pro-inflammatory effect of fibrinogen and FDP on vascular
smooth muscle cells by IL-6, TNF-α and iNOS. Life Sci. 88:839–845.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hadrava V, Tremblay J and Hamet P:
Abnormalities in growth characteristics of aortic smooth muscle
cells in spontaneously hypertensive rats. Hypertension. 13:589–597.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davalos D and Akassoglou K: Fibrinogen as
a key regulator of inflammation in disease. Semin Immunopathol.
34:43–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bini A, Fenoglio JJ Jr, Mesa-Tejada R,
Kudryk B and Kaplan KL: Identification and distribution of
fibrinogen, fibrin and fibrin(ogen) degradation products in
atherosclerosis Use of monoclonal antibodies. Arteriosclerosis.
9:109–121. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Maat MPM, Pietersma A, Kofflard M,
Sluiter W and Kluft C: Association of plasma fibrinogen levels with
coronary artery disease, smoking and inflammatory markers.
Atherosclerosis. 121:185–191. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lominadze D, Dean WL, Tyagi SC and Roberts
AM: Mechanisms of fibrinogen-induced microvascular dysfunction
during cardiovascular disease. Acta Physiol (Oxf). 198:1–13. 2010.
View Article : Google Scholar
|
|
26
|
Guo M, Sahni SK, Sahni A and Francis CW:
Fibrinogen regulates the expression of inflammatory chemokines
through NF-kappaB activation of endothelial cells. Thromb Haemost.
92:858–866. 2004.PubMed/NCBI
|
|
27
|
Sen U, Tyagi N, Patibandla PK, Dean WL,
Tyagi SC, Roberts AM and Lominadze D: Fibrinogen-induced
endothelin-1 production from endothelial cells. Am J Physiol Cell
Physiol. 296:C840–C847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jensen T, Kierulf P, Sandset PM,
Klingenberg O, Joø GB, Godal HC and Skjønsberg OH: Fibrinogen and
fibrin induce synthesis of proinflammatory cytokines from isolated
peripheral blood mononuclear cells. Thromb Haemost. 97:822–829.
2007.PubMed/NCBI
|
|
29
|
Smiley ST, King JA and Hancock WW:
Fibrinogen stimulates macrophage chemokine secretion through
toll-like receptor 4. J Immunol. 167:2887–2894. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soskić SS, Dobutović BD, Sudar EM,
Obradović MM, Nikolić DM, Zarić BL, Stojanović SD, Stokić EJ,
Mikhailidis DP and Isenović ER: Peroxisome proliferator-activated
receptors and atherosclerosis. Angiology. 62:523–534. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Plutzky J: Medicine. PPARs as therapeutic
targets: Reverse cardiology? Science. 302:406–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ricote M, Li AC, Willson TM, Kelly CJ and
Glass CK: The peroxisome proliferator-activated receptor-gamma is a
negative regulator of macrophage activation. Nature. 391:79–82.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang C, Ting AT and Seed B: PPAR-gamma
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galis ZS, Sukhova GK, Lark MW and Libby P:
Increased expression of matrix metalloproteinases and matrix
degrading activity in vulnerable regions of human atherosclerotic
plaques. J Clin Invest. 94:2493–2503. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tayebjee MH, Tan KT, MacFadyen RJ and Lip
GYH: Abnormal circulating levels of metalloprotease 9 and its
tissue inhibitor 1 in angiographically proven peripheral arterial
disease: Relationship to disease severity. J Intern Med.
257:110–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaneider NC, Mosheimer B, Günther A,
Feistritzer C and Wiedermann CJ: Enhancement of
fibrinogen-triggered pro-coagulant activation of monocytes in vitro
by matrix metalloproteinase-9. Thromb J. 8:2–9. 2010. View Article : Google Scholar : PubMed/NCBI
|