Introduction
Disseminated intravascular coagulation (DIC) is
frequently associated with a variety of conditions, such as severe
infection, hematological malignancies and advanced tumors (1). In addition to these diseases, numerous
pathological conditions provoke DIC. Sepsis-induced DIC is
life-threatening and is characterized by the systemic activation of
blood coagulation that generates intravascular fibrin leading to
multiple organ dysfunction syndrome and even fatality (2,3).
The mortality rate in patients exhibiting DIC was
higher than in those without DIC, and DIC treatment was shown to
reduce mortality among patients with DIC (4–6). Thus,
treatment of DIC together with therapy for the underlying diseases
may be important to increase survival rates and improve the
prognosis of patients with DIC.
Anticoagulant therapy to address systemic
pathological thrombus formation is the principle of DIC treatment.
In addition to unfractionated heparin (the mainstay of DIC
treatment), low molecular weight heparin, heparinoid, synthetic
protease inhibitors and antithrombin concentrates have been
developed and used for the treatment of DIC.
Recombinant human soluble thrombomodulin (rTM) is a
new anticoagulant agent that exerts anticoagulant and
anti-inflammatory effects through the activated protein C (APC)
pathway. Several animal studies demonstrated a reduction in
mortality with the administration of rTM in a severe sepsis model
(7,8);
however, clinical studies have been limited. rTM was approved and
is being used clinically for treatment of DIC in Japan, whereas
this agent is currently being evaluated in other countries.
In a phase III randomized controlled trial in Japan,
significant improvement in the rate of DIC resolution in the rTM
group was shown in comparison with the heparin group (9). Numerous gastrointestinal diseases cause
DIC. However, the efficacy of rTM in sepsis-induced DIC remains a
matter of dispute as there is limited evidence that it improves
clinical outcomes.
The aim of the present study was to reveal the
efficacy of rTM in patients with DIC in the gastroenterology
field.
Materials and methods
Patients
Among all the patients admitted to Juntendo
University Hospital between January 2009 and February 2014,
consecutive patients who developed sepsis-induced DIC were
analyzed. All the patients were treated for DIC. Protease
inhibitors, low molecular heparin, antithrombin III, γ globulin,
danaparoid sodium, albumin, fresh frozen plasma and rTM were
administered. The patients were divided into two groups: Those
receiving rTM in addition to one or more of the other agents (rTM
group) and those not receiving rTM (control group).
According to the Japanese Association for Acute
Medicine (JAAM) criteria (shown in Table
I), DIC was defined by a score of ≥4 and DIC was considered
resolved if the score reached ≤3. All the patients with severe
sepsis met the criteria for DIC. rTM was used at the discretion of
the attending physician. In the rTM group, rTM was principally
administered intravenously at a dose of 0.06 mg/kg/day and the
infusion was continued until the DIC state was resolved.
 | Table I.Japanese Association of Acute Medicine
criteria for acute disseminated intravascular coagulation
(DIC). |
Table I.
Japanese Association of Acute Medicine
criteria for acute disseminated intravascular coagulation
(DIC).
| Characteristics | Score |
|---|
| Systemic inflammatory
response syndrome criteria |
|
| ≥3 | 1 |
| 0–2 | 0 |
| Platelet count,
x109/l |
|
| <80 or
>50% decrease within 24 h | 3 |
| ≥80 and
<120, or >30% decrease within 24 h | 1 |
| ≥120 |
|
| Prothrombin time
(value of patient/normal value) |
| ≥1.2 | 1 |
|
<1.2 | 0 |
| Fibrin/fibrinogen
degradation products, mg/l |
| ≥25 | 3 |
| ≥10 and
<25 | 1 |
|
<10 | 0 |
| Diagnosis |
| ≥4
points | DIC |
Comparisons between the two
groups
The variables used for comparisons of the two groups
were age, gender, site of infection and DIC scores. Platelet
counts, prothrombin time-international normalized ratio (PT-INR),
fibrin/fibrinogen degradation products (FDP) and C-reactive protein
(CRP) were measured before drug infusion and on days 3 and 7 after
treatment. The JAAM DIC scores were recorded on the day the patient
met the inclusion criteria for baseline values and on days 3 and 7
after study entry.
Statistical analysis
Continuous variables are expressed as group mean
with standard deviation and were compared between groups with the
Mann-Whitney U test. Categorical variables were analyzed by the
χ2 test or Fisher's exact test, as appropriate. Changes
in laboratory data and DIC scores of the two groups were analyzed
by repeated measures analysis of variance adjusted for the baseline
values as a covariate and by the post hoc Bonferroni test.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed with SPSS version
17.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics
During the study period, 53 consecutive patients
were analyzed. The rTM group comprised 25 patients and the control
group comprised 28 patients. Baseline characteristics of the study
population and therapeutic interventions are shown in Table II. DIC scores based on JAAM criteria
were significantly higher in the control compared to the rTM group.
However, there were no significant differences in gender, age and
site of infection between the two groups. For the rTM group, the
mean duration of rTM administration was 3.6 days (range, 1.0–7.0
days).
 | Table II.Baseline characteristics of the study
patients and therapeutic interventions. |
Table II.
Baseline characteristics of the study
patients and therapeutic interventions.
| Characteristics | rTM group (n=25) | Control group
(n=28) | P-value |
|---|
| Gender |
|
| 0.71 |
| Male | 13 | 16 |
|
|
Female | 12 | 12 | 0.71 |
| Age, years |
|
|
|
| Mean ±
SD | 71.6±14.7 | 75.6±8.7 | 0.22 |
|
Range | 21–85 | 56–93 |
|
| Infection site |
|
|
|
| Biliary
tract | 15 | 20 | 0.38 |
|
Intestinal tract | 7 | 2 | 0.06 |
|
Pneumonia | 1 | 4 | 0.26 |
|
Others | 2 | 2 | 0.90 |
| DIC scores | 5.0±1.0 | 5.9±1.3 | 0.005 |
| Combination
therapy |
|
|
|
| Gabexate
mesilate | 15 | 26 | 0.005 |
|
Unfractionated heparin | 8 | 6 | 0.38 |
|
Antithrombin concentrates | 13 | 14 | 0.88 |
|
γ-globulin agent | 15 | 18 | 0.49 |
| Albumin
preparation | 16 | 15 | 0.44 |
| Fresh
frozen plasma | 1 | 4 | 0.21 |
| Duration of rTM
administration | 3.6±1.4 |
|
|
Effect of treatment on inflammation
and coagulation data
Serial changes in platelet counts, FDP, PT-INR and
CRP in the two groups are shown in Table
III. Significant intra-group improvement was observed for all
parameters except for FDP in the two groups. However, there were no
significant inter-group differences in any of the parameters
examined.
 | Table III.Serial changes in the blood
examination results. |
Table III.
Serial changes in the blood
examination results.
| Characteristics | Day 0 | Day 3 | Day 7 |
|---|
| Platelet count,
104/µl |
|
|
|
| rTM |
11.1±6.5 |
10.5±4.7 |
17.9±9.0a |
|
Control |
10.6±6.9 |
8.4±5.7 |
14.9±8.7b |
| PT-INR |
|
|
|
| rTM |
1.39±0.32 |
1.18±0.16b |
1.21±0.22b |
|
Control |
1.43±0.32 |
1.22±0.30b |
1.20±0.19b |
| FDP, µg/ml |
|
|
|
| rTM |
32.3±19.4 |
19.8±24.5 |
17.6±13.7 |
|
Control |
37.4±34.1 |
24.9±14.5 |
22.2±13.0 |
| CRP, mg/dl |
|
|
|
| rTM |
14.1±8.8 |
9.7±5.4a |
6.6±5.6b |
|
Control |
14.5±7.6 |
12.2±6.1 |
7.5±5.2b |
Effect of treatment on DIC
parameters
Serial changes in the DIC scores are shown in
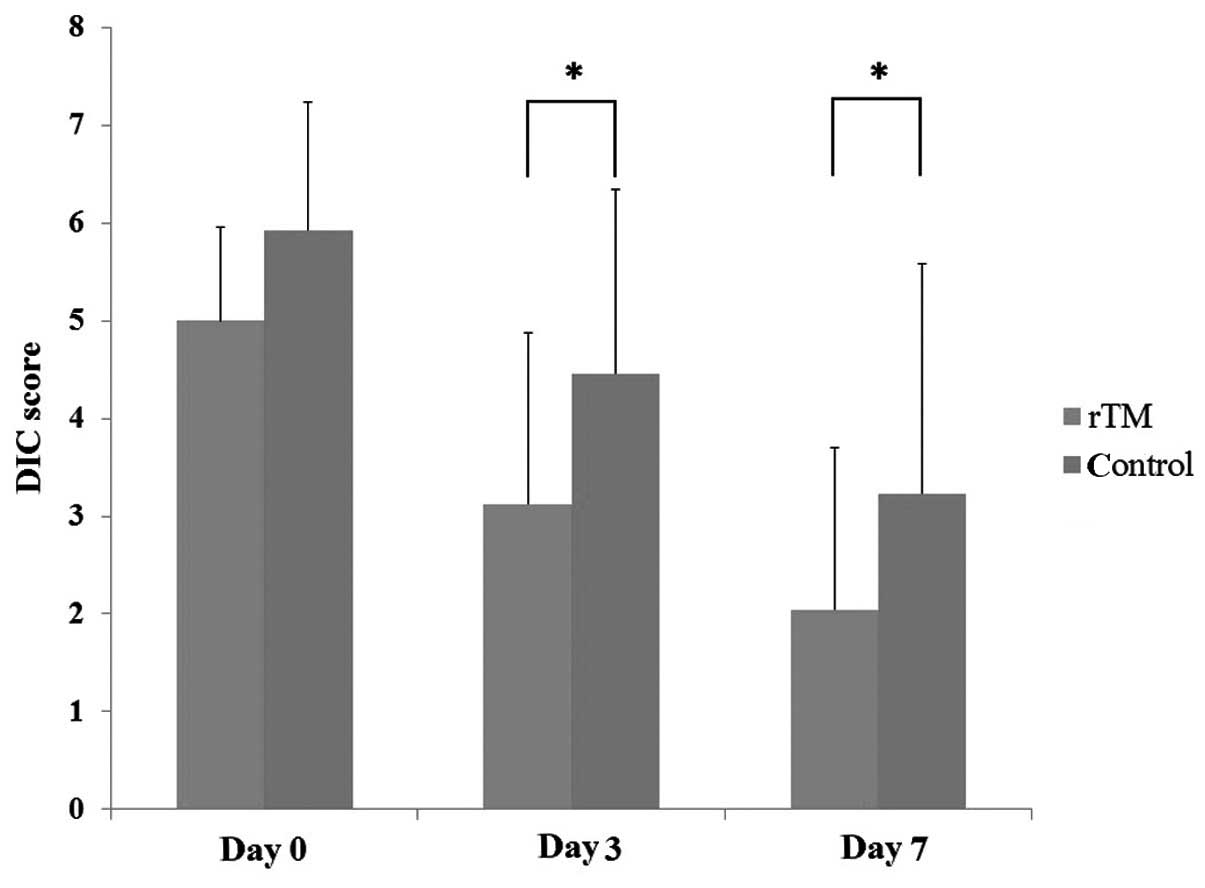
Fig. 1. The DIC scores improved
significantly in the rTM treatment group (P=0.001). The DIC
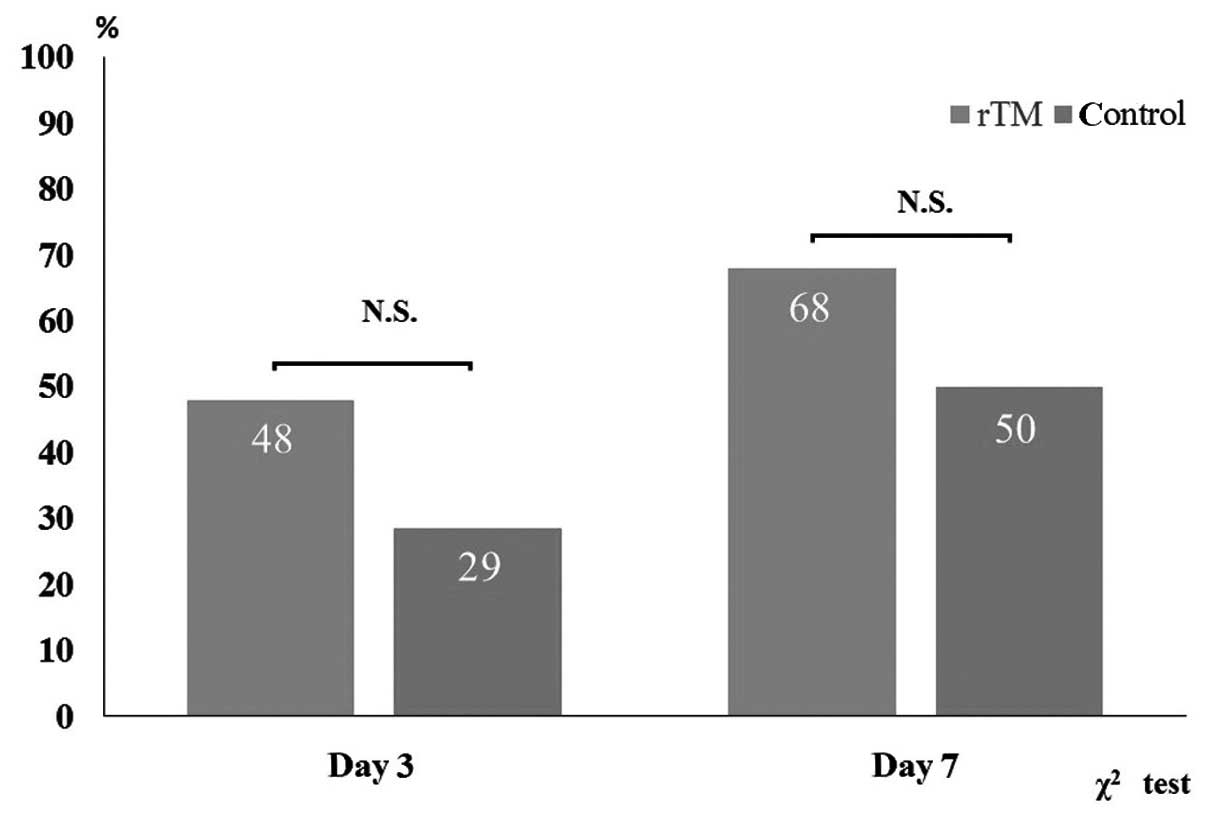
resolution rate was assessed using JAAM criteria (Fig. 2). DIC resolution rates in the rTM group
and the control group were 48 and 29% on days 3 and 68, and 50% on
day 7, respectively. However, between group differences were not
significant.
Adverse events
One adverse event associated with bleeding occurred
in the rTM group but no adverse event occurred in any control group
patient. The bleeding event in the rTM group was melena and did not
require endoscopic hemostatic treatment. However, there was no
significant difference in the incidence of this adverse event
between groups.
Discussion
As DIC is a fatal disease, and immediate resolution
of the DIC state should be critical. However, thus far no study has
evaluated the therapeutic efficacy of rTM in the early phase. To
the best of our knowledge, this is the first study to show that rTM
induced early phase improvement in DIC compared to conventional
therapy.
Gabexate mesilate (GM) was prescribed empirically to
treat DIC and it had a positive effect. GM is a serine proteinase
inhibitor that exerts an inhibitory effect on the clotting activity
of thrombin and inhibits the hydrolytic reactions of thrombin and
factor Xa with synthetic substrates; this agent was initially
approved to treat pancreatitis and was later approved to treat DIC
by the Japanese Ministry of Health, Labour and Welfare (JMHW). GM
was evaluated by several studies. Umeki et al (10) reported that GM was as effective as
heparin for the treatment of DIC and that it may be more successful
than heparin in the treatment of DIC accompanied by bleeding
diathesis. Conversely, Nishiyama et al (11) reported that GM did not inhibit
coagulation or fibrinolysis and did not improve the DIC score or
mortality rate in pre- or mild DIC in a limited number of patients.
The administration of GM is controversial; however, it is
frequently used in DIC patients even now as it does not induce
bleeding.
TM is a transmembrane glycoprotein predominately
expressed on the luminal surface of endothelial cells that line
normal blood vessels, which are in constant contact with blood
under physiological conditions. TM can bind to activated thrombin,
disabling its ability to activate platelets and cleave fibrinogen.
The TM-thrombin complex also cleaves protein C to APC, which in
turn degrades factor Va and factor VIIIa, thus preventing further
thrombin generation. Exposure of endothelial cells to inflammatory
cytokines results in downregulation of TM and increased
thrombogenicity, which could also contribute to the development of
DIC in the setting of infection.
However, only 1 study has compared the efficacy of
GM with that of rTM. Takazono et al (12) evaluated and compared the clinical
efficacies of rTM and GM for the treatment of sepsis-induced DIC.
In that study, the platelet counts of the rTM group significantly
improved compared to the GM group. The present results are
similar.
In a phase III randomized controlled trial in Japan,
the JMHW diagnostic criteria for DIC were used to diagnose and
assess DIC. DIC has been diagnosed using JMHW criteria for >20
years. However, the JAAM criteria were recently proposed for a
simple and early diagnosis and were validated in a prospective
study. Furthermore, JAAM criteria are used more frequently than
JMHW criteria in clinical settings. To the best of our knowledge,
this is the first study to evaluate the efficacy of rTM using JAAM
criteria.
Regarding causative disease, biliary tract infection
commanded a majority of the infection sites. Shimada et al
(13,14)
reported that sepsis induced biliary tract infection in highly
complicated DIC and/or multiple organ dysfunction. Therefore,
therapeutic management of DIC in these patients should be a
critical issue in the clinical setting.
In the two groups of the present study, GM was used
in a number of cases, although a larger number of patients used GM
in the control group. However, there were no significant
differences between the two groups with regard to the other agents
used.
In the study, DIC scores were significantly improved
on day 3 and this improvement was sustained on day 7 in the rTM
group compared to the control group. Regardless of the small number
of cases treated with GM in the rTM group, a good treatment outcome
was confirmed. This is the first study of an immediate improvement
in the DIC state due to administration of rTM, as confirmed by an
objective indicator, the JAAM criteria. These results indicated
that rTM would be a useful medicine for treatment of sepsis-induced
DIC.
Increased risk of bleeding is the greatest concern
with rTM administration as well as with rAPC. However, rTM is
thought to be associated with a reduction in bleeding complications
in comparison with rAPC. As rTM exerts its anticoagulant effect in
a thrombin-dependent manner, rTM does not activate protein C
following inhibition of thrombin generation. In the present study,
PT-INR and platelet counts improved significantly in the two
groups. Furthermore, there was no significant difference in
bleeding complications between the two groups. This suggested that
rTM was as safe as the other drugs for DIC treatment.
The present study has certain limitations. First,
this was a retrospective study and not a randomized controlled
study. Second, the number of patients in the study was small
compared with another larger trial, which was a randomized
controlled trial.
In conclusion, rTM may significantly benefit
patients with sepsis-induced DIC by achieving an early improvement
in DIC.
References
|
1
|
Levi M and Ten Cate H: Disseminated
intravascular coagulation. N Engl J Med. 341:586–592. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levi M and van der Poll T: Inflammation
and coagulation. Crit Care Med. 38:(Suppl 2). S26–S34. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esmon CT: The interactions between
inflammation and coagulation. Br J Haematol. 131:417–430. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kienast J, Juers M, Wiedermann CJ,
Hoffmann JN, Ostermann H, Strauss R, Keinecke HO, Warren BL and
Opal SM: KyberSept investigators: Treatment effects of high-dose
antithrombin without concomitant heparin in patients with severe
sepsis with or without disseminated intravascular coagulation. J
Thromb Haemost. 4:90–97. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wada H, Asakura H, Okamoto K, Iba T,
Uchiyama T, Kawasugi K, Koga S, Mayumi T, Koike K, Gando S, et al:
Japanese Society of Thrombosis Hemostasis/DIC subcommittee: Expert
consensus for the treatment of disseminated intravascular
coagulation in Japan. Thromb Res. 125:6–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dhainaut JF, Yan SB, Joyce DE, Pettilä V,
Basson B, Brandt JT, Sundin DP and Levi M: Treatment effects of
drotrecogin alfa (activated) in patients with severe sepsis with or
without overt disseminated intravascular coagulation. J Thromb
Haemost. 2:1924–1933. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagato M, Okamoto K, Abe Y, Higure A and
Yamaguchi K: Recombinant human soluble thrombomodulin decreases the
plasma high-mobility group box-1 protein levels, whereas improving
the acute liver injury and survival rates in experimental
endotoxemia. Crit Care Med. 37:2181–2186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iba T, Nakarai E, Takayama T, Nakajima K,
Sasaoka T and Ohno Y: Combination effect of antithrombin and
recombinant human soluble thrombomodulin in a lipopolysaccharide
induced rat sepsis model. Crit Care. 13:R2032009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saito H, Maruyama I, Shimazaki S, Yamamoto
Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M,
et al: Efficacy and safety of recombinant human soluble
thrombomodulin (ART-123) in disseminated intravascular coagulation:
Results of a phase III, randomized, double-blind clinical trial. J
Thromb Haemost. 5:31–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Umeki S, Adachi M, Watanabe M, Yaji S and
Soejima R: Gabexate as a therapy for disseminated intravascular
coagulation. Arch Intern Med. 148:1409–1412. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishiyama T, Matsukawa T and Hanaoka K: Is
protease inhibitor a choice for the treatment of pre- or mild
disseminated intravascular coagulation? Crit Care Med.
28:1419–1422. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takazono T, Nakamura S, Imamura Y,
Yoshioka S, Miyazaki T, Izumikawa K, Sawai T, Matsuo N, Yanagihara
K, Suyama N, et al: A retrospective comparative study of
recombinant human thrombomodulin and gabexate mesilate in
sepsis-induced disseminated intravascular coagulation patients. J
Infect Chemother. 20:484–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimada H, Nakagawara G, Kobayashi M,
Tsuchiya S, Kudo T and Morita S: Pathogenesis and clinical features
of acute cholangitis accompanied by shock. Jpn J Surg. 14:269–277.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimada H, Nihmoto S, Matsuba A and
Nakagawara G: Acute cholangitis: A histopathologic study. J Clin
Gastroenterol. 10:197–200. 1988. View Article : Google Scholar : PubMed/NCBI
|